Prognostic Factors of Malignant Pleural Effusion among Palliative Care Outpatients: A Retrospective Study
Address for correspondence: Dr. Jenifer Jeba, Department of Medical Oncology, Christian Medical College Hospital, Vellore - 632 004, Tamil Nadu, India. E-mail: jenifermugesh@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Malignant pleural effusion (MPE) has varied survival and indicates advanced disease. LENT prognostic score is the first validated score used for MPE. This study assessed the role of LENT among palliative care cancer patients and assessed different patient, tumor, and treatment related factors that may affect survival.
Methods:
A retrospective study of advanced cancer patients with MPE, seen in palliative care outpatient clinic (2013–2015) until death, was done. LENT prognostic score could be calculated in 15 patients. Patient, tumor, and treatment related factors that affect survival were assessed.
Results:
The study included 48 patients (70.8% female; 29.2% male) with a median age of 53 years. Lung (41.7%) was the most common primary, and adenocarcinoma (44.7%) was the most common histology. The median overall survival (OS) was 14.5 months (interquartile range [IQR]: 5.25–32.75) and median survival time (ST) was 3 months (IQR: 1–7.75). ST was significantly low with poor Eastern Cooperative Oncology Group (ECOG) performance status (P = 0.002), bilateral effusion (P < 0.001), and with no oncological treatment after MPE diagnosis (P < 0.001). OS and ST were significantly low with lung primary (P = 0.006 and 0.02, respectively). Age, gender, breathlessness, tumor histology, lung metastasis, and interventions for MPE did not significantly affect survival. The median ST in the moderate and high risk LENT groups was 6 and 3 months, respectively (P = 0.16).
Conclusion:
ECOG performance status, bilateral effusion, and no oncological treatment after diagnosis of MPE were associated with poor ST. Lung primary was associated with shorter OS and ST. Small numbers precluded any definitive conclusion on the prognostic value of LENT in our group of patients, and hence larger studies are recommended.
Keywords
Malignant pleural effusion
LENT
survival
prognosis
INTRODUCTION
Malignant pleural effusion (MPE) occurs in approximately 15% of cancer patients.[1] MPE indicates advanced disease with a median survival of about 3–12 months.[2] The mortality rates as reported by DeBiasi et al. are 37% and 77% at 30 days and 1 year, respectively.[3] The management of MPE includes different therapeutic options: therapeutic pleural tap, intercostal tube drainage and pleurodesis, indwelling pleural catheter, oncological treatment, or best supportive care.[4]
With the heterogeneity in the group of patients with MPE, there is a challenge to predict prognosis and survival. With improved techniques in the management of pleural effusion and better oncological options, there is an increasing need for good prognostication to tailor the most appropriate treatment. Many factors affect survival including the tumor type, performance, and systemic inflammatory markers.[5] Clive et al. analyzed 14 predefined prognostic variables that affected survival across three large international cohorts and developed and validated the LENT prognostic score. LENT score [Table 1] is the first validated prognostic score in MPE, calculated based on pleural fluid lactate dehydrogenase, Eastern Cooperative Oncology Group (ECOG) performance score, neutrophil-to-lymphocyte ratio, and tumor type. Based on the scores, patients were stratified to low, medium, or high risk.[6] The advantages of LENT score are that it is cheap, objective, clinically easy to use, and has been reported to be more accurate than ECOG alone.[6] Good prognostic scoring systems can aid in therapeutic decision-making.

This study was designed to study the usefulness of LENT prognostic score among palliative care cancer patients with MPE. Other patient, tumor, and treatment related factors that may affect survival in this group of patients were also assessed.
METHODS
This retrospective study included advanced cancer patients with MPE, seen in the palliative care outpatient clinic, during a period of 2 years from 2013 to 2015, until death. In 15 patients, necessary parameters were available and LENT score was calculated at the time of diagnosis of MPE. In the other 33 patients, LENT score was not calculated as one or more parameters needed for its calculation were not available. Overall survival (OS – time from diagnosis of cancer to death) and survival time (ST – time from diagnosis of MPE to death) were calculated.
Statistical analysis
Categorical variables were presented using frequencies with percentages. Continuous variables were summarized using mean with standard deviation or median with interquartile range (IQR) depending on the distribution which was assessed using quantile-quantile plot. The comparison of survival curves was done using log-rank test.
Statistical analysis was done using SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc and P < 0.05 was considered statistically significant.
RESULTS
The study included 48 patients (70.8% female; 29.2% male) with a median age of 53 years (mean: 56.25; range: 33–86 years). Table 2 summarizes the demographic and clinical characteristics of the study population. MPE was unilateral in 76.7% [Table 3]. The most common primary was lung (41.7%), followed by breast (27.1%). Adenocarcinoma (44.7%) was the most common histology. Breathlessness was the major symptom at diagnosis and at the last visit in 52.6% and 41.6%, respectively. Lung parenchymal metastasis was present in 39.6%. The mode of diagnosis of MPE was a confirmed malignancy with pleural effusion in 70.8% and a positive pleural fluid cytology in 27.1%. Of the 18 patients who had pleural fluid tested for cytology, positive results were obtained in 72.2% (13 patients). Two-third of the patients were ECOG performance status ≤2 and one-third were ECOG performance status 3. There was only one patient in this group who was ECOG performance status 4 [Table 3]. Metastases at other sites were found in 81.3% of patients with MPE.
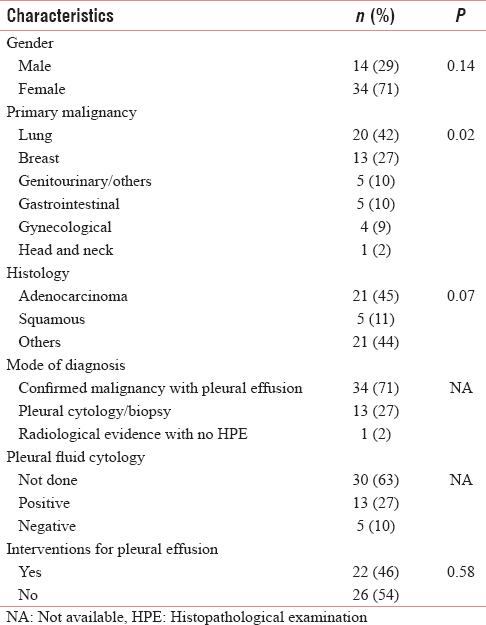

Among patients with MPE, 54.2% did not need any intervention for the pleural effusion [Table 2]. Among those who needed some intervention, 59.1% underwent intercostal chest tube drainage and pleurodesis. The agent most commonly used for pleurodesis was iodopovidone (84.6%) and 92.3% had successful pleurodesis. There were no major complications; infection was seen in 12.5%. The mean hospital stay of patients who had pleurodesis was 6.54 ± 6.7 days. After an intervention for MPE, 37.4% of patients went on to have some oncological treatment with chemotherapy and hormonal or targeted therapy.
The median OS was 14.5 months (IQR: 5.25–32.75). The median ST from diagnosis of MPE to death (ST) was 3 months (IQR: 1–7.75). LENT prognostic score could be calculated in 15 patients (moderate risk – 10, high risk – 5). The median ST in the moderate and high risk groups was 6 and 3 months, respectively, showing a nonsignificant trend of shorter survival in the high risk group (P = 0.16). The percentage of patients alive at 3 months in the moderate and high risk group was 80% and 60%, respectively. At 6 months, 50% and 20% of patients were alive among the moderate and high risk groups, respectively.
Among the various factors studied, ST was significantly low with poor ECOG performance status (P = 0.002) [Figure 1], bilateral effusion (P< 0.001) [Figure 2], and with no oncological treatment after diagnosis of MPE (P< 0.001) [Figure 3]. The ST and OS [Figure 4] were significantly low with lung primary (P = 0.02 and P = 0.006, respectively). The median ST with IQR, in patients with different ECOG performance status, LENT score groups, and unilateral/bilateral effusion, is provided in Table 3. ST was not affected by other factors such as age (P = 0.934), gender (P = 0.136), presence of breathlessness (P = 0.886), tumor histology (P = 0.07), lung metastasis (P = 0.882), and interventions for MPE (0.582). It is of interest to note that the last visit before death for most patients was to the outpatient department (60.4%) as compared to the inpatient unit (29.2%) and to the accident and emergency department (10.4%).
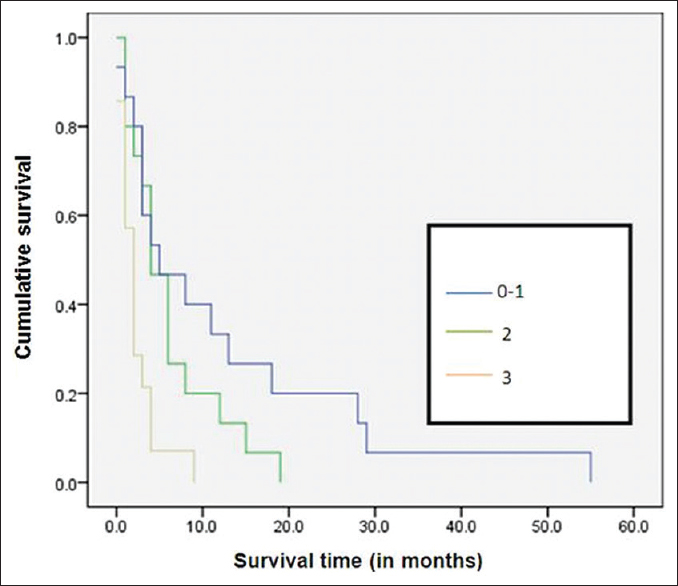
- Kaplan–Meier survival curves according to Eastern Cooperative Oncology Group performance status

- Kaplan–Meier survival curves for unilateral and bilateral pleural effusion

- Kaplan–Meier survival curves with and without oncological treatment

- Kaplan–Meier survival curves according to diagnosis
DISCUSSION
MPE signifies advanced disease and is often associated with poor prognosis. The most common cancer in our group of patients with MPE was lung (41.7%), followed by breast (27.1%). This finding is similar to what is known that MPE occurs most commonly in lung, followed by breast.[7] Lung cancer is the most common cause of MPE in men and breast cancer in women.[8] MPE occurs in about 8%–38% of patients with lung cancer.[2910]
In the LENT score validation study,[6] all patients had presented with symptomatic pleural effusion; however in our study, there was a good proportion (47%) of patients who did not have breathlessness at diagnosis of MPE. The yield from pleural fluid cytology in our study was 72.2%, much higher than what is reported by DeBiasi et al. as 60%. In their study, the 30-day and 1-year mortality rates in patients with malignant cytology positive versus negative effusions were not different.[3]
The agent most commonly used for pleurodesis in our study was iodopovidone (84.6%), with a high success rate of 92.3%. A systematic review on iodopovidone for pleurodesis suggests it to be safe and effective in recurrent pleural effusions and pneumothoraces.[11] A recent Cochrane review suggests talc poudrage as the most effective pleurodesis method for MPE, but also states that there may not be randomized trials on many other agents and local availability; global experience needs to be considered in the choice of the sclerosant.[12]
The median survival for patients in the low, moderate, and high risk groups in the LENT validation study was 319, 130, and 44 days, respectively.[6] The survival rate of those in the moderate risk group was 81%, 59%, and 47% at 1, 3, and 6 months, respectively. The survival rate in the high risk group was 65%, 13%, and 3% at 1, 3, and 6 months, were respectively.[6] LENT score was also significantly better than ECOG performance status at predicting survival at 1, 3, and 6 months.[6] Different factors have been studied that affect survival of patients with MPE. Multiple studies [13141516] have identified performance status as a significant factor that affects survival in MPE, as in our study. Patients with bilateral effusions had significantly low ST in our study. DeBiasi et al., in their analysis of a cohort of patients who underwent thoracocentesis, also reported higher mortality at 30 days in patients with bilateral MPE.[3] Patients who did not have oncological treatment after diagnosis of MPE had significantly low ST. This group of patients may have had also had poor ECOG status or failed previous oncological treatment.
Patients with lung cancer had the shortest ST, both in the LENT validation cohort (2.5 months)[6] and in the present study (7 months). The better ST and survival rates in our study group as compared to the LENT validation study cohort could be because all patients in the LENT validation study cohort had symptomatic MPE and may have been seen later in the course of the illness. Anevlavis et al. report the survival with non-small cell lung cancer and small cell lung cancer to be 9.5 and 6 months, respectively.[16] Presence of pleural effusion at initial presentation in lung cancer has been associated with shorter survival, in both non-small cell and small cell lung cancers.[171819] Among the different cancers, lymphoma is reported to have the best median survival (26 months), followed by breast and ovarian cancers (18 and 15 months, respectively).[16]
The limitations of our study include the small sample size and the retrospective design. LENT prognostic scoring was possible only in 31%. This group represents only those who were referred and followed up until death in palliative care outpatient clinic and does not represent the entire group of patients with MPE.
Implications for clinical practice
LENT prognostic score is a simple score that is possible in patients in whom pleural fluid analysis is available. Factors such as the severity of symptoms and access to care are other important factors to consider in choosing between pleurodesis and less invasive interventions, especially in high risk patients. LENT prognostic score can be used to guide decision-making in the treatment of MPE, especially in multidisciplinary meetings and in routine clinical care.[20] In appropriate high risk patients, pleurodesis should be considered, as majority of them lived for more than a month. In those with extremely poor prognosis, less invasive interventions such as therapeutic pleural aspiration or indwelling pleural catheter, to try and minimize discomfort, and hospital stay at end of life should be considered, where resources and expertise are available.[21]
Implications for research
There is a need for larger prospective studies to study the role of LENT prognostic score among palliative care patients in hospital, community, and hospice settings. The usefulness of LENT score should be translated to oncological decision-making, and risk-based treatment algorithm should be developed to aid the management of MPE.
CONCLUSION
Poor ECOG performance status, bilateral effusion, and no oncological treatment after diagnosis of MPE were factors associated with poor survival. Lung cancer was associated with shorter OS and ST. As highlighted, there is a need for larger multicenter prospective studies to study the role of LENT prognostic score among palliative care patients in different settings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J. 1989;2:366-9.
- [Google Scholar]
- Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii32-40.
- [Google Scholar]
- Mortality among patients with pleural effusion undergoing thoracocentesis. Eur Respir J. 2015;46:495-502.
- [Google Scholar]
- Cochrane corner: Interventions for the management of malignant pleural effusions. Thorax. 2016;71:964-6.
- [Google Scholar]
- Pleural effusions as markers of mortality and disease severity: A state-of-the-art review. Curr Opin Pulm Med. 2016;22:386-91.
- [Google Scholar]
- Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax. 2014;69:1098-104.
- [Google Scholar]
- Pleural diseases related to metastatic malignancies. Eur Respir J. 1997;10:1907-13.
- [Google Scholar]
- The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31:85-97.
- [Google Scholar]
- Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
- [Google Scholar]
- The positive pleural effusion. A retrospective study of cytopathologic diagnoses with autopsy confirmation. Acta Cytol. 1992;36:329-32.
- [Google Scholar]
- Efficacy & safety of iodopovidone pleurodesis: A systematic review & meta-analysis. Indian J Med Res. 2012;135:297-304.
- [Google Scholar]
- Interventions for the management of malignant pleural effusions: A network meta-analysis. Cochrane Database Syst Rev. 2016;5:CD010529.
- [Google Scholar]
- Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10:e335-41.
- [Google Scholar]
- Predicting survival in patients with recurrent symptomatic malignant pleural effusions: An assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117:73-8.
- [Google Scholar]
- Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med. 2015;15:29.
- [Google Scholar]
- Prognostic factors in patients presenting with pleural effusion revealing malignancy. Respiration. 2014;87:311-6.
- [Google Scholar]
- Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J. 2013;41:1409-18.
- [Google Scholar]
- Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol. 2014;32:960-7.
- [Google Scholar]
- Minimal pleural effusion in small cell lung cancer: Proportion, mechanisms, and prognostic effect. Radiology. 2016;278:593-600.
- [Google Scholar]
- Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA. 2012;307:2383-9.
- [Google Scholar]






