Translate this page into:
A Reflection on the Experience with Conducting a Clinical Audit Aimed at Optimizing Pain Assessment in Cancer Patients in Sri Lanka
Address for correspondence: Dr. Gunasekara Vidana Mestrige Chamath Fernando, 185, Vijaya Kumaratunga Mawatha, Colombo 000500, Sri Lanka. E-mail: chemetf@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
One of the principle obstacles identified in suboptimal management of pain in worldwide cancer patients is inadequate assessment of pain which in turn leads to poor management. In Sri Lanka, this is heralded by the lack of medical or nursing professionals qualified in Palliative Medicine/Care to date in Sri Lanka.
Aim:
The aims of this clinical audit were to raise awareness and optimize the assessment of pain among resident patients of a tertiary care cancer hospital by oncology doctors.
Methods:
A simple “pain and associated symptom chart” was designed for the doctors of the tertiary care cancer institution to document pain experienced by resident cancer patients in terms of intensity, both upon admission and on daily clerking. The expected standards were 100% documentation for each, regardless of the presence of pain on a visual analog scale (0–10). Documentation of the site and character of pain were expected to be 80% each.
Results:
Despite conducting three audit cycles with staff training and clarifications in between, the pain assessment practices did not be improve significantly (P > 0.05). In the third/ultimate audit cycle, it was noted that 23.5% of the charts were marked as “0” pain intensity upon admission and have been neglected thereafter.
Conclusions:
Pain assessment practices of the tertiary care oncology unit concerned was suboptimal. Therefore, it is of utmost importance to explore obstacles and incorporate pain assessment as a mandatory routine practice in clinical oncology units.
Keywords
Cancer pain
clinical audit
pain management
pain measurement
palliative care
INTRODUCTION
Pain is recognized to be the most distressing of symptoms experienced by cancer patients after lack of energy.[1] A systematic review carried out with pooled data over the past 40 years revealed that more than a half of patients with cancer suffer pain[23] which reaches a 75% among patients with advanced cancer.[4]
Although 90% of patients with cancer-related pain have the potential of attaining relief with the available medical interventions,[5] there is evidence that management of pain in cancer patients worldwide remains suboptimal.[6] Inadequate “pain assessment” was identified as the single most important barrier,[78] mitigation of which has been found to optimize pain control.[9]
I am a lecturer in family medicine in a medical school of the country with a special interest in palliative care. I work in the palliative care unit that was established in the tertiary cancer institution in 2015 on a voluntary basis in the capacity of a physician with institutional permissions. I aim to improve the quality of palliative care delivered to the patients in liaison with the consultant oncologists of the institution.
In Sri Lankan clinical settings ranging from primary care to oncology intensive care where I have personal work experience, use neither validated pain assessment tools nor perform routine clinical audit. Information from colleagues working in oncology units in different regions of the island confirmed these facts. Lack of published evidence on clinical audits aimed at pain assessment in local oncology settings also informed the decision to embark on this exercise. Hence, the dual challenge of introducing pain assessment as well as clinical audit process was apparent at the very outset.
Therefore, this audit was aimed to support the development of a standardized “assessment of pain” of patients in an in-ward facility at a tertiary care cancer institution in Sri Lanka, thus improving patients’ quality of life as a result of better pain management. This was expected to honor the international human right of each person for pain relief and palliative care.[10111213]
SUBJECTS, METHODS, AND RESULTS IN EACH AUDIT CYCLE
Setting and permissions
The audit was conducted with permissions from the director of the institution. Ethics approval was sought but was deemed unnecessary.[14] The in-patient oncology unit chosen was that of a consultant with whom I work in liaison. It was anticipated that this exercise would improve the quality of patient care. The entire clinical team (all the nurses and doctors) were instructed to participate as stakeholders in the audit team. In addition, the hospital director and myself were part of the team.
Initial meeting
The stakeholders were not familiar with the term clinical audit. This was explained at a team meeting as a process that helps to align the institutional practice to the current best evidence as opposed to a fault finding exercise. It was further appreciated that this would be an honorary opportunity for the medical officers in oncology to manage cancer pain effectively, in the absence of dedicated local hospital-based palliative care teams. There was no expressed objection from any member of the initially formed team toward participating in the audit process.
Setting criteria and standards
The standards and the procedure to follow were discussed and agreed. Standards were created based on the pain assessment tool “brief pain inventory” (BPI) since it has led to satisfactory results when used in combination with World Health Organization analgesic ladder for comprehensive assessment and management of cancer-related pain.[415]
Since “access to pain relief” is globally regarded as a fundamental human right,[12] inquiry of the presence of pain and its intensity were expected to be 100%. Other standards were set at the following levels in consensus among the team members, concerning the relative importance in management of pain as represented in Table 1.
| Criteria to be assessed ‘upon admission’ and during ‘daily clerking’ | Target (percentage of patients) | Strength of evidence | Exceptions |
|---|---|---|---|
| Presence or absence of pain | 100%* | The Brief Pain Inventory (BPI) developed by the Pain Research Group of the WHO Collaborating Centre for Symptom Evaluation in Cancer Care. BPI-Short Form (11) - a powerful tool with cross cultural reliability and validity | Patients who cannot comprehend and/or communicate due to dementia, impaired consciousness and children <12 years of age were excluded |
| Intensity assessment tools: the visual analogue scale (VAS), verbal rating scale, numerical rating scale (NRS) or equivalent (14).Verbal Rating Scale (VRS | 100%* | ESMO Clinical Practice Guidelines (17) further states the direct implications of assessing the ‘presence of pain’ and its ‘intensity’ using appropriate scales for optimum pain management; hence the level of standard set at 100% | |
| Character | 80%# | The character of pain will be drawn from BPI-Long Form as there are direct implications of knowing the ‘character’ of pain (e.g., neuropathic or nociceptive) to implement proper management (16,2); hence the level of standard set at 80% and so was ‘site’ of pain | |
| Site | 80%# | The site of pain was assessed to ascertain the potential tissue/organ system of origin of pain which has a bearing on management. (e.g., suprapubic pain → ? Bladder outflow obstruction) | |
| Relief with current analgesic medication | 50%^ | Despite their significance in proper management of pain (17,19), the ‘reviewing of analgesics’ and ‘assessment of interference of activity, psychosocial wellbeing and sleep’ with pain were considered to be of secondary importance | |
| Inquiry about the interference of pain with activity, psychosocial wellbeing and sleep. | 50%^ |
100%*: (mandatory to guide prescription of analgesics according to WHO analgesic ladder), 80%#: (required to ascertain the origin and pathophysiology of pain; nociceptive or neuropathic, to guide conventional and adjuvant analgesics), 50%^: (of secondary importance in the management of pain and the extensiveness of assessment was expected to contribute towards impaired clinician compliance with pain assessment guidelines; hence standards of which were set at 50% each. (considered to be removed following the preliminary audit cycle)
Data collection
Data collection was carried out by myself using a structured format based on BPI before implementation of any changes to the existing practice [Appendix 1].
Audit cycle 1
During the first audit cycle, the documentations made by medical officers on pain were explored inpatient records of all the in-ward patients in the unit. A total of 32 records were analyzed where documentation of pain upon admission [Figure 1] and daily clerking (on the day before the date of auditing) [Figure 2] and were compared with the agreed standards and communicated with the team.

- Expected and observed standards for pain assessment in in-ward cancer patients upon admission
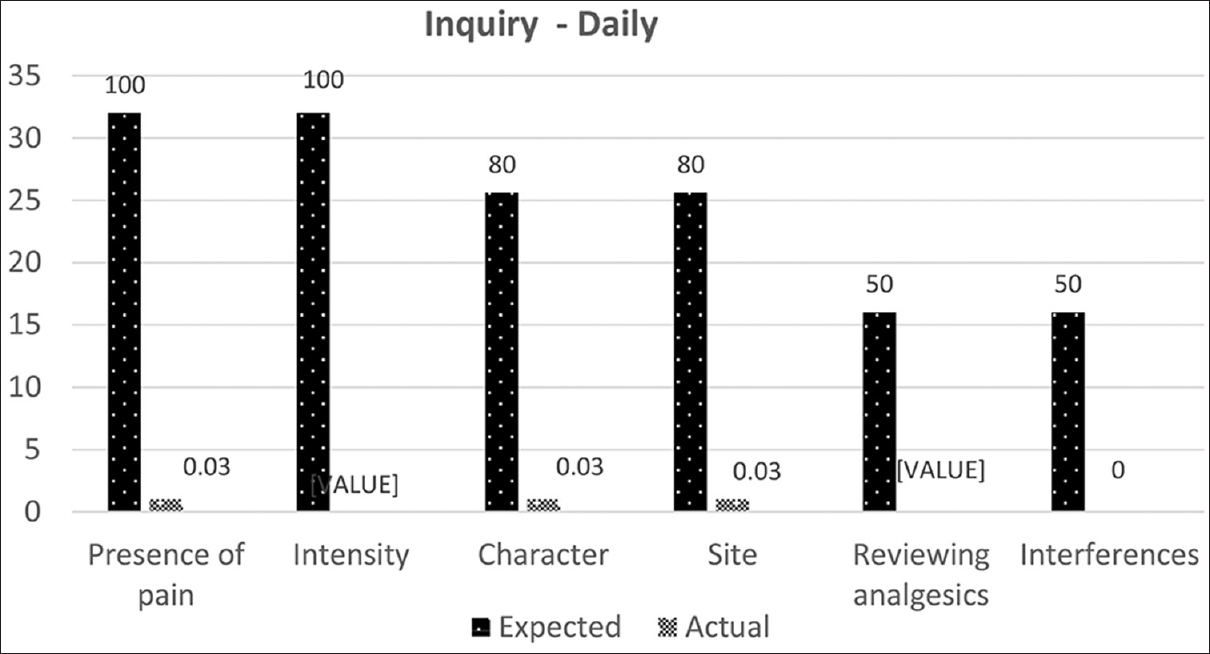
- Expected and observed standards for pain assessment in in-ward cancer patients upon daily clerking
An extremely low (22%) rate of documentation of the presence of pain upon admission and a poorer (0.03%) rate on daily clerking were noted. None of the records revealed information about pain intensity on any of the accepted scales, previous analgesic medication review, or interference of pain with well-being.
Implementing change – Phase 1
The stakeholders’ suggestions were obtained so as to devise a mechanism to ensure proper assessment of pain in cancer patients. The main concern of the team was the additional workload resulted by the introduction of the form.
Hence, a simplified version of BPI was proposed to be incorporated as a standard part of the patient records. The following collective decisions were arrived at:
-
To minimize written descriptions.
-
To use a pictorial diagram of human body to mark the site/s of pain
-
To use Wong–Baker pain scale (WBPS)[16] integrated with numbers to mark the pain score. Apart from its use in children, WBPS can also be used reliably in adult patients with cognitive impairment, limited education, low literacy rates, or dyslexia
-
To have the list “character of pain” extracted from BPI-long form for the doctors to select the most appropriate option from.
-
-
To monitor the “presence” and “intensity” of pain on a daily basis
-
To assess the “site” and “character” of pain upon admission only
-
Detailed assessment of pain was considered to be adding to the complexity of work, thus diminishing clinician compliance; hence omitted from the format
-
An optional area designated as “associated symptoms/notes” was decided to be added for reviewing adverse effects of drugs and to note down associated symptoms.
The “pain and associated symptom chart” devised is shown in Figure 3.
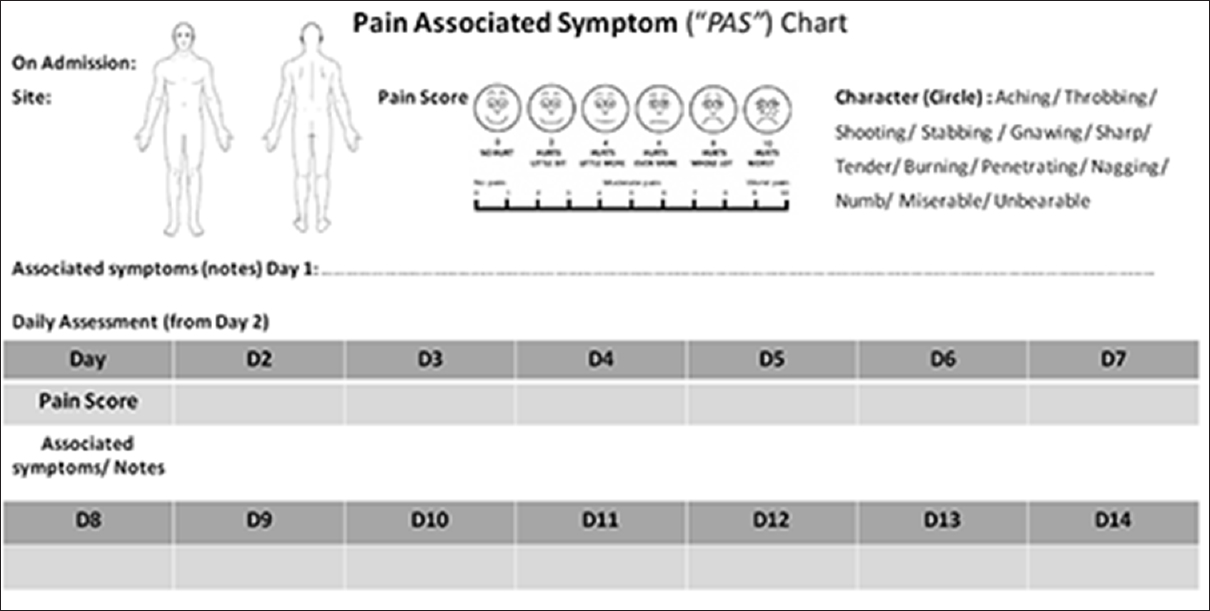
- Pain and associated symptoms chart
The new standards agreed upon are mentioned in Table 2.
| Areas to be assessed for documentation | Expected (%) |
|---|---|
| Annexure of chart by nursing staff | 100 |
| On admission - doctor | |
| Inquiry of presence of pain | 100 |
| Intensity of paina (in terms of VAS) | 100* |
| Site of paina | 80* |
| Character of paina | 80* |
| Daily clerking - doctor | |
| Intensity of painb (in terms of VAS) | 80 |
*Applicable only to those who have responded to be experiencing pain upon inquiry at any stage. If a patient was pain-free upon initial inquiry and it was marked as “0” in the chart, subsequent inquiry of the intensity, character, and site of pain were considered to be appropriately marked by the clinicians (although the fields were left blank). VAS: Visual analog scale. aParameters read upon admission,bParameters read upon daily clerking
Having distributed the printed forms, the nursing staffs were instructed to include a single chart for each new admission regardless of the presence of pain (since pain may occur later during the course of hospital stay). The doctors were trained to maintain charts up to date. My availability was offered throughout the duration for any clarification.
Regular contact maintained with the responsible on-site doctor assured me that the process was continuing without the need for clarifications.
Audit cycle 2
This was carried out on a random day of the 2nd week from commencement. The results pertaining to the total of 16 clinical records (all resident patients admitted after implementation of the new pain assessment form) accounted for in the analysis are briefed in Figure 4.
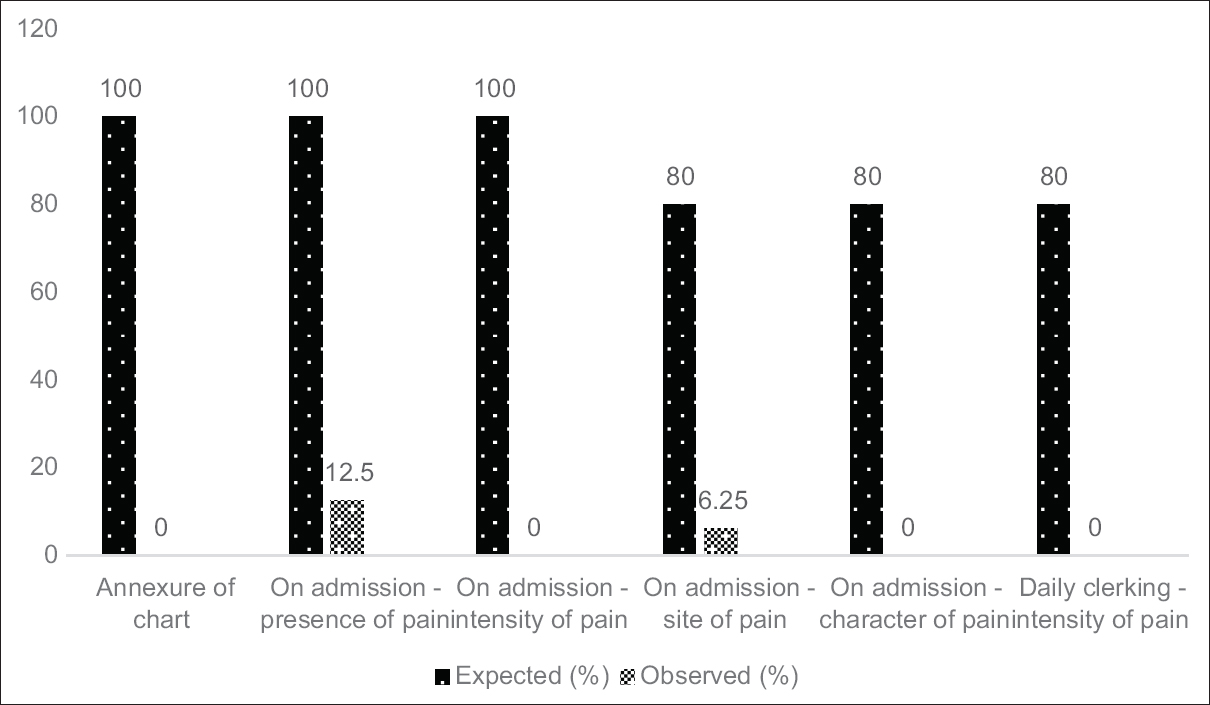
- Comparison between observed practice of pain assessment with agreed standards – Audit Cycle 2. If a patient was pain-free upon initial inquiry and it was marked as “0” in the chart, subsequent inquiry of the site, character, and intensity on daily clerking of pain were considered to be appropriately marked by the clinicians (even if the fields were left blank). #: Details. extracted from the patient records (not the standard form)
Since no forms were attached to the patients’ records, the pain-related details assessed were sought in the doctors’ notes. No patients were on analgesics including the 2 patients who were mentioned to be suffering pain on the records.
A further meeting was called where the barriers reflected in these poor results were discussed. Some team members stated that they did not think that the process was important. The team was given the option to terminate the audit. However, the consultant and the majority decided to continue as a team. The team decided that further modification of the pain assessment tool (Example: Diminishing the components in the chart) would yield inadequate information to sufficiently manage pain. They expected to improve outcomes with further training of the staff.
Implementing change – Phase 2
Predetermined standards were unaltered. It was made sure with succinctly written instructions and verbal clarifications that everyone in the team had understood their individual roles. The process was reaudited in the subsequent week and the results were analyzed in detail [Table 3].
| Areas to be assessed for documentation | Expected | Observed | |
|---|---|---|---|
| Annexure of chart by nursing staffs | 100% | 0% | |
| On Admission - doctor | Inquiry of presence of pain | 100% | #12.5% |
| Intensity of paina (in terms of VAS) | *100% | 0% | |
| Site of paina | *80% | #6.25% | |
| Character of paina | *80% | 0% | |
| Daily Clerking - doctor | Intensity of painb (in terms of VAS) | 80% | 0% |
Audit cycle 3
Of the 17 clinical records assessed, the PAS chart was only annexed to 6 (35.3%). Out of them, 4 (23.5% of all) were observed to be marked as “0” (absent) pain upon admission [Figure 5]. An additional analysis revealed that three of those who were not suffering pain according to the chart were on the analgesic combination of tramadol and paracetamol. In five other records, the presence of pain was documented elsewhere on the patient clinical record than the chart. Two of them did not receive any form of analgesia despite documentation of pain. None of the patients were assessed for pain on a daily basis.
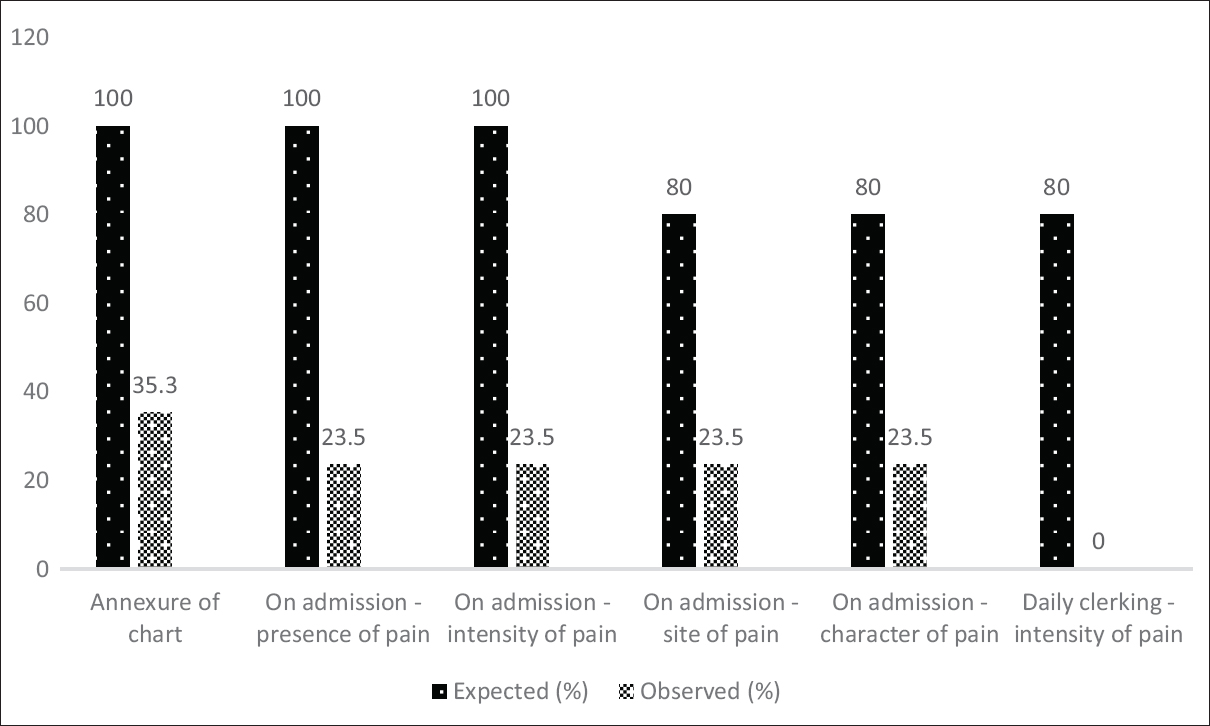
- Comparison between observed practice of pain assessment to agreed standards – Audit Cycle 3. ^: Since the intensity of pain was marked as “0,” the site and character of pain have been considered to be filled correctly although the fields were left blank
The assessment of the presence of pain upon admission did not alter among the doctors with the audit process (P > 0.05). These findings were presented to the team and assistance offered, should they wish to conduct clinical audits in the future.
DISCUSSION
The disappointing results of the audit process, despite the attempts to improve existing pain assessment practice led to identification of many potential obstacles. There appeared to be resistance from the professionals toward introduction of the novel concept of “clinical audit” and challenges around the attempt to initiate change by an authorized, yet voluntary, academic like myself with an ill-defined role in the particular institution.
My intention of engaging the hospital director was to persuade other units to optimize their pain assessment. However, he could not attend the meetings which he attributed to the overwhelming workload. I believe the fact that the clinical audit, not being among the routinely recommended processes by health authorities of Sri Lanka would have contributed significantly to their beliefs on its low importance.
The continuous availability of opioids and other analgesics is not generally a barrier in this tertiary care cancer institution. However, the lack of consultants in palliative care in the country, who would place symptom management within their first priorities, was perceived as a barrier to emphasize the importance of pain assessment and management to hospital authorities and the government. The limited bed strengths of this hospital would also have led to discouragement of the authorities to spare beds for symptom palliation as opposed to oncological treatment.
While a few members obviously avoided meetings, no alternative suggestions were made to the clinical audit to improve the existing practice to improve pain relief. Eventually, the oncologist expressed his view that his team was poorly trained to provide “quality of life” oriented care. Thus, the whole exercise appeared to demonstrate that incorporating even a single criterion of the audit which had been streamlined to the agreed standards involved many challenges. These included a lack of experience with, and engagement in, both the discipline of palliative care and the practice of clinical auditing and lack of recommended sets of local guidelines on pain assessment and clinical auditing.
In the United Kingdom, the care quality commission ensures the quality of health-care delivery that is partially achieved through clinical audits.[17] Similarly, certain fellow nations in the South Asian region as Sri Lanka have established regulatory frameworks for the clinical establishments; most notably the Clinical Establishments Act India although the institutional and legislative support to conduct clinical audits have been identified as insufficient.[18]
Before the introduction of clinical audit process as a mandatory requirement in Sri Lankan health-care systems, further qualitative research will be useful to identify barriers with key stakeholders including the patients. Many overseas models aiming for a change, reflect on such barriers and stress that role modeling behavior change is important for success.[192021] Inadequate expertise among clinicians in pain assessment and basic principles of management, poor consensus on the most appropriate pain assessment means, fear of adverse effects related to analgesic medications; especially “opiophobia,”[22] poor accessibility to narcotic analgesics, lack of referral from primary and secondary care to palliative care services, and lack of supportive care services have all been implicated in poor pain assessment and management among cancer patients.[58232425] Certain measures which have been implemented (Example: Assignment of pain as a 5th vital sign) have also led to less promising results than expected.[26] However, a rapid review of elements of palliative care models revealed that integration between specialist expertise and community resources are invaluable to empower transitions between different care settings from residential care to tertiary level.[27]
One of the senior consultants’ important roles also is to constantly strive to improve standards in clinical practice.[28] Of the symptoms concerned, assessment and management of physical pain would be an ideal and feasible objective to achieve initially, its higher incidence (>50%), ease of assessment, and the availability of effective behavioral, pharmacological, and interventional measures to alleviate it.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This audit was undertaken by the author as part of the MSc in Palliative Medicine at Cardiff University, United Kingdom. The director of the cancer care institution and the relevant consultant oncologist are kindly acknowledged for their support although the names of individuals and institutions will remain anonymized as per their request.
REFERENCES
- The symptom experience of patients with cancer. J Hosp Palliat Nurs. 2012;14:61-70.
- [Google Scholar]
- Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18:1437-49.
- [Google Scholar]
- Symptoms experienced by cancer patients during the first year from diagnosis: Patient and informal caregiver ratings and agreement. Palliat Support Care. 2010;8:313-24.
- [Google Scholar]
- Pain assessment and analgesic prescription for cancer patients in a medical ward: The influence of an educational intervention. Natl Med J India. 2009;22:177-80.
- [Google Scholar]
- Barriers to effective cancer pain management: A review of the literature. J Pain Symptom Manage. 1999;18:358-68.
- [Google Scholar]
- Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19:1985-91.
- [Google Scholar]
- Physician attitudes and practice in cancer pain management. A survey from the Eastern Cooperative Oncology Group. Ann Intern Med. 1993;119:121-6.
- [Google Scholar]
- The challenges of cancer pain assessment and management. Ulster Med J. 2013;82:40-2.
- [Google Scholar]
- Undertreatment of cancer pain: Barriers and remedies. Support Care Cancer. 1993;1:74-8.
- [Google Scholar]
- Palliative care as an international human right. J Pain Symptom Manage. 2007;33:494-9.
- [Google Scholar]
- 2017. Palliative Care as a Human Right:AFact Sheet. Open Society Foundations. Available from: https://www.opensocietyfoundations.org/publications/palliative-care-human-right-fact-sheet
- Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: A focused review. Indian J Palliat Care. 2011;17:108-15.
- [Google Scholar]
- The wong-baker pain FACES scale measures pain, not fear. Pediatr Emerg Care. 2013;29:17-20.
- [Google Scholar]
- 2017. Clinical Establishments Act India | Regulatory & Policy Framework for Hospitals and Clinical Establishments in India. Dr. Hempel Digital Health Network. Available from: https://www.dr-hempelnetwork.com/health-policies-in-india/clinical-establishments-in-india/
- Health Organization Regional Office for Europe W. World Health Organization Regional Office for Europe Better Palliative Care for Older People. Available from: www.euro.who.int
- Care Network P, of Health DW. WA Cancer and Palliative Care Network Palliat Care Model Care. 2008. Available from: https://ww2.health.wa.gov.au/~/media/Files/Corporate/generaldocuments/HealthNetworks/Palliativecare/Palliative-Care-Model-of-Care.pdf
- [Google Scholar]
- Opiophobia as a barrier to the treatment of pain. J Pain Palliat Care Pharmacother. 2002;16:105-9.
- [Google Scholar]
- Overcoming barriers to cancer pain management: An institutional change model. J Pain Symptom Manage. 2007;34:359-69.
- [Google Scholar]
- Barriers to cancer pain relief: Fear of tolerance and addiction. J Pain Symptom Manage. 1998;16:1-9.
- [Google Scholar]
- Barriers to pain assessment and management in cancer survivorship. J Cancer Surviv. 2008;2:65-71.
- [Google Scholar]
- Elements of effective palliative care models: A rapid review. BMC Health Serv Res. 2014;14:136.
- [Google Scholar]
- 2015. Consultant Physicians. London: RCP; Available from: https://www.rcplondon.ac.uk/education-practice/advice/consultant-physicians
APPENDIX
Appendix 1:
Hospital Unit: _____ Bed Number: _____
Bed-Head Ticket Number: _____ Site/Type of Malignancy: _____
| Dimension | Documented On admission | Documented Today (Daily clerking) | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Presence of “pain” | ||||
| Site of pain | ||||
| Character of pain | ||||
| The pain score* | XXXXXXXXXXX | XXXXXXXXXXXXX | ||
| When the pain is | ||||
| worst | ||||
| When the pain is | ||||
| least | ||||
| On average | ||||
| At the moment | ||||
| Reviewing of current medication | XXXXXXXXXXX | XXXXXXXXXXXX | ||
| Drug name | ||||
| Degree of pain | ||||
| relief with them | ||||
| Interference with activities within the past 24 hours | XXXXXXXXXXX | XXXXXXXXXXXX | ||
| General activity | ||||
| Mood | ||||
| Walking ability | ||||
| Employment/ | ||||
| household work | ||||
| Relationships with | ||||
| people | ||||
| Sleep | ||||
| Enjoyment of life | ||||
| Medicine prescribed | ||||
*Pain Score on Visual Analog Scale, Verbal Scale or Numeric Scale






