Translate this page into:
Comparison of Safety and Efficacy of Pregabalin versus Gabapentin for the treatment of Uremic Pruritus in Patients with Chronic Kidney Disease on Maintenance Haemodialysis
Address for correspondence: Dr. Rajesh Peringanazhi Kunnath, Flat No. 1E, Skyline Crescendo, Chevarambalam, Chevayur PO, Kozhikode - 673 017, Kerala, India. E-mail: rajeshpkunnath@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Uremic pruritus (UP) affects many patients suffering from chronic kidney disease (CKD) and has a negative impact on the quality of life. The severity of UP ranges from sporadic discomfort to complete restlessness during both day and night time. It has become increasingly evident that central transmission and sensitization processes similar to those observed in chronic pain are important mechanisms of pruritus.
Methodology:
This was a randomized single-blind prospective-interventional study carried out for 6 weeks. Male and female patients aged between 20 and 85 years with end-stage renal disease undergoing maintenance hemodialysis and who had substantial pruritus defined as persistent were included in our study. Informed consent was obtained from each patient before enrolling in the study. Patients were randomly divided into two groups (Group A and B), with Group A receiving pregabalin 25 mg and Group B receiving gabapentin 100 mg. The efficacy and safety of drugs were assessed after 6 weeks using visual analog scale and 5D itch scale.
Results:
We used gabapentin 100 mg or pregabalin 25 mg for 42 consecutive patients with CKD on maintenance hemodialysis with a mean age of patients in Group A (pregabalin 25 mg) 55.29 ± 14.58 and B (gabapentin 100 mg) 58.10 ± 11.09. Both gabapentin and pregabalin produced a significant difference in itching intensity with P < 0.001; however, there was no statistically significant difference between the effectiveness of two drugs in reducing itch. While receiving gabapentin, 11 of 21 patients (52.38%) reported fatigue, dizziness, somnolescence, which was statistically significant (P ≤ 0.001) and 4 of these patients discontinued use of the drug due to excessive somnolence, all after the first dose. A statistically significant difference was found in each domain of 5D-Pruritus scale after gabapentin and pregabalin therapy.
Keywords
Chronic kidney disease
hemodialysis
uremic pruritus
INTRODUCTION
Skin problems are one among the many frustrating manifestations of end-stage renal disease affecting 25%–90% of patients. Despite the availability of more efficient hemodialysis techniques, uremic pruritus (UP) remains a challenging problem for both clinicians and patients and reduces the quality of life in many patients.[1] Some of the factors implicated in the pathophysiology of UP are xerosis, divalent ions, calcium-phosphate product, interleukin-31, and hyperparathyroidism. Apart from this, the adequacy of dialysis and clearance of pruritogenic substances could influence the severity of pruritus.[2]
The severity of UP ranges from sporadic discomfort to complete restlessness during both day and night time. It is most severe during or immediately after dialysis in 25% of patients. Initially, skin appears normal; scratching with or without impetigo can occur as a secondary phenomenon and prurigonodularis or Kyrle's disease is observed rarely. Inter-individual differences in UP are reported. While some patients complain about generalized pruritus others are affected on back, the face, and the shunt arm.[3]
The main obstacle in the effort to create effective treatment modalities is the clinical heterogeneity of UP. Of the various hypothesis proposed, the most prominent concept focused on parathyroid hormone (PTH) because UP resolved after parathyroidectomy. The other two major pathophysiological concepts are “immuno-hypothesis” and “opioid hypothesis.”[3] Because of the lack of a sufficient number of randomized, placebo-controlled trials, effective treatment options are limited.[4] Even though no major renal societies have created guidelines for the management of UP in hemodialysis patients, gabapentin is recognized as a second-line or third-line agent for generalized UP.[5] Gabapentin, an anticonvulsant and centrally acting calcium channel blocker, is used in neuropathic pain.[4] As it is renally eliminated, the substantially increased half-life in hemodialysis patients is concerning.[5] In a study by Gunal et al. 300 mg of oral gabapentin administered three times weekly for 4 weeks was effective in reducing pruritus from 8.4 before treatment to 1.2 after 4 weeks of treatment according to visual analog scale (VAS). Another double-blind, controlled, crossover study involving 34 patients treated with 100 mg gabapentin orally three times a week also shows similar results. These beneficial effects empower it as a promising treatment option in relieving pruritus if topical treatments are ineffective.[4]
Drugs such as antihistamines, menthol, capsaicin, topical calcineurin inhibitors, cannabinoid agonists, and leukotriene antagonists affect itch transmission in the peripheral nervous system while antidepressants, μ opioid receptor antagonist and antiepileptics such as gabapentin and pregabalin act centrally. Pregabalin, a gamma aminobutyric acid analog, binds to the voltage-gated calcium channels and reduces the effects of substance P and excitatory neurotransmitters and calcitonin gene-related peptides. Reduction in neuropathic pain, anxiety, and controlling epilepsy are attributed to these effects, even though the exact mechanism of pregabalin in reducing itch sensation is not clear.[6]
METHODOLOGY
Patients
Male and female patients aged between 20 and 85 years with end-stage renal disease undergoing maintenance hemodialysis and who had substantial pruritus defined as persistent were included in our study. Patients with a history of dermatologic disease antedating renal failure and those with skin disease other than cutaneous findings of uremia and with systemic diseases such as malignancy and cholestatic liver disease were excluded from the study. Informed consent was obtained from each patient before enrolling in the study.
Study criteria
Inclusion criteria
-
Stage 5 chronic kidney disease (CKD) on maintenance hemodialysis
-
Kt/v >1.2
-
Hemoglobin (Hb), calcium, phosphorous, intact PTH targets achieved as per the KDIGO guidelines.
Exclusion criteria
-
Patients on peritoneal dialysis
-
Other cutaneous manifestations apart from UP.
Study design
This was a randomized single-blind prospective-interventional study carried out for 6 weeks in the dialysis unit, Department of Nephrology in a tertiary care teaching hospital, Pariyaram Medical College, Kerala, after getting approval from the Institutional Ethics Committee. The baseline characteristics (kt/v, serum calcium, phosphorous, intact PTH, Hb) of the patients so identified were analyzed, and those satisfying the inclusion criteria were enrolled in the study. Patients on anti-histamines were subjected to a washout period of 1 week before entering the study. Patients were randomly divided into two groups (Group A and B), with Group A receiving pregabalin 25 mg and Group B receiving gabapentin 100 mg [flow chart given in consort Flow Diagram 1]. The efficacy and safety of drug will be assessed after 6 weeks using VAS and 5D itch scale.

- CONSORT flow diagram
Ethical committee approval
Ethical committee approval for the research work obtained from Academy of pharmaceutical sciences; Pariyaram Medical College, Kannur (A1 / 1839/2017/APSC/IEC-10 / 2018).
RESULTS
Out of the 197 patients undergoing maintenance hemodialysis in our center, 42 participants (prevalence =21.3%) had moderate-to-severe pruritus; 31 men (73.8%) and 11 women (26.1%), most of them were experiencing it for the past 2–5 years. The mean age of the patient in Group A and B were 55.29 ± 14.58 and 58.10 ± 11.09, respectively. In Group A, 14 patients (66.6%) were male and 7 patients (33.3%) were female, and in Group B, 17 patients (80.90%) were male and 4 patients (19%) were female. Baseline laboratory tests for patients are depicted in Table 1.
| Mean±SD | |
|---|---|
| Ipth | 716.65±519 |
| kt/v | 1.50±0.87 |
| Calcium | 8.36±0.89 |
| Phosphorous | 4.74±1.54 |
| Hb | 9.95±1.56 |
SD: Standard deviation, Hb: Hemoglobin
VAS was used to analyze the intensity of itching before and after drug therapy. Median itch severity score before starting treatment with both the drugs were found to be identical, i.e., most of the patients reported a score of 7 in the VAS. This was reduced to 1 (VAS score) after 6 weeks of treatment with both the drugs. Both gabapentin and pregabalin produced a significant difference in itching intensity with P < 0.001 (Wilcoxon Signed-Rank Test). However, there was no statistically significant difference between the effectiveness of two drugs in reducing itch.
Each domain in the 5D-Pruritus scale was assessed before and after pregabalin and gabapentin therapy [Tables 2 and 3]. A statistically significant difference was found in each domain of 5D-Pruritus scale after both the therapy. This shows that the duration of itch was diminished, the intensity of itch was reduced, the impact of itch on sleep, social activities, housework and work was lessened, and the distribution of itch on the body was greatly decreased after pregabalin and gabapentin therapy. Regarding the duration of itch, the majority of the patients had discomfort 6–12 h/day and throughout the day. After treatment with either of the drug, there was a significant reduction (P ≤ 0.001) in the duration of itching to <6 h/day.
| Baseline burden of disease before drug therapy | Baseline burden of disease after drug therapy | P | ||
|---|---|---|---|---|
| Score | Frequency (%) | Score | Frequency (%) | |
| Duration of itching | Duration after itching | |||
| 1 | 4 (19.05) | 1 | 14 (66.67) | <0.001 |
| 2 | 5 (23.81) | 2 | 4 (19.05) | |
| 3 | 4 (19.05) | 3 | 1 (4.76) | |
| 4 | 2 (9.52) | 4 | 1 (4.76) | |
| 5 | 6 (28.57) | 5 | 1 (4.76) | |
| Degree of itching | Degree of itching | |||
| 1 | 0 (0.00) | 1 | 10 (47.62) | <0.001 |
| 2 | 1 (4.76) | 2 | 7 (33.33) | |
| 3 | 9 (42.86) | 3 | 2 (9.52) | |
| 4 | 8 (38.10) | 4 | 2 (9.52) | |
| 5 | 3 (14.29) | 5 | 0 (0.00) | |
| Direction | Direction | |||
| 1 | 0 (0.00) | 1 | 9 (42.86) | 0.001 |
| 2 | 0 (0.00) | 2 | 7 (33.33) | |
| 3 | 8 (38.10) | 3 | 3 (14.29) | |
| 4 | 8 (38.10) | 4 | 2 (9.52) | |
| 5 | 5 (23.81) | 5 | 0 (0.00) | |
| Disability | Disability | |||
| Leisure/social | Leisure/social | |||
| N/A | 0 (0.00) | N/A | 0 (0.00) | <0.001 |
| 1 | 6 (28.57) | 1 | 16 (76.19) | |
| 2 | 10 (47.62) | 2 | 3 (14.29) | |
| 3 | 2 (9.52) | 3 | 1 (4.76) | |
| 4 | 2 (9.52) | 4 | 1 (4.76) | |
| 5 | 1 (4.76) | 5 | 0 (0.00) | |
| Sleep | Sleep | |||
| 1 | 8 (38.10) | 1 | 17 (80.95) | 0.002 |
| 2 | 4 (19.05) | 2 | 2 (9.52) | |
| 3 | 3 (14.29) | 3 | 0 (0.00) | |
| 4 | 2 (9.52) | 4 | 2 (9.52) | |
| 5 | 4 (19.05) | 5 | 0 (0.00) | |
| Housework | Housework | |||
| N/A | 0 (0.00) | N/A | 0 (0.00) | <0.001 |
| 1 | 4 (19.05) | 1 | 15 (71.43) | |
| 2 | 11 (52.38) | 2 | 5 (23.83) | |
| 3 | 3 (14.29) | 3 | 0 (0.00) | |
| 4 | 2 (9.52) | 4 | 1 (4.76) | |
| 5 | 1 (4.76) | 5 | 0 (0.00) | |
| Work/school | Work/school | |||
| N/A | 14 (66.67) | N/A | 15 (71.43) | 0.041 |
| 1 | 2 (9.52) | 1 | 6 (28.57) | |
| 2 | 2 (9.52) | 2 | 0 (0.00) | |
| 3 | 0 (0.00) | 3 | 0 (0.00) | |
| 4 | 3 (14.29) | 4 | 0 (0.00) | |
| 5 | 0 (0.00) | 5 | 0 (0.00) | |
N/A: Not available
| Baseline burden of disease before drug therapy | Baseline burden of disease after drug therapy | P | ||
|---|---|---|---|---|
| Score | Frequency (%) | Score | Frequency (%) | |
| Duration of itching | Duration after itching | |||
| 1 | 4 (19.05) | 1 | 19 (90.48) | <0.001 |
| 2 | 5 (23.81) | 0 | 0 (0) | |
| 3 | 4 (19.05) | 0 | 0 (0) | |
| 4 | 4 (19.05) | 4 | 2 (9.52) | |
| 5 | 4 (19.05) | 5 | 0 (0) | |
| Degree of itching | Degree of itching | |||
| 1 | 0 (0) | 1 | 11 (52.38) | <0.001 |
| 2 | 1 (4.76) | 2 | 7 (33.33) | |
| 3 | 9 (42.86) | 3 | 1 (4.76) | |
| 4 | 9 (42.86) | 4 | 2 (9.52) | |
| 5 | 2 (9.52) | 5 | 0 (0.00) | |
| Direction | Direction | |||
| 1 | 0 (0.00) | 1 | 13 (61.90) | 0.001 |
| 2 | 3 (14.285) | 2 | 5 (23.81) | |
| 3 | 5 (23.81) | 3 | 2 (9.52) | |
| 4 | 12 (57.14) | 4 | 1 (4.76) | |
| 5 | 1 (4.76) | 5 | 0 (0.00) | |
| Disability | Disability | |||
| Leisure/social | Leisure/social | |||
| N/A | 2 (9.52) | N/A | 2 (9.52) | 0.001 |
| 1 | 6 (28.57) | 1 | 16 (76.19) | |
| 2 | 8 (38.10) | 2 | 3 (14.29) | |
| 3 | 1 (4.76) | 3 | 0 (0.00) | |
| 4 | 4 (19.05) | 4 | 0 (0.00) | |
| 5 | 0 (0.00) | 5 | 0 (0.00) | |
| Sleep | Sleep | |||
| 1 | 9 (42.86) | 1 | 18 (85.71) | 0.003 |
| 2 | 4 (19.05) | 2 | 3 (14.29) | |
| 3 | 2 (9.52) | 3 | 0 (0.00) | |
| 4 | 2 (9.52) | 4 | 0 (0.00) | |
| 5 | 4 (19.05) | 5 | 0 (0.00) | |
| Housework | Housework | |||
| N/A | 0 (0.00) | N/A | 0 (0.00) | <0.001 |
| 1 | 5 (23.81) | 1 | 19 (90.48) | |
| 2 | 9 (42.86) | 2 | 2 (9.52) | |
| 3 | 3 (14.29) | 3 | 0 (0.00) | |
| 4 | 4 (19.05) | 4 | 0 (0.00) | |
| 5 | 0 (0.00) | 5 | 0 (0.00) | |
| Work/school | Work/school | |||
| N/A | 9 (42.86) | N/A | 9 (42.86) | 0.001 |
| 1 | 1 (4.76) | 1 | 11 (52.38) | |
| 2 | 9 (42.86) | 2 | 1 (4.76) | |
| 3 | 1 (4.76) | 3 | 0 (0.00) | |
| 4 | 1 (4.76) | 4 | 0 (0.00) | |
| 5 | 0 (0.00) | 5 | 0 (0.00) | |
N/A: Not available
The intensity of itching which was moderate-to-severe resolved and ameliorated to mild after treatment. This reduction in itch was significant for both pregabalin and gabapentin (P ≤ 0.001). Of the 42 patients, 33 had unchanged pruritus status compared to the previous month, before starting treatment. The patients were much better and free of discomfort after treatment.
When patients were asked to rate the impact of itching on activities such as sleep, leisure, housework, and work/school, it was revealed that leisure/social activities and housework that was earlier rarely affected by pruritus, were never affected in majority of patients after treatment with pregabalin and gabapentin, P ≤ 0.001. While analyzing the impact of itching on sleep, although sleep was never affected in most of the cases (n = 17) prior treatment, some of them experienced occasional delay falling asleep (n = 8), frequent delay falling asleep (n = 5), occasionally wakes at night (n = 4), and frequently wakes up at night (n = 8) due to itch. Whereas following treatment sleep was never affected in 35 patients. There is also a positive impact of gabapentin on sleep (P = 0.002) compared with pregabalin (P = 0.003). This may be attributed to the most frequently reported adverse effect, sedation due to gabapentin. Evaluation of 5-D itch scale before after drug therapy for gabapentin and pregabalin is given in [Tables 1 and 2].
Evaluation of pattern of pruritus distribution using 5D scale showed most of the patients were affected on back, lower legs, thighs, head, and top of hands/fingers. Both the drugs showed a statistically significant reduction in itch in these areas [Graphs 1 and 2].
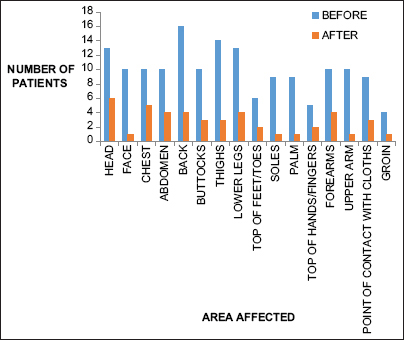
- Distribution of pruritus in patients treated with gabapentin before and after drug therapy
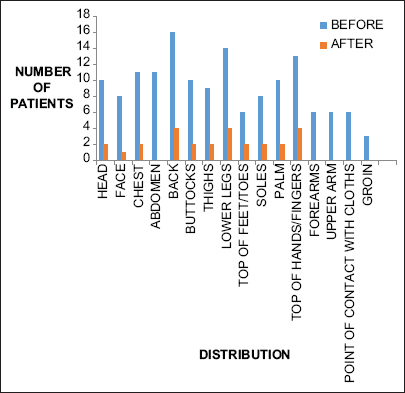
- Distribution of pruritus in patients treated with pregabalin before and after drug therapy
While receiving gabapentin, 11 of 21 subjects (52.38%) reported fatigue, dizziness, somnolescence, which was statistically significant (P ≤ 0.001) and 4 of these patients discontinued use of the drug due to excessive somnolence, all after the first dose.
DISCUSSION
We have demonstrated that both gabapentin 100 mg and pregabalin 25 mg improve pruritus in dialysis patients compared with baseline (no therapy). There were no significant differences observed between the two drugs. However, in our patient population, pregabalin was associated with fewer side effects than gabapentin. Therefore, our overall impression is that pregabalin 25 mg is a preferred agent for the treatment of dialysis pruritus.
CKD with pruritus is a common health problem, especially among the elderly who undergo dialysis. High incidence of pruritus was observed among the mean age of 56.09 ± 11.62 similar to the study conducted by Prabahar et al., 2017, in which the mean age was 53.36 ± 12.81 years.[7]
In our study, there was a decreased compliance to gabapentin when compared to pregabalin, i.e., 11 of 21 patients (52.38%) reported fatigue, dizziness, somnolescence which was statistically significant (P ≤ 0.001) and 4 of these patients discontinued use of the drug due to excessive somnolence, all after the first dose. Similarly, Rayner et al. in their study, UP: Relief of itching by Gabapentin and Pregabalin showed that gabapentin relieved itching in 47 patients (66%). Twenty-six patients (37%) suffered side effects from gabapentin.[8] The frequent intolerance to gabapentin is the most significant finding from the study conducted by Marquez et al.[9] Whereas study conducted by Amirkhanlou et al. revealed that four patients (15.4%) experienced drowsiness and 1 patient (3.8%) had dizziness after taking gabapentin and ketotifen which was not statistically significant (P = 1.00).[10] The majority of adverse drug reaction (ADR) was found between the age group of 30–60 years (29.17%) followed by >60 years (25%). All patients showed compliance with pregabalin, and no ADR was reported. Shavit et al. evaluated the use of pregabalin in the management of chronic UP in 12 patients, and they demonstrated dramatic improvement of long-standing pruritus after initiation of pregabalin. It was well tolerated with somnolence and dizziness developing in 2 patients.[11]
CONCLUSION
Clinicians should enquire about skin irritation and itching during consultations. Patients may be reluctant to admit to these symptoms due to embarrassment or a belief that they are caused by nonadherence to diet or phosphate binder therapy. In patients with CKD and itching that persists despite attempts to correct calcium and phosphate balance and treatment with emollient creams, we have demonstrated that both gabapentin 100 mg and pregabalin 25 mg improve pruritus in dialysis patients compared with baseline (no therapy). There were no significant differences observed between the two drugs. However, pregabalin was associated with fewer side effects than gabapentin. Therefore, our overall impression is that pregabalin 25 mg is a preferred agent for the treatment of dialysis pruritus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Comparison of pregabalin with doxepin in the management of uremic pruritus: A randomized single blind clinical trial. Hemodial Int. 2017;21:63-71.
- [Google Scholar]
- A comparison of uremic pruritus in patients receiving peritoneal dialysis and hemodialysis. Medicine (Baltimore). 2016;95:e2935.
- [Google Scholar]
- Uraemic pruritus--new perspectives and insights from recent trials. Nephrol Dial Transplant. 2002;17:1558-63.
- [Google Scholar]
- Gabapentin for uremic pruritus in hemodialysis patients: A qualitative systematic review. Can J Kidney Health Dis. 2016;3:14.
- [Google Scholar]
- Role of Pregabalin in Management of Pruritus: A Literature Review. J Pharm Pharm Sci. 2016;19:465-74.
- [Google Scholar]
- Assessment of quality of life and severity of itching pre and post doxepin therapy in dialysis patients with pruritus. J Applied Pharma Sci. 2017;7:119-25.
- [Google Scholar]
- Uraemic pruritus: Relief of itching by gabapentin and pregabalin. Nephron Clin Pract. 2012;122:75-9.
- [Google Scholar]
- Uremic pruritus in hemodialysis patients: Treatment with desloratidine versus gabapentin. J Bras Nefrol. 2012;34:148-52.
- [Google Scholar]
- Comparison of gabapentin and ketotifen in treatment of uremic pruritus in hemodialysis patients. Pak J Med Sci. 2016;32:22-6.
- [Google Scholar]
- Use of pregabalin in the management of chronic uremic pruritus. J Pain Symptom Manage. 2013;45:776-81.
- [Google Scholar]






