Translate this page into:
Is Karnofsky Performance Status Correlate with Better Overall Survival in Palliative Conformal Whole Brain Radiotherapy? Our Experience
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
Brain metastases (BMs) are a common event in the progression of many human cancers. The aim of this study was to evaluate the potential prognostic factors for the clinical identification of a subgroup of patients that could benefit from whole brain conformal radiotherapy (WBRT).
Materials and Methods:
From January 2010 to February 2014, 80 patients with a diagnosis of BMs underwent WBRT at our Radiation Oncology Department, San Luigi Hospital, Italy. Among them, 36 medical records were retrospective reviewed. Gender, age, Karnofsky performance status (KPS), number of BMs on computed tomography and/or magnetic resonance images, presence or absence of perilesional edema, presence or absence of necrosis pattern, and histology of primary tumor were analyzed. Univariate and multivariate analyses were performed.
Results:
In our cohort of patients, significant prognostic factors for 20 months overall survival was KPS> 70, while a statistical trend (P = 0.098) was registered regarding primary breast.
Conclusion:
WBRT can be still considered a standard and effective treatment in patients with BMs. High KPS and breast cancer primary tumor seem to be useful parameters for characterize a subgroup of patients with more favorable prognosis.
Keywords
Brain metastases
Karnofsky performance status
Prognostic factors
Whole brain radiotherapy
INTRODUCTION
Brain metastases (BMs) represent a significant healthcare problem. Tsao et al., estimated that 20–40% of patients with cancer will develop BMs during the course of their illness.[1] More than 80% of BMs are detected after the primary tumor has been diagnosed (metachronous metastases) and less frequently, they are the first manifestation of disease or are diagnosed at the same time as the primary tumor (synchronous metastases).
Schouten affirmed that literature data reported that, the 5 years cumulative incidence of BMs was approximately 16%, 10%, 7%, 5%, and 1% for lung cancer, renal cell cancer, melanoma, breast cancer, and colorectal carcinoma, respectively.[2]
Due to the advanced nature of disease at the time of presentation in patients with intracranial metastases, particularly if symptomatic, treatment options have been limited.
According to Borgelt et al., all patients with BMs typically receive corticosteroids, which will improve edema and neurologic symptoms in approximately 70% of patients, nonetheless, the median survival with steroids alone is approximately 2 months.[3]
The irradiation of the whole brain radiotherapy (WBRT) remains the most commonly undertaken treatment for multiple metastases and is associated with increases in the median survival, compared with steroids alone, to approximately 4–6 months. Because of WBRT leads to serious neurologic or neuro-cognitive deficits (motion and eloquio reduction), in selected subgroup of patients this technique must be discussed in relation to other therapeutic alternatives such as stereotactic radiotherapy (SRT) and radiosurgery (SRS) or the use of new chemotherapy drugs.[4]
However, for patients with short life expectancy and poor performance status, supportive care alone may be the more appropriate oncologic approach.
In according to data published on 2012 from “international practice survey on the management of BMs” different treatment schedules are administered in WBRT, particularly 30 Gy in 10 daily fraction is most used in USA and Europe while 20 Gy in five daily fractions is preferred Canada and Australia/New Zeeland.[356]
Survival prediction model that might lead decision making when choosing between best supportive care and WBRT were developed.[7]
The aim of our retrospective study was to analyze the outcomes of patients with newly diagnosis of multiple BMs referred to our Institution for WBRT, in order to identify the prognostic factors useful for clinical identification of a subgroup that really benefits from radiation therapy.
MATERIALS AND METHODS
From January 2010 to February 2014, 80 patients with diagnosis of BMs, not eligible for SRT or SRS, underwent WBRT at our Institution. In this preliminary study, were retrospective anlyzed 36 medical records of patients, with a mean age of 63 years (range from 30 to 79), selected because they had performed a radiation treatment comparable in terms of contouring, dose, and fractionation.
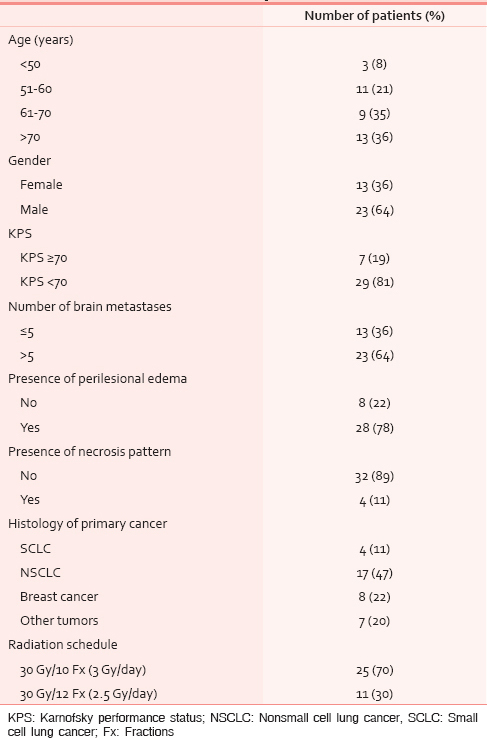
With the intent of detecting several prognostic factors useful to identify patients who really benefit from WBRT compared to those to be addressed to supportive care alone, were examined all the following clinical and radiological parameters: Gender, age (≤50 vs. 51–60 vs. 61–70 vs. >70 years), Karnofsky performance status (KPS) (KPS < 70 vs. KPS ≥ 70), number of BMs on computed tomography (CT) and/or magnetic resonance images (≤5 and >5), presence or absence of perilesional edema, presence or absence of necrosis pattern, and histology of primary tumor [Table 1]. In our retrospective study, these data were extrapolated from computerized medical records, which were recorded by the radiation oncologist at the time of the medical examination.
As a retrospective study, the Ethics Committee approval was not required.
Although crucial in the choice of treatment strategy, predicting accurately the length of survival of BMs cancer patients is difficult because systemic therapies often led patients’ survival beyond estimated survival rates. Therefore, in this first analysis status of systemic disease was not considered. Steroid therapy was administrated in all patients along WBRT course, with a tailoring dosage in relation to the neurological symptoms and signs reported before, during, and after treatment.
Patients will be immobilized in the supine position using an individually customized thermoplastic face mask (Klarity Medical and Equipment Co. Ltd., Guangzhou, China). For treatment planning a noncontrast-enhanced CT for virtual simulation (AcqSim, Philips, the Netherlands) was performed, including whole brain down to the inferior plate of the II cervical vertebral body. Images were transferred to a treatment planning system (Oncentra MasterPlan v3.3, SP 3, Nucletron, Elekta, Atlanta, GA, USA). Planning target volume (PTV) was generated adding 5 mm margin to the whole brain clinical target volume. The selected organ at risks such as a lens, optic nerves, eyeballs, and spinal cord were outlined. WBRT was administered using two opposite shaped latero-lateral fields with 45° collimator rotation. The field-in-filed technique was implemented in order to reduce the percentage of PTV receiving more than 110% (V 110%) and 107% (V 107%) prescribed dose, as well as Dmax. A dose of 2.5–3 Gy was delivered with six megavoltage machine, 5 days/week, for 12–10 fractions for a total dose of 30 Gy.
All patients alive at the time of analysis were censored with the date of last follow-up. Survival was calculated from the 1st day after the end of WBRT.
Statistical univariate analysis (Kaplan-Meier method and log-rank test) and multivariate analysis (Cox hazards proportional model) were performed in order to identify prognostic factors influencing survival in case of WBRT administration. A P = 0.05 was considered statistically significant.
RESULTS
Males and females constituted the 64% and 36% of the group, respectively. Nonsmall cell lung cancer (NSCLC) represented the most common histological type (47.5%) followed by breast cancer (22%) [Table 1]. All patients completed the WBRT planned course.
The overall median survival time was 6 months [Figure 1]. The 6, 12, and 20 months overall survival (OS) rate were 47%, 19%, and 17%, respectively.

- Median overall survival time
In our clinical records, patients with breast cancer had a better median survival of 9 months compared with 3.5 months for NSCLC.
On univariate analysis, high KPS score, absence of perilesional edema and the number of BMs were <5 were significant prognostic factors correlated with OS (P = 0.0003, P = 0.03 and P = 0.037, respectively) [Figures 2–4 and Table 2].

- Karnofsky performance status and overall survival

- Presence/absence of edema around metastatic lesions and overall survival correlation

- The number of brain metastases on radiological images and overall survival relation

On multivariate analysis, performed with cox regression, of all potential prognostic factors for OS only KPS has been confirmed statistically significant (P = 0.006), while a trend was showed for breast cancer primary tumor (P = 0.098) [Table 3 and Figure 5].


- OS in relation to histology of primary tumor
During the treatment course, 44.4% of patients had a tolerable toxicity profile whereas 28% experienced symptomatic intracranial hypertension syndrome and neurological deficits that required inpatients supportive setting. After WBRT, the most frequents adverse events were headache and dizziness (25% of incidence in both cases) whereas 25% of treated patients showed no symptomatic effects. Only two patients discontinued definitively steroid therapy after WBRT. Finally, nevertheless the radiation dose to the scalp ranged between 28 and 31 Gy in all patients, in four cases we registered. Hair regrowth after 3 months, from the end of WBRT probably, due to individual biological parameters. No treatment-related deaths were identified.
DISCUSSION
Patients with BMs are a heterogeneous population and treatment depends on the individual clinical setting. There is a lack of high-quality randomized evidence to clarify the value of WBRT versus supportive care alone in patients with BMs, while no guidelines are available for clinicians to identify which subsets of patients should be managed with supportive care alone, without WBRT.
The aim of this retrospective study was to assess the effectiveness of WBRT in adult patients with newly diagnosed BMs and to individuate potential prognostic factors useful to drive therapeutic choice that is, WBRT versus supportive care. In the present study, all patients were treated as homogeneously as possible. The limitations of our preliminary study are related to inadequate consideration about the information on the status of systemic disease at the moment of WBRT and the extensive range of time in which patients were retrospectively enrolled.
Historically, WBRT has been utilized as the main treatment modality for the management of BMs.[8] The addition of WBRT to steroids extended median survival to 3–6 months,[91011] but in the last decade there has been mounting evidence regarding the toxic effects of WBRT, especially serious neurocognitive impairments.[11] Therefore, the clinical indication of WBRT must be the result of the appropriate balance between the potential benefits and possible sequelae, taking into account the clinical implementation of modern radiation therapy approaches, such as SRS or hippocampal-avoidance WBRT, and patients performance status, toward a more tailored and disease-specific treatment management.[6]
Numerous factors in addition to the presence of active systemic disease have been correlated with poor patient survival: KPS < 70 poor neurologic status, number of BMs, age, time from primary to BMs, and tumor histology.[1213]
In patients affected with BMs, literature data show that histology of primary tumor must be considered in order to optimize the therapeutic choice, because there are histological subtypes associated with more favorable OS, such as breast cancer versus others more radio-resistant correlated with poor patient survival, such as lung cancer or melanoma.[14]
In particular, to date, diagnosis of central nervous system involvement in breast cancer patients is becoming more common because of treatment strategies are improving with a longer OS.[15]
In order to individualized a subgroup of patients that benefit from WBRT, Gaspar et al., analyzed 1200 patients from three consecutive radiation therapy oncology group (RTOG) trials, which tested several different WBRT fractionation schemes and radiation sensitizers, using the RTOG recursive partitioning analysis (RPA) prognostic system. A prognostic subgroup of patients was identified according to age at diagnosis, absence or presence of extracranial disease, KPS score and status of primary cancer. On the basis of this analysis, the median survival of patients with BMs ranges from 2.3 to 7.1 months, opening the debate regarding whether the patients in the best prognosis category should or should not be treated with more aggressive therapies to control intracranial disease.[16] However, the RPA classification failed to correctly predict long-term survival in the majority of patients.[17]
Gállego et al., in a recent publication confirmed the importance of performance status assessment in the therapeutic decision. In patients with widely disseminated cancer and short life expectancy, WBRT did not significantly improve survival compared with supportive palliative care alone. By contrast, in patients with the extra cranial controlled systemic disease, good performance status, and a limited number of BMs WBRT may be a beneficial therapeutic option both for the quality of life and survival.[18]
Multivariate analysis of our preliminary data shows that the KPS correlates with significant evidence to a better prognosis while a statistically significant trend is highlighted for breast cancer, and this is in agreement with literature data. Regarding the number of BMs was not detected statistical significance, as it could be seen from the literature, perhaps for the small power of our sample.
Knowledge of prognostic factors of patients with BMs is crucial for appropriate therapeutic choice and our preliminary study indicate that, even if in this heterogeneous group of few patients, KPS and breast cancer primary tumor are parameters useful to help clinicians to individualize well-selected patients with good life expectancy, in according with literature. Especially KPS, beyond the small number of patients of our preliminary study was anyway an important prognostic factor, immediate and easily available, useful in daily clinical practice everywhere.
CONCLUSIONS
Whole brain conformal radiotherapy continues to be an efficacious treatment in the management of BMs. There is clear recognition that not all BMs patients that undergo WBRT have survival outcome equivalently. Patients with poor performance status, extensive brain disease, and unfavorable primary tumor histology have less evident beneficial effects from WBRT and probably are best managed with supportive care alone.[7]
Taking into account specific prognostic factors, in our preliminary, high KPS, and breast cancer primary tumor seem to characterize a subgroup of patients with favorable prognosis.
ACKNOWLEDGMENTS
The authors thank the individuals who participate in this study. None of the authors had financial or personal conflict of interest with regard to this study.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869.
- [Google Scholar]
- Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698-705.
- [Google Scholar]
- The palliation of brain metastases:Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1-9.
- [Google Scholar]
- Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260-6.
- [Google Scholar]
- International practice survey on the management of brain metastases: Third International Consensus Workshop on Palliative Radiotherapy and Symptom Control. Clin Oncol (R Coll Radiol). 2012;24:e81-92.
- [Google Scholar]
- Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7:1633-8.
- [Google Scholar]
- Future directions in treatment of brain metastases. Neuro-Oncology - A Supplement to Surgical Neurology International. 2013;4(Suppl 4)
- [Google Scholar]
- Best supportive care in patients with brain metastases and adverse prognostic factors: Development of improved decision aids. Support Care Cancer. 2013;21:2671-8.
- [Google Scholar]
- Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-34.
- [Google Scholar]
- Brain metastases treated with radiosurgery alone: An alternative to whole brain radiotherapy? Neurosurgery. 2003;52:1318-26.
- [Google Scholar]
- Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-72.
- [Google Scholar]
- Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys. 1998;42:155-9.
- [Google Scholar]
- Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795-803.
- [Google Scholar]
- The accuracy of predicting survival in individual patients with cancer. J Neurosurg. 2014;120:24-30.
- [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp
- [Google Scholar]
- Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-51.
- [Google Scholar]
- A new survival score for patients with brain metastases who received whole-brain radiotherapy (WBRT) alone. Radiother Oncol. 2013;108:123-7.
- [Google Scholar]






