Translate this page into:
5-Fluorouracil-induced Reversible Encephalopathy
*Corresponding author: T. M. Varun, Department of Palliative Medicine, Tata Memorial Hospital, Homi Bhabha National Institute, Mumbai, Maharashtra, India. varuntmv95@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Varun TM, Prasun P, Amte D. 5-Fluorouracil-induced Reversible Encephalopathy. Indian J Palliat Care. doi: 10.25259/IJPC_12_2025
Abstract
The management of altered mental status in patients with metastatic cancer is a complex task because of various potential causes such as electrolyte imbalances, brain metastasis, cancer treatments and delirium. Anti-cancer drugs such as methotrexate and cisplatin can cause delirium, and it is crucial to recognise and manage these cases early because of the associated increased morbidity and mortality. This case report presents a rare hyperammonaemic encephalopathy induced by 5-fluorouracil (5FU), a pyrimidine uracil analogue used in gastrointestinal cancers. A 60-year-old woman with metastatic pancreatic adenocarcinoma experienced altered sensorium, incoherent speech and ataxia after chemotherapy, including high-dose 5FU infusion. Her symptoms, along with the exclusion of other common causes, led to a diagnosis of 5FU-induced encephalopathy. Palliative care, supportive care and specific treatments resulted in symptom reversal and discharge with improved functional status. This report emphasises the importance of recognising 5FU-induced encephalopathy for its timely and effective management in clinical practice.
Keywords
Altered mental status
Encephalopathy
Geriatric palliative care
Medical resident education
Oncological issues in palliative care
INTRODUCTION
The treatment of altered mental status in patients with metastatic cancer is a challenge. Several causes of altered mentation include electrolyte and metabolic abnormalities, brain metastasis, cancer treatment or delirium due to the disease itself. In addition to these, the use of some anti-cancer drugs such as Methotrexate, Cytarabine, Cisplatin, Vincristine and Ifosfamide is associated with delirium as an adverse effect.[1] The early recognition and management of delirium is important, as it may lead to increased morbidity and mortality in patients.
5-fluorouracil (5FU), a pyrimidine uracil analogue, is an antineoplastic agent commonly used to treat gastrointestinal cancers, such as carcinomas of the rectum, colon, stomach and pancreas. It is frequently associated with adverse effects such as neutropenia and thrombocytopenia due to bone marrow suppression, nausea, vomiting and diarrhoea.[2] However, hyperammonaemic encephalopathy is a rare adverse effect of 5FU, with only limited mention in the literature. Through this case report, we narrate our experience of managing hyperammonaemic encephalopathy due to 5FU at a palliative care unit composed of specialist palliative medicine physicians, palliative care nurses, psychologists and social workers functioning in a tertiary cancer hospital in India and hope to provide insights into this diagnostic dilemma for health professionals.
CASE REPORT
Our patient was a 60-year-old woman, a retired Chemistry professor, with pancreatic adenocarcinoma with metastasis to the liver. She had other comorbidities of type 2 diabetes mellitus and hypertension with a single kidney. She was on the 5th cycle of folinic acid, fluorouracil, irinotecan (FOLFIRI) chemotherapy when she was brought to the emergency department of our tertiary cancer care centre with complaints of altered sensorium, incoherent speech, ataxia and dysarthria. The patient had received 5FU at 2,400 mg/m2 as an infusion over 46 h, irinotecan 180 mg/m2 and leucoverin 300 mg 5 days prior.
The caregivers also reported nausea, with 3–4 episodes of vomiting per day and decreased oral intake for the past 3 days. They also gave a history of a minor fall in the washroom 1 day prior. On further probing, a history of a similar but milder episode after the first dose of the same chemotherapy was obtained. There was no history of fever, seizures or evidence of brain metastasis. At presentation, her Glasgow coma scale (GCS) score was 10/15 with spontaneous eye opening (E4), no verbal response (V1) and localising pain (M5), eastern cooperative oncology group (ECOG) - Performance Status 4, pulse rate - 88 beats/min, blood pressure - 140/90 mmHg and saturation on room air - 98%, normal electrocardiography. The non-contrast computed tomography brain was performed on an emergency basis and was found to be normal.
Her laboratory investigations revealed that the Hb level of moderate anaemia with haemoglobin-8.2 - 8.2 mg/dL, thrombocytopenia with a platelet count of 56,000 and the rest of the laboratory investigations were normal.
The patient was promptly referred to the Department of Palliative Medicine for further management of her symptoms. Her poor general condition and performance score (ECOG) made it unlikely for her to benefit from further aggressive disease-directed treatment by the Medical Oncology team.
On admission to the Acute Palliative Care unit, the patient was started on intravenous fluids, blood investigations were sent, and a magnetic resonance imaging (MRI) was done. The serum ammonia level was borderline high, 90 (Normal = 18–86). The diffusion-weighted (DW) MRI revealed minimal symmetrical T2/fluid-attenuated inversion recovery hyperintensities in the bilateral globus pallidus, as shown in Figure 1.
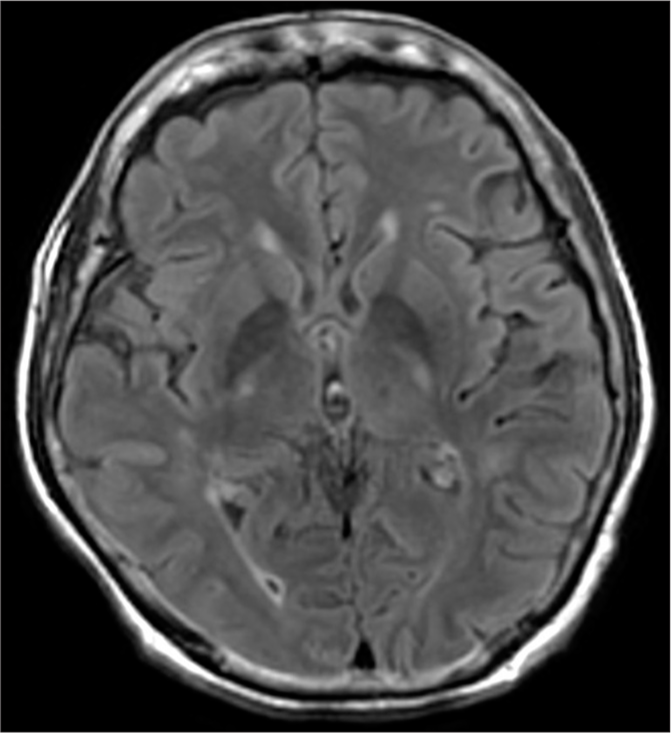
- Minimal symmetrical T2/fluid-attenuated inversion recovery hyperintensities in the bilateral globus pallidus.
Based on previous history, presentation, exclusion of other common causes and investigations, the patient was diagnosed to have developed 5FU-induced encephalopathy. Intravenous hydration was continued, and in addition, she received a thiamine infusion and haloperidol for restlessness, nausea and vomiting. The patient was initiated on frequent orientation measures concurrently with patient-tailored cognitive exercises to stimulate the brain and improve concentration, such as simple jigsaw puzzles, colouring and chemistry basics, as the patient was a chemistry professor. Physical activities were resumed as early as day 2 with the help of a physiotherapist, along with other supportive care measures.
The caregivers, who were in psychological distress due to the patient’s condition, were reassured and provided psychological support and counselling services by the psychologist in our team, which helped them cope with the present condition.
The patient’s mental status returned to baseline in 48 h with a GCS score of 15/15. Her ataxia and slurring of speech and functional status improved over 4 days, and we were able to discharge her on the 5th day with an ECOG performance status (PS) of 2 and satisfied caregivers.
DISCUSSION
5-FU, initially developed in the 1950s by Heidelberger et al., is presently a widely utilised anti-neoplastic and anti-metabolite medication for the treatment of breast, head and neck and gastrointestinal malignancies. The toxicities associated with 5FU are influenced by both dose and schedule.[2] The key effects of this medication are primarily focused on rapidly growing tissues, particularly the mucosal layer of the gastrointestinal system and the bone marrow. Myelosuppression is influenced by the administration schedule and is more likely to occur when 5-FU is administered as a bolus dose. Gastrointestinal toxicity is a common manifestation of 5-FU therapy, which is characterised by ulceration of the epithelial layer, such as mucositis, esophagitis, gastritis and colitis, often accompanied by diarrhoea.[3] In addition, 5-FU therapy can cause various dermatological toxicities, such as alopecia and changes in fingernails, as well as a pruritic erythematous rash. Ocular toxicity and, less commonly, cardiotoxicity, are also associated with 5-FU therapy. One of the infrequent adverse effects associated with 5-FU treatment is neurotoxicity, which may manifest in both acute and delayed forms.[4]
5-FU-induced encephalopathy has been observed in 5.7% of patients receiving high-dose 5-FU chemotherapy.[5] Two distinct mechanisms are recognised as contributing to its progression. The first involves dihydropyrimidine dehydrogenase (DPD) deficiency. DPD is a crucial enzyme that inactivates 5-FU, and patients with insufficient DPD may experience symptoms associated with 5-FU accumulation. Approximately 2.7% of cancer patients are reported to have DPD deficiency, which is hypothesised to result from a mutation in the gene encoding the DPD enzyme. In such instances, elevated levels of 5-FU in DPD-deficient individuals penetrate the cerebrospinal fluid, leading to rapid demyelination of neurons. The second is the milder form of 5-FU catabolite-induced encephalopathy. This mechanism suggests that high doses of 5-FU cause fluoroacetate to build up, which then directly hinders the Krebs cycle. As a result, a temporary increase in blood ammonia levels occurs due to the impairment of the adenosine triphosphate-dependent urea cycle.[6]
An alternative hypothesis regarding the neurological adverse effects of 5-FU treatment involves the potential thiamine deficiency induced by the medication. The active form of the vitamin, thiamine pyrophosphate, exhibits elevated levels upon exposure to 5-FU. This observation suggests that 5-FU may accelerate thiamine metabolism within cells, potentially exacerbating thiamine deficiency. Evidence supporting this theory stems from the similarity between the symptoms of Wernicke–Korsakoff syndrome, including ataxia, nystagmus, mental confusion and cognitive alterations and the neurotoxic manifestations of fluorouracil.[7]
Yi et al. reported the case of 5-FU-induced encephalopathy without lesions in DW-MRI, encephalopathy with hyperammonaemia within 24 h of the start of 5-FU infusion and resolving within 48 h of supportive care.[8]
Mehta et al. reported a case of 5-FU-induced acute leucoencephalopathy with diffusion-restricted lesions in the bilateral deep white matter, cerebellar peduncles and splenium of corpus callosum 2 weeks after receiving the third cycle of 5-FU-based chemotherapy.[9]
The identification of 5-FU-induced encephalopathy is contingent upon the fulfilment of three criteria. The first criterion is the occurrence of encephalopathy either during or shortly after the administration of 5-FU treatment. Second, it is necessary to eliminate other metabolic factors that could potentially affect a patient’s cognitive abilities and consciousness. These factors include hypoglycaemia, organ failure, dyselectrolytemia, septicaemia and cancer involvement in the central nervous system. Finally, the third criterion involves ruling out any adverse effect that may arise from other medications being taken concurrently.[5]
5-FU-induced encephalopathy is a reversible condition if recognised and treated early. At present, there is no definitive treatment available. Withdrawal of the offending drug (if the infusion is still ongoing), ensuring adequate hydration and managing restlessness, nausea and vomiting, followed by supportive measures like thiamine infusion, can help reverse the condition and decrease the associated morbidity. Given the patient’s improvement, we opted not to administer lactulose enema or branched-chain amino acids. However, certain studies have demonstrated remarkable outcomes in patients suffering from severe hyperammonaemia when using these treatments.[10]
CONCLUSION
5FU-induced encephalopathy is a rare but potentially reversible condition seen in 5FU-based chemotherapy. Although it is a rare adverse event, this should be promptly considered amongst patients on 5FU who present with neurological complaints. Conservative management with supportive care helps in reversal, decreasing morbidity and improving the quality of life for patients. Through our case report, we wish to increase awareness of this condition amongst healthcare providers, oncologists, palliative and supportive care professionals, which will help in its early diagnosis and timely management.
Acknowledgements:
I would like to express sincere gratitude to my professors, colleagues, patient and the caregiver.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship: Nil.
References
- Neurotoxic Complications of Chemotherapy in Patients with Cancer: Clinical Signs and Optimal Management. Drugs. 2003;63:1549-63.
- [CrossRef] [PubMed] [Google Scholar]
- Fluorinated Pyrimidines, a New Class of Tumour-Inhibitory Compounds. Nature. 1957;179:663-6.
- [CrossRef] [PubMed] [Google Scholar]
- A Case of 5-Fluorouracil Induced Encephalopathy. Cancer Res Treat. 2010;42:118.
- [CrossRef] [PubMed] [Google Scholar]
- Fluorouracil-Induced Neurotoxicity. Ann Pharmacother. 2000;34:35-8.
- [CrossRef] [PubMed] [Google Scholar]
- High-Dose 5-Fluorouracil Infusional Therapy is Associated with Hyperammonaemia, Lactic Acidosis and Encephalopathy. Br J Cancer. 1997;75:464-5.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemical Basis for Fluorouracil Neurotoxicity. The Role of Krebs Cycle Inhibition by fluoroaCetate. Arch Neurol. 1970;23:155-60.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of the Fluoropyrimidines 5-Fluorouracil and Doxifluridine on Cellular Uptake of Thiamin. Anticancer Res. 1989;9:1073-7.
- [Google Scholar]
- Acute Hyperammonemic Encephalopathy After 5-Fluorouracil Based Chemotherapy. Ann Surg Treat Res. 2016;90:179.
- [CrossRef] [PubMed] [Google Scholar]
- Teaching Neuroimages: 5-FU-Induced acute Leukoencephalopathy. Neurology. 2013;80:e191-1.
- [CrossRef] [Google Scholar]
- A case of Hyperammonemic Encephalopathy in a Patient with Recurrent Colon Cancer Treated with Modified FOLFOX6. Gan To Kagaku Ryoho. 2009;36:867-9.
- [Google Scholar]







