Translate this page into:
Ultrasound-Guided Dry Needling As a Treatment For Postmastectomy Pain Syndrome – A Case Series of Twenty Patients
Address for correspondence: Dr. Lakshmi Vas, Ashirvad Institute for Pain Management and Research, Plot No. 117, Shubh Ashirvad, Hindu Colony, 5th Road, Dadar (E), Mumbai - 400 014, Maharashtra, India. E-mail: lakshmi@paincareindia.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Existing interventions for postmastectomy pain syndrome (PMPS) address the neural component while overlooking a possible myofascial component.
Aim:
The aim of the study is to investigate the myofascial contribution to PMPS, by examining the effectiveness of myofascial trigger point release by ultrasound-guided dry needling (USGDN).
Patients and Methods:
This retrospective review assessed the efficacy of USGDN in addressing myofascial pain in twenty consecutive patients with treatment-refractory PMPS. Patients in Group 1 (n = 16) received USGDN after neural interventions (NIs) such as neuraxial blocks, intrathecal pump implant, or pulsed radiofrequency, while those in Group 2 (n = 4) received USGDN alone. Outcome measures were changes in Numerical Rating Scale (NRS), PainDETECT (PD), Disabilities of Arm, Shoulder, and Hand (DASH), Patient Health Questionnaire-9 (PHQ-9) scores, and opioid use.
Results:
In Group 1, the mean (standard deviation) NRS and PD scores (9.6 [0.9] and 28.3 [4.3], respectively, at baseline) reduced to 5.2 (1.1) and 16.1 (3.7) at 1-week post-NI. The post-NI DASH reduction was below the cutoff for clinical relevance (80.9 [10.5] at baseline vs. 71.1 [10.5] post-NI). The opioid dose remained unchanged. Following USGDN, NRS, PD, and DASH scores further reduced to 2.3 (0.8), 6.6 (1.2), and 34.6 (14.4), respectively. Patients receiving USGDN alone also showed reduction in NRS, PD, and DASH (7.8 [1.7], 20.0 [8.0], and 61.0 [14.4] at baseline vs. 1.3 [0.5], 6.0 [1.6], and 22.5 [10.4] post-USGDN, respectively). In all patients, opioid use and PHQ-9 scores reduced only post-USGDN.
Conclusions:
USGDN reduced pain, disability, and opioid use, whereas NI reduced only pain. This suggests a myofascial contribution to pain and disability in PMPS.
Keywords
Myofascial pain
neuromyopathy
neuropathic pain
opioids
postmastectomy pain syndrome
ultrasound-guided dry needling
INTRODUCTION
Postmastectomy pain syndrome (PMPS), defined as a chronic post-surgical pain lasting over 3 months, is a common condition that develops in 25%–60% of patients who have undergone breast surgery.[123] PMPS is characterized by stinging pain, burning, tingling, numbness, hypersensitivity, and electric shocks.[4] Risk factors for PMPS include younger age, radiotherapy, axillary lymph node dissection, as well as pre- and post-operative pains.[56] PMPS incidence also increases with an increase in the length of the post-surgical period, and it has been reported in 23% of patients at 3 months, 42% at 5 years, and 37% at 9 years.[478]
While the etiology of PMPS is unclear, it is primarily considered a neuropathy[9] arising due to nerve damage during surgery or later induced by tissue scarring. Neuropathic pain by definition is confined to that affecting the somatosensory system.[10] Unfortunately, this definition excludes the possibility of motor efferent nerves supplying the muscles as well as the abundant nociceptive afferent nerves from muscles getting affected by neuropathy and giving rise to myofascial pain. We have previously observed myofascial pain to play a significant role in multiple pain syndromes attributed solely to neuropathy.[111213141516] Myofascial pains have also been described in cancer[17] and specifically in PMPS.[181920] Many major nerves supplying the upper limb, shoulder, and chest wall muscles traverse through muscle planes where they are vulnerable to damage from surgery as well as radiation. The fibrotic changes could give rise to entrapments of the nerves that traverse these muscle planes. Indeed, it has been our clinical experience that myofascial pains play a major role in post-surgical neuropathy (after mastectomy or lumpectomy), postradiation neuropathy, and postchemotherapy neuropathy in breast cancer patients (unpublished observations). We propose that increased firing from nerves carrying muscle efferent fibers damaged by surgery, radiation, or chemotherapy could presumably give rise to increased firing at the neuromuscular junction (NMJ). The increased spontaneous electrical activity (SEA) at the NMJ can generate myofascial trigger points (MTrPs) and taut bands in muscle, giving rise to pain and stiffness as described by Simons et al. and Kuan et al.[212223] Intense, unrelenting, and constant spasm of these muscles could lead to resource depletion and energy crisis as described by Simmons et al., eventually leading to fibrosis. We have termed this neuropathy of motor nerves as neuromyopathy and postulate that it may play an important role in PMPS by giving rise to MTrPs in the various muscles of the chest, shoulder, and neck. Treatment with dry needling has been shown to release MTrPs in various forms of myofascial pains,[242526272829] and we set out to examine if it could improve PMPS. However, needling of deep-seated muscles such as the serratus anterior, subscapularis, and pectoralis minor and major (in patients who have undergone breast conservation procedures) carries a risk of damaging the pleura and vascular structures. Needling under ultrasound guidance allows the visualization of pleura and vascular structures in the axilla and removes this risk. One pilot study also reports the efficacy of ultrasound-guided trigger point injections in the pectoralis and subscapularis muscles as a treatment for PMPS.[30]
In this retrospective case series of twenty patients, we examine the efficacy of ultrasound-guided dry needling (USGDN) per se, or as the main component of a multimodality treatment regimen to relieve the pain and disability associated with PMPS.
PATIENTS AND METHODS
Patients
Twenty consecutive patients presenting at our clinic with treatment-refractory PMPS between 2004 and 2016 were included in this retrospective case series. A diagnosis of PMPS was made based on clinical findings [Table 1]. Informed consent was obtained from all patients prior to the start of treatments.
| Patient number | Age, years | PMPS duration, years | Symptoms | Medications | Intervention |
|---|---|---|---|---|---|
| 1 | 42 | 6 | Plexopathy; chest wall, neck, and shoulder pain; lymphedema; RROM | M, FP, P, NM | CBPB + USGDN |
| 2 | 32 | 2 | Plexopathy; chest wall, neck, and shoulder pain; UE CRPS; lymphedema; arm tethered to chest; RROM | M, FP, P, NM, S | CBPB + USGDN |
| 3 | 60 | 5 | Plexopathy; chest wall, neck, and shoulder pain; UE CRPS; lymphedema; arm tethered to chest; RROM | M, FP, P, NM, S | CBPB + USGDN |
| 4 | 69 | 2 | Neck, shoulder, and UE pain; shocks; UE CRPS; lymphedema | T, P, NM | CBPB + USGDN |
| 5 | 70 | 3 | Numbness and pain in the arm, forearm, and hand; UE CRPS; lymphedema; RROM | C, T, P, NM, S | CBPB + USGDN |
| 6 | 68 | 6 | Plexopathy; chest wall, neck, and shoulder pain; UE CRPS, lymphedema; RROM | M, FP, P, NM, S | CBPB + USGDN |
| 7 | 56 | 10 | Chest wall, neck, and shoulder pain; UE CRPS; lymphedema; RROM | M, FP, P, NM, S | CBPB + USGDN |
| 8 | 63 | 6 | Plexopathy; chest wall, neck, and shoulder pain; UE CRPS; lymphedema; RROM | M, FP, NM | CBPB + USGDN |
| 9 | 70 | 6 | Neck and shoulder pain; UE CRPS; lymphedema; RROM | T, P, N, NM | SGB + USGDN |
| 10 | 52 | 5 | Neck and shoulder pain; RROM | M, N, NM | SGB + USGDN |
| 11 | 52 | 4 | Plexopathy; neck, shoulder, and UE pain | M, FP, P, NM, S | ITP + USGDN |
| 12 | 48 | 8 | Plexopathy; chest wall, neck, shoulder, and UE pain; back pain | M, FP, P, NM, S | ITP + USGDN |
| 13 | 36 | 14 | Chest wall, neck, and shoulder pain; RROM | M, N, NM | sPRF + USGDN |
| 14 | 54 | 15 | Chest wall, neck, and shoulder pain | T, N, NM | sPRF + USGDN |
| 15 | 59 | 1 | Chest wall, neck, and shoulder pain | B, T, P, NM | sPRF + USGDN |
| 16 | 43 | 3 | Plexopathy; chest wall, neck, and shoulder pain; hard, enlarged, discolored breast | FP, T, P, NM | sPRF + USGDN |
| 17 | 70 | 10 | Chest wall, neck, and shoulder pain | FP, T, P, NM | USGDN alone |
| 18 | 63 | 3 | Supra-, infra-, and interscapular areas and arm pain; RROM | M, T, P, NM | USGDN alone |
| 19 | 32 | 3 | Chest wall, neck, and shoulder pain; hard, enlarged, and discolored breast | C, T, P, NM, S | USGDN alone |
| 20 | 56 | 3 | Supra-, infra-, and interscapular area and arm pain; enlarged and hard breast; RROM | M, T, P, NM | USGDN alone |
B: Buprenorphine, C: Codeine, UE CRPS: Upper extremity complex regional pain syndrome, CBPB: Continuous brachial plexus block, FP: Fentanyl patch, ITP: Intrathecal pump, M: Morphine, N: Nonsteroidal anti-inflammatory drugs, NM: Neuromodulators, P: Paracetamol, PMPS: Postmastectomy pain syndrome, USGDN: Ultrasound-guided dry needling, RROM: Restricted range of motion, S: Sedatives, sPRF: Shoulder pulsed radiofrequency treatment, SGB: Stellate ganglion block, T: Tramadol
Treatments
Most patients (16/20) initially received one of the following NIs to address the neuropathic component: stellate ganglion block (SGB); continuous brachial plexus block (CBPB) from a posterior paravertebral approach; opioid delivery via an intrathecal pump (ITP); or ultrasound-guided pulsed radiofrequency (PRF) treatment of the cervical plexus, spinal accessory, suprascapular, axillary, radial, and musculocutaneous nerves (in patients with shoulder pain). One week after treatment with the NI, USGDN was initiated and continued for up to 45 days, as required by the patient. Four patients refused NIs and opted for only USGDN.
Neural interventions
Single-shot stellate ganglion block
This procedure was performed in two patients under fluoroscopic or ultrasound guidance [Figure 1, top row, left, and center] to inject 3 mL 0.5% bupivacaine and 40 mg triamcinolone (Solucort®).
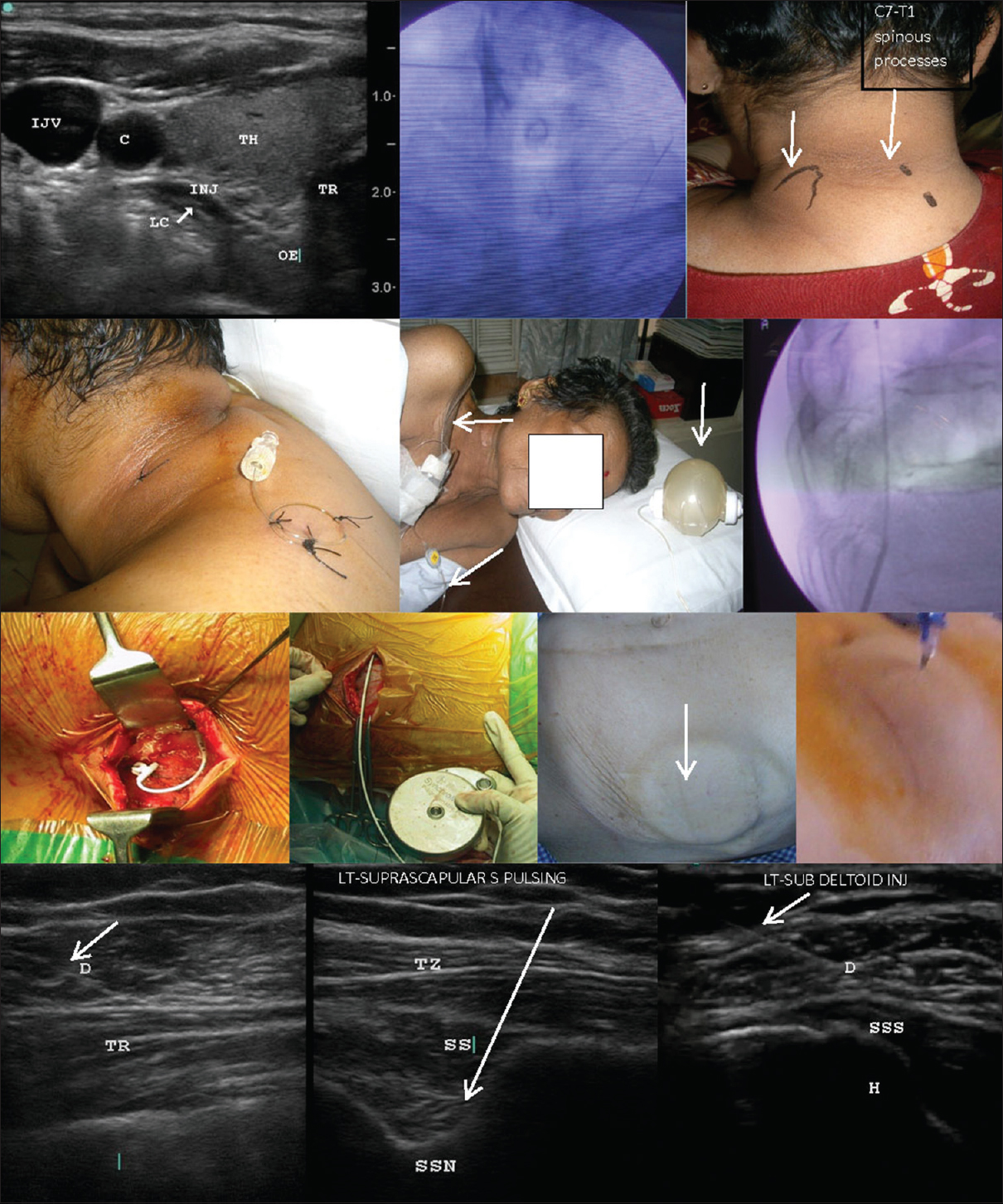
- Neural interventions. First row, left: Ultrasound-guided stellate ganglion block showing the outline of the injectate anterior to the longus colli muscle; First row, center: Dye spread during fluoroscopy-guided stellate ganglion block at the sixth cervical vertebra, two levels above the first rib (arrow); First row, right: placement of the continuous brachial plexus block needle, introduced between the trapezius and levator scapulae (arrows) for posterior paravertebral brachial plexus block; Second row, left and center: the tunneled continuous brachial plexus block catheter is secured with stitches, looped over the shoulder, and connected to an elastomeric pump (center, arrows); Second row, right: fluoroscopic view of the intrathecal catheter emerging from the needle; Third row, left and center left: figures show the anchoring of the intrathecal catheter to the paravertebral fascia; Third row, center right and right: the silastic catheter is subcutaneously tunneled across the flank to be connected to the titanium pump subcutaneously implanted in the abdominal wall; the arrow indicates the refill port of the pump seen under the skin and the refill process; Fourth row: shoulder pulsed radiofrequency and injections under ultrasound guidance at the axillary nerve between the deltoid and trapezius (left), the suprascapular nerve in suprascapular notch (center), and the subdeltoid bursa between deltoid and supraspinatus tendon (right). The arrows indicate needles. C: Carotid artery, H: Humerus, IJV: Internal jugular vein, INJ: Injectate, LC: Longus colli, OE: Esophagus, D: Deltoid, TH: Thyroid, TR (first row): Trachea, TR (forth row): Triceps; TZ: Trapezius, SS: Supraspinatus, SSN: Suprascapular notch, SSS: Supraspinatus tendon.
Posterior-approach continuous brachial plexus block
This procedure was performed in eight patients. A nerve-stimulating catheter was placed in the posterior paravertebral brachial plexus and was connected to an infusion pump [Figure 1]. An infusion of 0.125% bupivacaine (1–2 mL/h) with a bolus of 3–4 mL with a 2-h lockout interval was programmed. Patients were sent home with the following instructions: twice-daily oral cefoperazone (500 mg), bupivacaine boluses for breakthrough pain >3 Numerical Rating Scale (NRS), home physical therapy, and directions to stop patient-controlled analgesia if sensory/motor deficits developed. Pump refill and wound dressing were done every week. CBPB was maintained for 21–38 days in three patients and for 3–4 months in four patients at their request until their demise.
Intrathecal pump
SynchroMed (Medtronic Inc., Minneapolis, MN) was used in two patients. A silastic catheter was placed at the T1–2 intrathecal space and connected to the titanium pump filled with 40 mL of concentrated morphine (20 mg/mL). It was externally programmed to deliver the daily dose of morphine [Figure 1]. Subsequent refills were given through the injection port of the pump.
Pulsed radiofrequency
This procedure was performed in four patients with shoulder pain and movement restrictions. Ultrasound-guided nerve location was confirmed by motor stimulation of the suprascapular, accessory, axillary, and musculocutaneous nerves before PRF treatment [40°C for 10 min; Figure 1]. In addition, patients received steroid injections at the subdeltoid bursa, the bicipital groove, and the origin of short head of biceps at the coracoid.
Ultrasound-guided dry needling
This procedure was initiated 1-week post-NIs. The rationale for starting after a week was to assess the effect of NIs before starting USGDN. We also wanted to confirm whether USGDN was warranted at all or whether NI alone could provide complete symptom relief. We systematically introduced 25-, 40-, 50-, or 75-mm 32-G needles into muscles contributing to myofascial pain in PMPS under ultrasound guidance. The muscles to be needled were selected on the basis of the pain diagram, which indicated the likelihood of pain arising from the underlying muscles, tenderness over the muscle, and pain caused by a movement involving that muscle. The length of the needle was determined by muscle thickness and depth, as visualized on ultrasound. Some patients required an immediate-release diclofenac/tramadol/fentanyl lollipop to reduce the procedural pain of USGDN. Patients with CBPB received a 5-mL bolus to reduce the pain associated with USGDN. USGDN was performed at several points along the muscles – one needle every 1 cm along the length as well as breadth of the muscle. Notably, a hard, grating sensation was perceived at advancement of the needle in the affected muscles, unlike the easy painless entrance into a normal muscle. Some muscles were so tough that the needles would buckle and bend, but waiting 1–2 min allowed further introduction following a perceptible relaxation of the muscle. Incremental advancements of 1–2 mm were required in these cases. This kind of toughness in muscle was found in radiated areas or around surgical scars. One patient who had undergone a breast conservation procedure had such a hardening of breast tissue itself that it was difficult to even introduce needles through the skin, let alone reach the underlying pectoral muscles. However, after 2–3 sessions, the hardness disappeared, and the breast tissue lost the edematous swelling and regained its normal suppleness. The pectoral muscle needling could be done subsequently to relieve her pain in the breast and anterior chest. The following muscles were targeted:
-
Neck – Trapezius, sternocleidomastoid, scalenes, levator scapulae, splenius and semispinalis, the cervicis and capitis sections of the longissimus, and iliocostalis cervicis
-
Shoulder – Supraspinatus and infraspinatus, latissimus dorsi, teres major and minor, subscapularis, pectoralis major and minor, deltoid, and coracobrachialis
-
Chest wall and limb girdle – serratus anterior, serratus posterior superior and inferior, rhomboids, and intercostal muscles
-
Upper arm – Biceps, coracobrachialis, brachialis brachioradialis, and triceps
-
Forearm – Flexor carpi radialis and ulnaris, flexor digitorum superficialis and profundus, pronator teres and quadratus (anterior approach), brachioradialis and extensors of the wrist and fingers, and supinator and anconeus (posterior approach).
Outcome measures
Patients were administered the following questionnaires: the 10-point NRS;[31] the neuropathic pain-specific painDETECT (PD) questionnaire;[32] the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire to evaluate disability;[33] and the Patient Health Questionnaire (PHQ-9) to assess depression.[34] Questionnaires were provided at three points: at baseline, at 1-week post-NIs, and at 4-week post-USGDN (5 weeks after the start of treatment). Patients were followed up for up to 1 year after the end of treatment to determine the sustainability of pain relief and change in medications.
RESULTS
Patients
Baseline characteristics for the twenty patients are shown in Table 1. In total, 17/20 patients had metastasis and 19/20 patients had undergone radiation. All patients had received chemotherapy. Nine patients had swelling of the breast, arm, and forearm, presumed to be lymphedema. Twelve patients had a restricted range of motion (RROM) with pain and stiffness on flexion, abduction, or internal and external rotation of the shoulder. Eight patients had symptoms that conformed to the Budapest Criteria for complex regional pain syndrome (CRPS) of the upper extremity. Two patients had severe stiffening of pectoral girdle muscles that tethered their arms to the chest and felt hard on palpation. Three patients with breast conservation procedures showed a disparity compared to the normal breast in that they were enlarged and felt hard on palpation. All had sleep disturbances despite sedatives such as diazepam, alprazolam, and zolpidem.
Complications
One patient with CBPB who had an accidental catheter extrusion at 10 days when the pain was still 5–6 NRS (reduced from a score of 10 at baseline) failed to complete USGDN. She received her first session but did not return for further treatment. In another patient with CBPB, the catheter had to be removed at 1 month due to infection. There were no complications associated with SGB, ITP, PRF, or USGDN.
Posttreatment change in pain and disability scores
Pre- and post-treatment NRS, PD, and DASH scores are shown in Table 2. SGB produced a reduction of upper-extremity swelling [Figure 2] and rest pain [Table 2], without improving the DASH score [Table 2] or reducing opioid consumption. Among the patients receiving NIs before USGDN (n = 16), the mean (standard deviation [SD]) NRS score (9.6 [0.9] at baseline) reduced to 5.2 (1.1) at 1-week posttreatment (45.8% reduction by NI). Similarly, the mean (SD) PD scores reduced from 28.3 (4.3) at baseline to 16.1 (3.7) at 1-week posttreatment (43.1% reduction by NI). However, patients needed to continue opioids at the baseline dose to maintain pain relief, and the mean (SD) DASH score (71.1 [10.5] at 1-week post-NI) was only slightly reduced from baseline (80.9 [10.5]) (12.1% reduction by NI). At baseline, based on PHQ-9 scores, all patients were depressed, with 12 (60.0%), 5 (25.0%), and 3 patients (15.0%) reporting mild, moderate, and severe depression, respectively. One week after NIs, 12 (60%) and 6 patients (30.0%) reported mild and moderate depression, respectively, and the patients with severe depression also had improved to scores of moderate depression. PHQ-9 scores were unavailable for two patients at this time point.
| Patient number | Intervention group† | NRS | PD | DASH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-NI | Post-USGDN | Baseline | Post-NI | Post-USGDN | Baseline | Post-NI | Post-USGDN | ||
| 1 | CBPB + USGDN | 10 | 5 | 2 | 26 | 16 | 6 | 84 | 76 | 26 |
| 2 | CBPB + USGDN | 10 | 6 | 2 | 32 | 24 | 8 | 90 | 90 | 70 |
| 3 | CBPB + USGDN | 10 | 6 | 3 | 30 | 20 | 8 | 90 | 78 | 53 |
| 4 | CBPB + USGDN | 10 | 6 | 3 | 28 | 18 | 6 | 72 | 66 | 32 |
| 5 | CBPB + USGDN | 10 | 6 | 2 | 34 | 14 | 4 | 90 | 72 | 20 |
| 6 | CBPB + USGDN | 10 | 7 | 3 | 32 | 16 | 8 | 88 | 80 | 47 |
| 7 | CBPB + USGDN | 10 | 4 | 1 | 30 | 18 | 8 | 86 | 77 | 49 |
| 8 | CBPB + USGDN | 10 | 6 | 3 | 28 | 12 | 6 | 80 | 71 | 40 |
| 9 | SGB + USGDN | 10 | 5 | 3 | 26 | 20 | 8 | 86 | 80 | 34 |
| 10 | SGB + USGDN | 7 | 4 | 2 | 24 | 12 | 6 | 60 | 56 | 22 |
| 11 | ITP + USGDN | 10 | 4 | 1 | 34 | 12 | 6 | 90 | 64 | 40 |
| 12 | ITP + USGDN | 10 | 7 | 3 | 30 | 20 | 8 | 88 | 76 | 32 |
| 13 | sPRF + USGDN | 9 | 4 | 1 | 20 | 12 | 6 | 76 | 72 | 24 |
| 14 | sPRF + USGDN | 10 | 5 | 2 | 30 | 16 | 6 | 86 | 72 | 27 |
| 15 | sPRF + USGDN | 8 | 4 | 3 | 28 | 16 | 6 | 60 | 47 | 18 |
| 16 | sPRF + USGDN | 9 | 4 | 3 | 20 | 12 | 6 | 68 | 60 | 20 |
| 17 | USGDN alone | 6 | - | 1 | 16 | - | 4 | 50 | - | 15 |
| 18 | USGDN alone | 8 | - | 2 | 16 | - | 6 | 58 | - | 37 |
| 19 | USGDN alone | 10 | - | 1 | 32 | - | 8 | 82 | - | 23 |
| 20 | USGDN alone | 7 | - | 1 | 16 | - | 6 | 54 | - | 15 |
†SGB, CBPB, sPRF, and ITP are grouped together as NIs. CBPB: Continuous brachial plexus block, DASH: Disabilities of arm, shoulder, and hand, ITP: Intrathecal pump, NRS: Numerical Rating Scale, USGDN: Ultrasound-guided dry needling, sPRF: Shoulder pulsed radiofrequency, SGB: Stellate ganglion block, PD: PainDETECT, NIs: Neural interventions
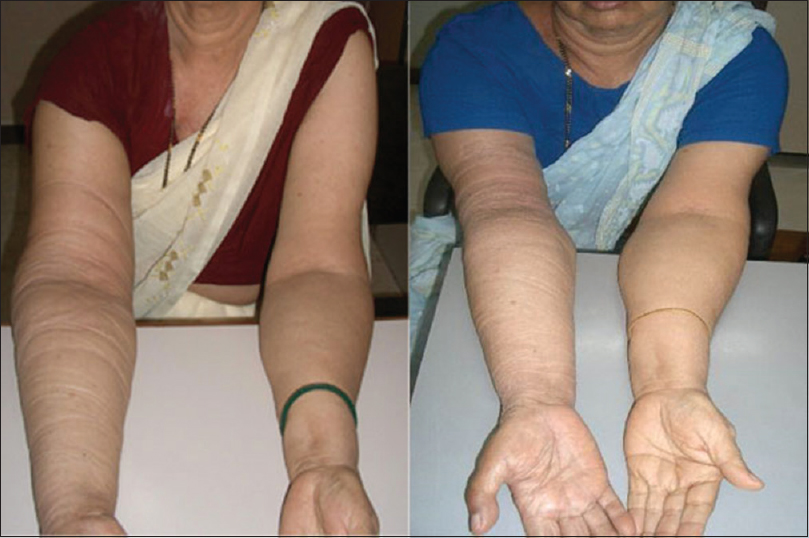
- Effect of stellate ganglion block on lymphedema. Left: image was taken at baseline, showing swelling in the right arm. Right: image was taken 1-week poststellate ganglion block before the start of ultrasound-guided dry needling, showing reduction in swelling.
CBPB reduced rest pain in the extremity, but patients still had chest wall pain at rest and upper-extremity pain upon movement. The response to USGDN revealed that the residual chest wall pains were arising from the serratus anterior and subscapularis muscles. Movement pain in the extremity was also reduced after systematic USGDN of the shoulder, arm, and forearm muscles. ITP produced general pain relief, but patients reported becoming more aware of focal myofascial pains in the shoulder girdle muscles after this procedure. It was only after USGDN of these muscles that they expressed satisfaction with pain relief that allowed them to rest well. The shoulder injections and PRF provided relief of rest pain, but stiffness and movement pain persisted with continued disability. Again, it was after USGDN that there was relief of pain on movement and reduction of stiffness.
After starting USGDN, pain relief, reduction of swelling, and improvement in the range-of-motion were observed with every session. After 4 weeks of biweekly USGDN sessions, NRS and PD scores further reduced to 2.3 (0.8) and 6.6 (1.2), respectively (a reduction by 55.8% for NRS and 59% for PD compared to post-NI scores). The DASH score was reduced by 51% to 34.6 (14.4) by USGDN. PHQ-9 scores were further improved by USGDN, with six (30.0%) and four patients (20.0%) reporting mild and moderate depression, respectively, and ten patients (50.0%) reporting no depression. Overall, patients who received ITP (n = 2) and CBPB (n = 8) required 4–6 sessions of USGDN to become pain free. Patients who had no NIs (n = 4) and those with SGB (n = 2) or PRF (n = 4) required about 12–15 sessions.
By the end of treatment with USGDN, of the patients on oral morphine (n = 12), eight patients (50.0%) discontinued medications and two patients (16.7%) continued at 50% of the initial dose. Of the patients on fentanyl patches (n = 9), two patients (22.2%) stopped medication and three patients (33.3%) continued at 50% of the initial dose. All patients continued treatment with neuromodulators and adjunct analgesics such as paracetamol.
DISCUSSION
PMPS is widely regarded to be a purely neuropathic disorder. The definition of neuropathy by Treede et al. as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” limits the etiopathogenic origin of pain solely to the somatosensory nervous system, to exclude possible contributions from the musculoskeletal system.[10] However, our experience has shown that motor nerve involvement or neuromyopathy contributes to multiple pain syndromes that are considered to be purely neuropathic.[111213141516] In this study of twenty patients with severe pains from PMPS, we examined the effectiveness of targeting the neuromyopathic component of PMPS, in addition to the sensory neuropathic component. Targeting of only the sensory neuropathic component via nerve blocks or PRF treatment reduced pain without reducing the opioid medication dosage or disability, with the slight 9.8 - point reduction in the mean DASH score falling below the threshold for clinical relevance.[35] In contrast, targeting the neuromyopathic component via USGDN, which deactivates MTrPs, produced sustained relief from pain as well as disability and allowed reduction or discontinuation of opioids. These results suggest that the PMPS pain in this patient cohort was an expression of myofascial pain that developed secondary to neuromyopathy.
In agreement with the findings of this study, Fernández-Lao et al. have described MTrPs in the neck and shoulder muscles in patients with PMPS.[18] Ultrasound-guided MTrP injections in the subscapularis and/or pectoralis were found to relieve pain in PMPS.[30] In addition, several reports have shown that Botox, which acts at the motor end plate,[36] can relieve PMPS pain.[373839]
Importantly, while NI could reduce pain, it had negligible-to-no effect on opioid consumption or disability – both these outcome measures were improved only post-USGDN. Opioid consumption was unchanged after NI but reduced substantially post-USGDN: 50% of patients discontinued morphine and 16.6% showed a 50% reduction in opioid dose. Of those patients on fentanyl, 22.2% discontinued the patch and 33.3% had a 50% reduction in fentanyl dose. The DASH reduction was only 12% after NI (below the cutoff for clinical relevance[35]) but was reduced by 51% by USGDN. Furthermore, pain, which was reduced by NI, decreased further post-USGDN: Compared to post-NI scores, NRS and PD were further reduced by 55.8% and 59%, respectively, by USGDN. Overall, these findings indicate that NI treated the neural contribution to PMPS, whereas USGDN treated both neural and myofascial contributions to PMPS as it further reduced pain and produced a tangible reduction in disability. In addition, while NI reduced depression in our patient cohort, this parameter was further improved by USGDN. Thus, USGDN appeared to provide the most symptom relief in this cohort. It took the patients to a level of comfort that they could appreciate while reducing the opioid consumption. It is a modality that can be repetitively performed, with distinct relief that the patient could identify with. For example, a patient presenting with chest wall pain responded to needling of the serratus anterior, whereas a patient presenting with shoulder pain responded to needling of shoulder muscles. It should be emphasized that PMPS has a heterogeneous array of pain generators, and the treatment has to be multimodal where each modality will contribute to a percentage of the eventual relief experienced by the patient. In addition, some of these patients present with such severe pain that it would be kinder to start with NIs such as PRF or brachial plexus catheter for a rapid control of pain.
The origins of MTrP development remain unclear, and the mechanism underlying the development of MTrPs in patients who have undergone breast surgery, radiation, or chemotherapy warrants examination. As outlined in Figure 3, we hypothesize that efferent motor nerve irritability (secondary to surgery, radiation, or chemotherapy) may result in increases in the electrical activity and end-plate potentials at the NMJs culminating in the development of MTrPs and subsequently, the symptoms of PMPS. In support of this theory, the nerve has been shown to be vulnerable to the effects of radiation.[40] An increased electrical activity (SEA) has been associated with MTrPs and is thought to arise from the motor end plate. This has been named “end-plate noise” by Simons et al.[23] Overall, there are some reports demonstrating MTrP association with increased electrical activity and end-plate noise.[21414243] Taken together, these findings add to a growing body of evidence,[111213141516] suggesting a connection between motor nerve irritability and MTrP formation in various pain conditions, which requires further exploration.
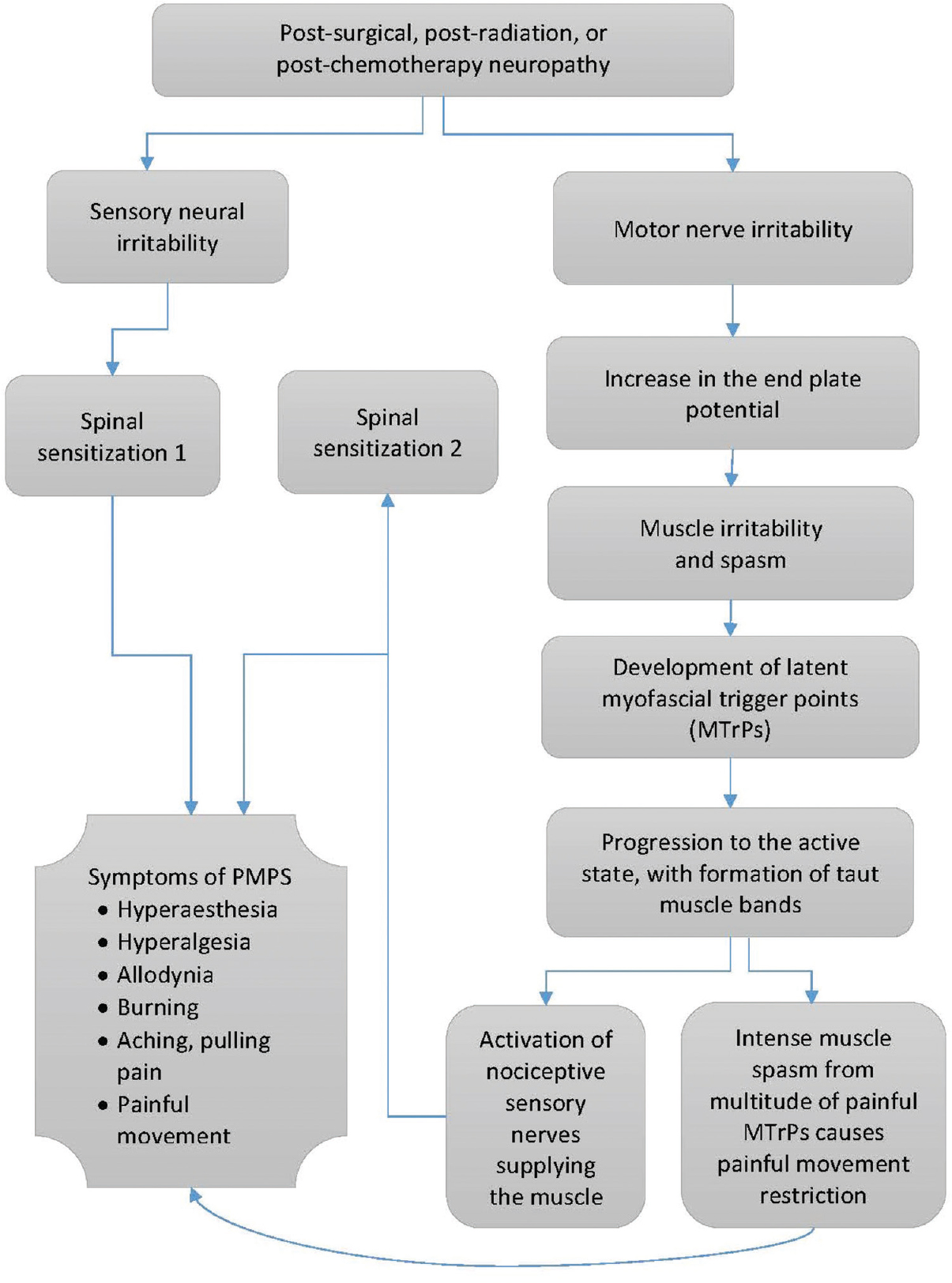
- Proposed mechanism of contribution of motor nerve neuropathy to the development of postmastectomy pain syndrome.
Once the MTrPs are formed, they may progress from the latent to active state,[44] at which point spontaneous pain is experienced. The intense contraction at the MTrP results in a sensory phenomenon of localized, exquisite pain, which is transmitted by musculosensory nerve endings in the muscle to the neuraxis, thereby setting up a process of central sensitization [Figure 3].[21] We refer to the nociceptive sensory afferents from the muscle as musculosensory nerves for the convenience of understanding and to distinguish them from the sensory nerves of the somatosensory nervous system. It is possible that painful afferent impulses from MTrPs and shortened bands give rise to stiffness, which impedes muscle movements and causes a pulling sensation at the tendinous insertions. This muscle pain escalates with the establishment of spinal sensitization, to give rise to hyperalgesia; allodynia; hyperesthesia, the severe burning pain; and movement restriction from stiffness.[4546] All these features are present in PMPS patients. An important characteristic of active MTrPs is referred pain, with a characteristic of spread of nociceptive activation in the central nervous system, specifically in the spinal cord. Pain referral makes diagnosis more difficult because arm and hand pain can originate from the neck or shoulder muscles.[21]
It can be sometimes challenging to establish the presence of an MTrP, especially in patients with allodynia and hyperalgesia where palpation would produce intense pain. The introduction of a needle into the MTrP causes a local twitch response (LTR), which elicits a reflex relaxation of the muscle harboring the MTrP through the mediation of a spinal reflex response.[47] Elicitation of an LTR establishes the presence of an MTrP.[44] We routinely observed LTRs in the muscles underlying and adjacent to the surgically scarred or radiated areas in our patient cohort (data not shown). In the patients with CRPS (as determined by Budapest criteria), these LTRs were followed by continuous fasciculation of muscles, which subsided after 15–20 min. Of note, many of these LTRs were of a low enough intensity that they could only be captured by ultrasound monitoring during USGDN but would have escaped detection by the naked eye or felt by the hand during dry needling. The lower-intensity LTRs were more frequently observed than the higher-intensity events, suggesting that the majority may be missed during routine dry needling as compared to USGDN.
We propose that the lasting relief of pain following MTrP deactivation by USGDN is attributable to the specific spinal reflex relaxation that follows an LTR, allowing improvement of muscle function, ROM, and disability. A proposed sequence of events in response to USGDN is shown in Figure 4. With successive USGDN sessions, the LTR intensity and frequency as well as muscle fasciculation (seen in patients with CRPS) were reduced, coinciding with reports of pain relief in the concerned areas. These included the chest wall (in particular, the intercostal muscles and the overlying serratus anterior or rhomboid muscles), upper extremity muscles, and neck muscles. Patients diagnosed with costochondritis displayed a superficial skin allodynia in the intercostal space, in addition to deep intercostal muscle tenderness. Both symptoms responded to USGDN of the intercostal muscles and the overlying serratus anterior or rhomboid muscles. In many patients, the needle had to reach the internal intercostal muscle just superficial to the pleura [Figure 5] to achieve lasting pain relief.
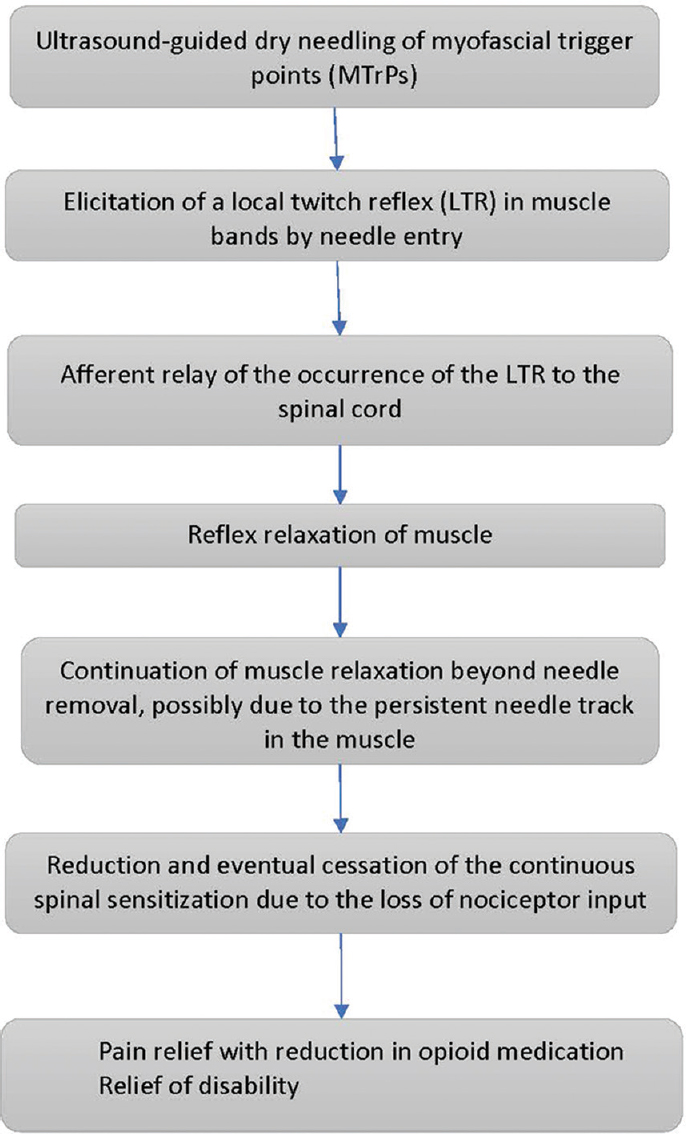
- Proposed mechanism of action of ultrasound-guided dry needling.
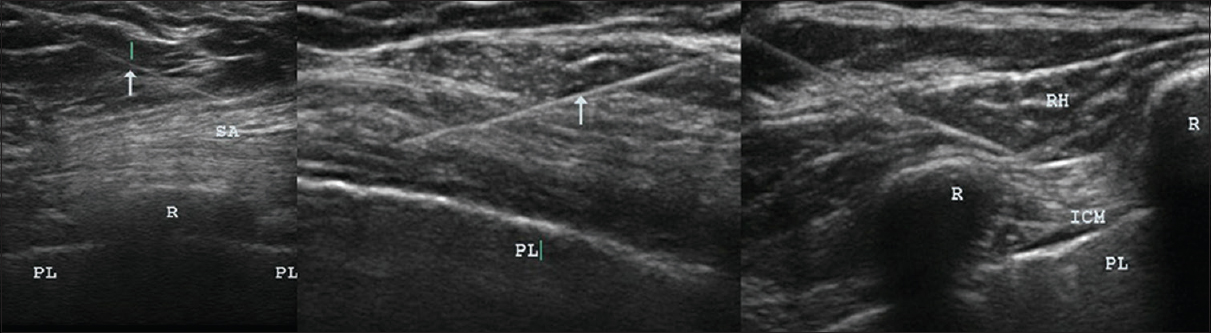
- Ultrasound images showing needles in muscles. Ultrasound images were taken during USGDN, with arrows indicating needles in the serratus anterior overlying the rib and the pleura in the intercostal spaces (left); in the pectoralis major and minor overlying the pleura (center); and in the rhomboid and intercostal muscles (right). USGDN, ultrasound-guided dry needling.
The majority of patients in this study had allodynia, hyperesthesia, dysesthesia (all considered as cutaneous manifestations of sensory neuropathy), and lymphedema.[484950] These were eliminated only after USGDN although the NIs of SGB and CBPB reduced them to some extent. Similar results were obtained in the four patients who received only USGDN, supporting the theory that the underlying mechanisms are not limited to the somatosensory system. Relief of superficial allodynia following USGDN of the underlying muscles led us to surmise elsewhere[14] that allodynia may be attributable to intense spasm of the underlying dermal motor elements such as erector pili, which could also be the cause of the “Peau d’orange” appearance of the skin in these areas. In addition, skin hardening and loss of elasticity described in PMPS, with the latter commonly seen in those who had undergone breast conservation procedures, was reversed by 1–2 sessions of USGDN. Our clinical experience suggests that the skin hardening is probably the result of an intense, but reversible spasm of the erector pili muscles, which responded to needling with relaxation. We have suggested elsewhere that erector pili spasm is responsible for allodynia (a classical symptom of neuropathy) in CRPS.[14] Fibrosis of the anterior and posterior axillary fold muscles (pectoralis major, coracobrachialis, teres major, and latissimus dorsi) in PMPS results in tethering of the upper arm to the chest and restricts shoulder, scapular, and clavicular movements. The constant pull of these muscles at both ends on the pain-sensitive tendinous insertion leads to the severe pains described by these patients.[50] It is probably this myofascial component that makes the so-called neuropathic/neuromyopathic pains respond better to NSAIDs than to opioids. Notably, USGDN resulted in a perceptible normalization of hardened muscle that was evident to both the patient and the doctor performing USGDN, along with improvements in the muscle contractility, RROM, and the DASH score.
In summary, the symptoms of PMPS are considered to be neuropathic in origin, yet medications and treatments designed to treat sensory neuropathy are only partially effective in relieving the pain.[234] We propose that, in addition to the NIs, the myofascial component plays an important role in the causation of PMPS, which is amenable to reversal by USGDN, which was the sole treatment for 20% of the patients in this cohort. We recommend that dry needling treatment for PMPS should be only done under ultrasound guidance, especially for needling over the chest wall, since the needles enter anatomical structures distorted by the dual effects of surgical procedures and radiation. The lungs and pleura are perilously close to the surface, necessitating needle entry under adequate visualization for safety and accuracy.
The findings of this study are limited by retrospective study design and lack of control groups. Prospective, well-controlled studies in larger cohorts comparing USGDN alone or in the combination of NIs with a sham-needled group are needed to confirm these findings.
CONCLUSIONS
PMPS appears to have a combination of neural and myofascial contributions to pain. USGDN, a treatment that deactivates MTrPs, provided sustained symptom relief with reduction or discontinuation of opioid medication, suggesting that a significant proportion of the neuropathy in PMPS may actually be neuromyopathy, which contributes to the pain and disability observed. These preliminary results indicate a need for a reexamination of the accepted etiology of PMPS and suggest that multimodal treatment regimens with NIs which reduce the pain intensity be performed initially to facilitate the performance of USGDN. It appears that in PMPS, it is not that one treatment modality scores over the effect of another, but that a multimodal approach is more likely to take the patient as close to normalcy as possible. This paradigm change in perspective may improve future outcomes in patients with PMPS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Medical writing support was provided by Jaya Vas, Ph.D. and funded by Ashirvad Institute of Pain Management and Research.
REFERENCES
- Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985-92.
- [Google Scholar]
- Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92:225-30.
- [Google Scholar]
- Impairments, disabilities and health related quality of life after treatment for breast cancer: A follow-up study 2.7 years after surgery. Disabil Rehabil. 2004;26:78-84.
- [Google Scholar]
- Predictors of persistent pain after breast cancer surgery: A systematic review and meta-analysis of observational studies. CMAJ. 2016;188:E352-61.
- [Google Scholar]
- Pilot study of a survey to identify the prevalence of and risk factors for chronic neuropathic pain following breast cancer surgery. Oncol Nurs Forum. 2012;39:E141-9.
- [Google Scholar]
- Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain. 2009;13:478-85.
- [Google Scholar]
- Post-breast surgery pain syndrome: Establishing a consensus for the definition of post-mastectomy pain syndrome to provide a standardized clinical and research approach – A review of the literature and discussion. Can J Surg. 2016;59:342-50.
- [Google Scholar]
- Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630-5.
- [Google Scholar]
- Successful reversal of complex regional pain syndrome type 1 of both upper extremities in five patients. Pain Med. 2012;13:1253-6.
- [Google Scholar]
- Reversal of complex regional pain syndrome type 2 and the subsequent management of complex regional pain syndrome type 1 occurring after corrective surgery for residual ulnar claw. Pain Med. 2014;15:1059-63.
- [Google Scholar]
- Complex regional pain syndrome-type 1 presenting as deQuervain's stenosing tenosynovitis. Pain Physician. 2016;19:E227-34.
- [Google Scholar]
- Improvement in CRPS after deep dry needling suggests a role in myofascial pain. Pain Med. 2018;19:208-12.
- [Google Scholar]
- Myofascial trigger points as a cause of abnormal cocontraction in writer's cramp. Pain Med. 2015;16:2041-5.
- [Google Scholar]
- A new perspective of neuromyopathy to explain intractable pancreatic cancer pains; dry needling as an effective adjunct to neurolytic blocks. Indian J Palliat Care. 2016;22:85-93.
- [Google Scholar]
- Prevalence of myofascial pain syndrome in patients with incurable cancer. J Bodyw Mov Ther. 2018;22:328-32.
- [Google Scholar]
- Myofascial trigger points in neck and shoulder muscles and widespread pressure pain hypersensitivtiy in patients with postmastectomy pain: Evidence of peripheral and central sensitization. Clin J Pain. 2010;26:798-806.
- [Google Scholar]
- Psychophysical examination in patients with post-mastectomy pain. Pain. 2000;87:275-84.
- [Google Scholar]
- Incidence of myofascial pain syndrome in breast cancer surgery: A prospective study. Clin J Pain. 2010;26:320-5.
- [Google Scholar]
- The myofascial trigger point region: Correlation between the degree of irritability and the prevalence of endplate noise. Am J Phys Med Rehabil. 2007;86:183-9.
- [Google Scholar]
- Do endplate noise and spikes arise from normal motor endplates? Am J Phys Med Rehabil. 2001;80:134-40.
- [Google Scholar]
- Travell & Simons’ myofascial Pain and Dysfunction: Upper Half of Body. Vol 1. Lippincott Williams & Wilkins; 1999.
- [Google Scholar]
- Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: A randomized, double-blinded, placebo-controlled trial. Evid Based Complement Alternat Med. 2013;2013:694941.
- [Google Scholar]
- Treatment of nonspecific thoracic spine pain with trigger point dry needling and intramuscular electrical stimulation: A case series. Int J Sports Phys Ther. 2014;9:699-711.
- [Google Scholar]
- The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: A case report. Int J Sports Phys Ther. 2013;8:145-61.
- [Google Scholar]
- Trigger point dry needling and proprioceptive exercises for the management of chronic ankle instability: A randomized clinical trial. Evid Based Complement Alternat Med. 2015;2015:790209.
- [Google Scholar]
- Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil. 2010;89:133-40.
- [Google Scholar]
- A critical overview of the current myofascial pain literature – April 2018. J Bodyw Mov Ther. 2018;22:184-91.
- [Google Scholar]
- Application of ultrasound-guided trigger point injection for myofascial trigger points in the subscapularis and pectoralis muscles to post-mastectomy patients: A pilot study. Yonsei Med J. 2014;55:792-9.
- [Google Scholar]
- Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20:88-93.
- [Google Scholar]
- PainDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911-20.
- [Google Scholar]
- Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602-8.
- [Google Scholar]
- The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-13.
- [Google Scholar]
- Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH) J Orthop Sports Phys Ther. 2014;44:30-9.
- [Google Scholar]
- Incobotulinum toxin-A improves post-surgical and post-radiation pain in cancer patients. Toxins (Basel). 2016;8 pii: E22
- [Google Scholar]
- OnabotulinumtoxinA for treatment of focal cancer pain after surgery and/or radiation. Pain Med. 2012;13:1029-33.
- [Google Scholar]
- Treatment of post mastectomy pain syndrome after mastopexy with botulinum toxin. J Plast Reconstr Aesthet Surg. 2014;67:873-4.
- [Google Scholar]
- Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys. 1995;31:1309-18.
- [Google Scholar]
- Decreased spontaneous electrical activity and acetylcholine at myofascial trigger spots after dry needling treatment: A pilot study. Evid Based Complement Alternat Med. 2017;2017:3938191.
- [Google Scholar]
- Effect of botulinum toxin on endplate noise in myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2002;81:512-20.
- [Google Scholar]
- Myofascial trigger points show spontaneous needle EMG activity. Spine (Phila Pa 1976). 1993;18:1803-7.
- [Google Scholar]
- Myofascial trigger points then and now: A Historical and scientific perspective. PM R. 2015;7:746-61.
- [Google Scholar]
- Scratching the surface: The processing of pain from deep tissues. Pain Manag. 2016;6:95-102.
- [Google Scholar]
- Symptom report in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press). 2015;7:345-52.
- [Google Scholar]
- Clinical manifestations and diagnosis of postmastectomy pain syndrome. In: Chagpar A, ed. UpToDate. Waltham, MA: UpToDate; 2015.
- [Google Scholar]
- Chronic arm morbidity after curative breast cancer treatment: Prevalence and impact on quality of life. J Clin Oncol. 2002;20:4242-8.
- [Google Scholar]






