Translate this page into:
Comparison of Efficacy and Safety of Prophylactic Use of Metoclopramide and Haloperidol on Morphine-induced Nausea and Vomiting in Cancer Patients: A Comparative, Randomised, Prospective Study
*Corresponding author: Yogendra Singhal, Department of Palliative Medicine, Sawai Man Singh Medical College, Jaipur, Rajasthan, India. singhalyogi@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Singhal Y, Pingoliya SK, Saji S, Gaurav RP. Comparison of Efficacy and Safety of Prophylactic Use of Metoclopramide and Haloperidol on Morphine-induced Nausea and Vomiting in Cancer Patients: A Comparative, Randomised, Prospective Study. Indian J Palliat Care. 2024;30:375-9. doi: 10.25259/IJPC_141_2024
Abstract
Objectives:
Morphine is the cornerstone of pain management in a palliative care setting. Nausea with or without vomiting usually occurs when patients are initiated on morphine for the 1st time or when the dose is substantially increased.
Materials and Methods:
A total of 90 patients fulfilling the inclusion criteria were randomly allocated into two groups of 45 each. Group M received a tablet of metoclopramide 10 mg orally 3 times a day; Group H received a tablet of haloperidol (2.5 mg) orally at night. All the patients were provided NCI CTCAE V4.3 NAUSEA AND VOMITING SCALE and asked to mark their response from day 1 to day 7. At the follow-up visit on the 7th day, the form was collected, and data were analysed.
Results:
In Group M and Group H, the mean nausea score was between 1 and 2 and the difference was statistically insignificant. In Group M, the maximum vomiting score was 1.28 on day 5, while in Group H, the maximum score was 2 on day 5. The difference between the two groups was statistically significant.
Conclusion:
Metoclopramide and haloperidol are equally efficacious in preventing nausea, but metoclopramide was found to be more effective with lesser side effects than haloperidol for morphine-induced vomiting in cancer patients when used prophylactically.
Keywords
Nausea
Vomiting
Haloperidol
Metoclopramide
Morphine
INTRODUCTION
Morphine is the mainstay of pain management in the palliative care setting. Successful pain management with morphine requires adequate analgesia without excessive side effects. Approximately 10–30% of patients treated with morphine do not have a successful outcome due to either excessive side effects and inadequate pain relief or a combination of both.[1,2] All opioids have the potential for side effects, which may compel some patients to decrease or discontinue opioids. Most of the side effects caused by morphine are self-limiting, and no interventions are required. These include nausea and vomiting, sedation and drowsiness.[3,4]
Nausea is defined as an unpleasant feeling of the need to vomit, often accompanied by autonomic symptoms.[5] Vomiting is the forceful expulsion of gastric content through the mouth.[6] Nausea with or without vomiting usually occurs when patients are initiated on morphine for the 1st time or when the dose is substantially increased. Amongst patients treated with morphine, 8–35% reported nausea and 14–40% suffered from vomiting.[7] In most patients, this responds well to antiemetic medication and disappears spontaneously within 3 or 4 days.[8]
Morphine can cause nausea and vomiting through a number of different possible mechanisms. Morphine stimulates the emetic centre through D2 receptors of the chemoreceptor trigger zone (CTZ) in the area postrema.[9] It reduces gastrointestinal motility, causing gastroparesis and constipation.[10] In some patients, it can be so severe that they choose to suffer with significant pain rather than endure the nausea and vomiting. Hence, the drugs which can block dopamine receptors in CTZ and the gastrointestinal system might show promising results in morphine-induced vomiting. Several different antiemetic medications can be used as dopamine receptor antagonists, but haloperidol and metoclopramide are widely used drugs amongst them.[11]
Review for similar articles on Google scholar, pubmed and Scopus didn’t found any of studies which compared anti nausea and antiemetic profiles of these two drugs. Hence, the present study was planned to assess the efficacy of metoclopramide and haloperidol for the prevention of nausea and vomiting after receiving morphine in cancer patients.
MATERIALS AND METHODS
This comparative randomised prospective study was conducted in the palliative care outpatient department of tertiary care hospitals after approval by the local institutional ethical committee Ref. No.152 MC/EC/2023/April 01, 2023. The patient was informed about risks and benefits, and written informed consent was taken.
All the patients between the age group 20–70 years of both sexes who reported to the palliative care outpatient department having histologically diagnosed cancer disease with pain severity 7–10 on a numerical rating scale and patients to be prescribed a tablet of morphine 5 mg orally every 4 hourly 1st time for pain management were included in the study. Patients having dyselectrolytemia, deranged renal and liver function test, pregnant and lactating females, electrocardiogram (EKG) changes suggestive of cardiac disease and QTc prolongation, and known hypersensitivity to study drugs were also excluded from the study.
The sample size was calculated as 34 subjects for each of the two groups at alpha error 0.05 and power 80%, assuming cessation of nausea and vomiting in 45% and 78.1% of the cancer patients after giving metoclopramide and haloperidol, respectively. Considering attrition of 20%, 45 cancer patients were decided to be allocated to each group. Ninety patients were enrolled in this study after satisfying inclusion criteria and randomly allocated into two groups of 45 each by an opaque sealed envelope method using a computer-generated table of random numbers.
Group M (metoclopramide) was prescribed tablet metoclopramide 10 mg orally 3 times a day.
Group H (haloperidol) was prescribed a tablet of haloperidol (2.5 mg) orally at night.
As per study protocol, all the patients were interviewed, briefed and counselled about the drugs. Previous medication history, clinical examination and investigations were reviewed, and the vitals of all the patients were recorded. Along with the tablet of morphine, all the patients were also prescribed a tablet of paracetamol 650 mg orally 3 times a day, a tablet of gabapentin 300 mg orally once a day, a tablet of bisacodyl 10 mg orally at night and a capsule of omeprazole 20 mg orally for 7 days to cover other neuropathic pain and constipation in cancer patients.
All the patients were provided NCI CTCAE V4.3 nausea and vomiting scale. This scale is used to measure the severity of nausea and vomiting. This scale contains all the parameters related to the severity of nausea and is categorised into 3 grades (Grade-1 [mild] stands for loss of appetite without alteration in eating habits, Grade-2 [moderate] stands for oral intake decreased without significant weight loss, dehydration or malnutrition and Grade-3 [severe] stands for inadequate oral caloric or fluid intake tube feedings, total parenteral nutrition (TPN) or hospitalisation may be indicated). The severity of vomiting is categorised into 5 grades (Grade-1 [mild] stands for 1–2 episodes in 24 h; Grade-2 [moderate] stands for 3–5 episodes in 24 h; Grade-3 [severe] stands for >6 episodes in 24 h; tube feeding, TPN or hospitalisation indicated; Grade-4 [life threatening] stands for life-threatening consequences; urgent intervention indicated, and Grade-5 stands for death).
All the patients were asked to mark their responses from day 1 to day 7. At the follow-up visit on the 7th day, the form was collected, and the patients who stopped to take treatment were excluded from the study. Any adverse effects marked by the patient were also recorded. Pre-intervention and post-intervention EKGs were compared.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and compared using the student’s t-test, whereas categorical data were presented as numbers (Proportion) and compared using the Chi-square test. Data were analysed using the Statistical Package for the Social Sciences version 28.0 with P < 0.05 considered as statistically significant.
RESULTS
A total of 90 patients were included in the study, out of which 5 patients in Group M and 5 patients in Group H were lost to follow-up. Hence, data from 40 patients of each group were analysed [Figure 1].
- CONSORT flow diagram.
There were no statistical differences between the two groups regarding demographic parameters. The mean age in Group M was 52.27 ± 9.97 (38–69 years) years, while in Group H, it was 52.37 ± 10.16 years (35–70 years). The mean weight in Group M and Group H was 51.2 ± 7.07 and 53.8 ± 9.23 kg, respectively [Table 1].
| Patients parameters | Group-M | Group-H | P-value |
|---|---|---|---|
| Age | 52.27±9.94 | 52.32±10.16 | 0.98 |
| Sex | Male/Female (21/19) 52.5/47.5% n=40 | Male/Female (28/12) 70/30% n=40 | |
| Weight | 51.2±7.07 | 53.8±9.23 | 0.161 |
The most common types of malignancy in Group-M cases were lung 10 (25%) followed by buccal mucosa 8 (20%) and tongue 6 (15%). The most common types of malignancy in Group H were buccal mucosa 12 (30%), followed by lung 10 (25%) and G.B. 4 (10%) [Table 2].
| Malignancy | Group-M (n=40) | Group-H (n=40) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Buccal mucosa | 8 | 20 | 12 | 30 |
| Lung | 10 | 25 | 10 | 25 |
| GB | 3 | 7.5 | 4 | 10 |
| Rectum | 2 | 5 | 0 | 0 |
| Larynx | 1 | 2.5 | 0 | 0 |
| Tongue | 6 | 15 | 4 | 10 |
| Sarcoma | 3 | 7.5 | 2 | 5 |
| Breast | 3 | 7.5 | 3 | 7.5 |
| Urinary bladder | 1 | 2.5 | 0 | 0 |
| Prostate | 1 | 2.5 | 1 | 2.5 |
| Gynic (ovary, cervix) | 1 | 2.5 | 1 | 2.5 |
| Oesophagus | 1 | 2.5 | 0 | 0 |
| RCC | 0 | 0 | 2 | 5 |
| Parotid | 0 | 0 | 1 | 2.5 |
| Total | 40 | 100 | 40 | 100 |
GB: Gall bladder, RCC: Renal cell carcinoma
In Group M and Group H, the mean nausea score was between 1 and 2 [Figure 2]. In both groups, the difference between the mean nausea scores from day 1 to day 7 was insignificant [Table 3].
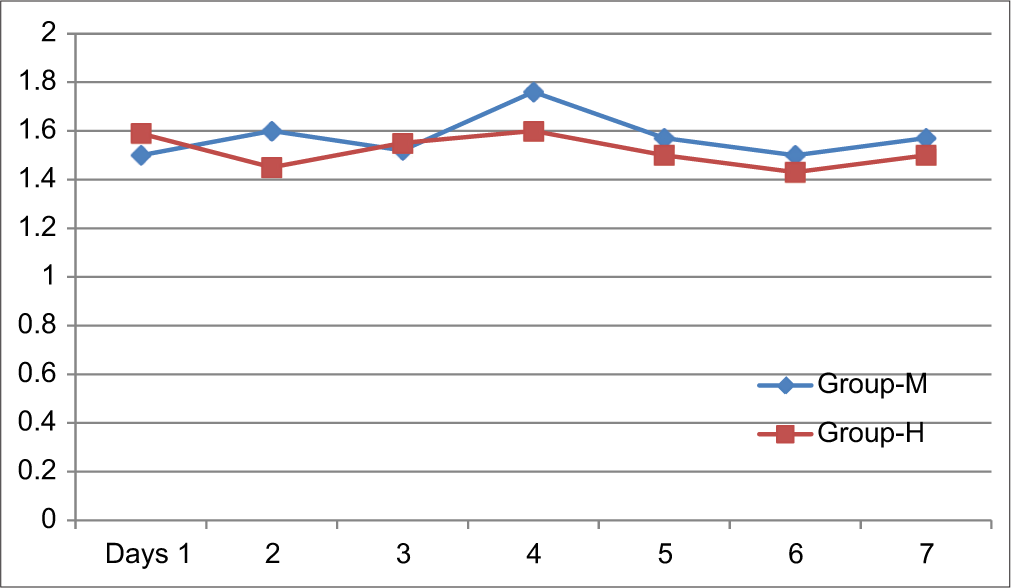
- Distribution of patients between different groups according to mean nausea score.
| Outcome parameter (Days) | Group-M | Group-H | P-value |
|---|---|---|---|
| 1 | 1.5±0.65 | 1.59±0.71 | 0.55 |
| 2 | 1.6±0.66 | 1.45±0.49 | 0.25 |
| 3 | 1.52±0.69 | 1.55±0.49 | 0.82 |
| 4 | 1.76±0.57 | 1.6±0.61 | 0.22 |
| 5 | 1.57±0.62 | 1.5±0.5 | 0.5 |
| 6 | 1.5±0.61 | 1.43±0.49 | 0.5 |
| 7 | 1.57±0.62 | 1.5±0.5 | 0.5 |
In Group M, the mean vomiting score was less compared to Group H. In Group M, the maximum score was 1.28 on day 5, while in Group H, the maximum score was 2 on day 5 [Figure 3]. The difference between the two groups was statistically significant [Table 4].
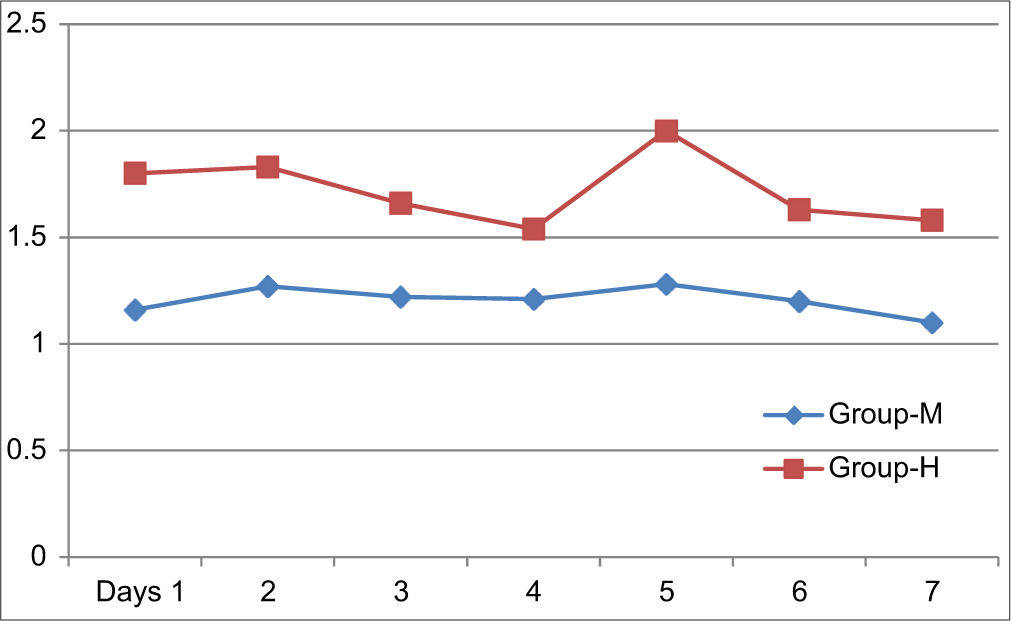
- Distribution of patients in different groups according to mean vomiting score.
| Outcome parameter (days) | Group-M | Group-H | P-value |
|---|---|---|---|
| 1 | 1.16±0.37 | 1.8±0.82 | P<0.0001 |
| 2 | 1.27±0.44 | 1.83±0.68 | P<0.0001 |
| 3 | 1.22±0.49 | 1.66±0.69 | 0.0015 |
| 4 | 1.21±0.48 | 1.54±0.78 | 0.025 |
| 5 | 1.28±0.45 | 2±0.89 | P<0.0001 |
| 6 | 1.2±0.4 | 1.63±0.77 | 0.0024 |
| 7 | 1.1±0.3 | 1.58±0.75 | 0.0003 |
The most common adverse events after the 7th day of medication in Group M were dizziness 5 (12.5%), headache 3 (7.5%), tiredness 2 (5%), drowsiness 1 (2.5%) and extrapyramidal symptoms 1 (2.5%). 28 (70%) patients in Group M had no adverse events following medication. The most common adverse events in Group H cases were dry mouth 5 (12.5%), palpitation 5 (12.5%), dizziness 2 (5%), drowsiness 2 (5%) and tremor 1 (2.5%). 25 (62.5%) patients in Group H were free from any adverse events.
DISCUSSION
Morphine is a cornerstone in the management of severe pain, especially in cancer patients. However, its use is frequently associated with nausea and vomiting, which can significantly impact patient quality of life and adherence to pain management regimen. To mitigate these adverse effects, antiemetic agents such as haloperidol and metoclopramide are often employed. This discussion examines the efficacy and safety of these two medications in preventing morphine-induced nausea and vomiting in cancer patients. Haloperidol is an antipsychotic of the butyrophenones class that acts primarily as a dopamine (D2) antagonist. It binds to the D2 receptors in the CTZ[12] and produces antiemetic effects. It has a long half-life (16 h) and can thus be given as a once- or twice-daily dose. Metoclopramide is a prokinetic agent which antagonises dopamine type 2 receptors in CTZ located in the area postrema of the brain. It is also a weak 5HT3 receptor antagonist and 5HT4 receptor agonist.[13] In the present study, we compared their efficacy in the prevention of nausea and vomiting caused by morphine in cancer patients.
In the present study, we compared the efficacy of oral metoclopramide and haloperidol for the prevention of nausea and vomiting caused by oral morphine in cancer patients. We found that both metoclopramide and haloperidol are equally efficacious in preventing nausea in cancer patients when used prophylactically with morphine administration. The difference was statistically insignificant. The average nausea score was between 1.5 and 1.75 in the metoclopramide group, while it was 1.43 to 1.6 in the haloperidol group. Both groups had mild nausea, according to the NCCN nausea scale, which was easily tolerated. No patient in either group developed severe nausea as assessed by the scale and had to stop taking morphine. Throughout the study period, the differences in nausea scores were statistically insignificant between the two groups.
In this study, the mean vomiting score was less in the metoclopramide group as compared to the haloperidol group, which was found to be significant statistically. Vomiting score remained constantly low throughout the study period in the metoclopramide group compared to the haloperidol group, although in a mild category. No patient in either group stopped treatment due to vomiting. The higher efficacy of metoclopramide over haloperidol to prevent morphine-induced vomiting might be explained by the fact that it acts on multiple receptors, including dopamine type 2 receptor, 5HT3 and 5HT4 receptors.
Hardy et al.[14] conducted a study to evaluate the efficacy of methotrimeprazine versus haloperidol in palliative cancer patients with cancer-related nausea. They found that response to treatment at 72 h was 75% in the haloperidol arm and 63% in the methotrimeprazine arm. The complete response rate was 56% in the haloperidol arm, while it was 51% in the methotrimeprazine arm.
Hardy et al.[15] conducted another study to assess the efficacy of haloperidol as an antiemetic in patients with cancer and nausea/vomiting not related to cancer treatment. They found that at day 2, 33 of 42 (79%) treated patients were assessed for response. Eight (24%) patients had complete control of nausea/vomiting, and 12 (36%) had partial control, giving an overall response rate of 61%. On day 5, 23 patients were assessed for response. The overall response rate was 17 of 23 (74%). If all patients were included in the response analysis, the overall response rates on days 2 and 5 were 47% and 40%, respectively. The low response rate by haloperidol in the above studies compared to the present study might be due to multiple aetiologies that contribute to being associated with nausea and vomiting in cancer patients. These include gastric stasis, drug-induced, brain metastasis, pain, radio/chemotherapy-induced, paraneoplastic syndrome, and metabolic causes like hypercalcaemia. Due to complex aetiologies, no single agent can be efficacious in the management of nausea and vomiting in cancer patients. While we used haloperidol to prevent morphine-induced nausea and vomiting only, we got a higher response rate by haloperidol.
Gralla et al.[16] compared the antiemetic efficacy of metoclopramide with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. They observed that metoclopramide was superior to placebo and prochlorperazine in reducing the volume of emesis and was more effective than placebo in shortening the duration of nausea and vomiting.
In the metoclopramide group, the most common adverse event noted was dizziness, headache and drowsiness. Only one patient developed extrapyramidal symptoms. Most of the patients were free from any adverse events. In the haloperidol group, the most common adverse event was dry mouth, palpitation and drowsiness. Most of the patients did not develop any adverse events.
Hardy et al.[17] also found that the incidence of adverse events was very low in the metoclopramide groups.
Limitation
The present study was a single-centre study with a small sample size; further, multi-centre studies should be conducted on large sample sizes. We did not include a placebo group to compare the incidence of nausea and vomiting when no drugs were prescribed prophylactically with morphine administration. Future clinical trials should be conducted to study and compare various other drugs to manage morphine-induced nausea and vomiting in cancer cases. In our study, we used fixed doses of drugs instead of comparing different dose ranges by titration. Thus, future studies should be conducted to assess the efficacy of drugs at various dose ranges.
CONCLUSION
Metoclopramide and haloperidol are equally efficacious in preventing nausea, but metoclopramide was found to be more effective with lesser side effects than haloperidol for morphine-induced vomiting in cancer patients when used prophylactically.
Ethical approval
The research/study was approved by the Institutional Review Board at Sawai Man Singh Medical College Jaipur, number 152MC/EC/2023/April 01, 2023, dated April 1, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Palliative Care and Cancer Pain In: Pain Care Essentials and Innovations. Netherlands: Elsevier; 2021. p. :91-111.
- [CrossRef] [Google Scholar]
- Strategies to Manage the Adverse Effects of Oral Morphine: An Evidence-based Report. J Clin Oncol. 2001;19:2542-54.
- [CrossRef] [Google Scholar]
- A Survey of Chronic Noncancer Pain Patients Prescribed Opioid Analgesics. Pain Med. 2003;4:340-51.
- [CrossRef] [Google Scholar]
- Management of Opioid Side Effects in Cancer-related and Chronic Noncancer Pain: A Systematic Review. J Pain. 2003;4:231-56.
- [CrossRef] [Google Scholar]
- What is Nausea? A Historical Analysis of Changing Views. Auton Neurosci. 2017;202:5-17.
- [CrossRef] [Google Scholar]
- Phase III Multicenter Randomized Trial of Oxaliplatin Added to Chronomodulated Fluorouracil-leucovorin as First-line Treatment of Metastatic Colorectal Cancer. J Clin Oncol. 2000;18:136-47.
- [CrossRef] [Google Scholar]
- Opioids and GI Motility-Friend or Foe? Curr Treat Options Gastroenterol. 2016;14:478-94.
- [CrossRef] [Google Scholar]
- Treating Nausea and Vomiting in Palliative Care: A Review. Clin Interv Aging. 2011;6:243-59.
- [CrossRef] [Google Scholar]
- Antipsychotic Drugs In: Advances in Neuropharmacology: Drugs and Therapeutics. USA: Apple Academic Press; 2020. p. :297-316.
- [CrossRef] [Google Scholar]
- Pharmacological Agents Affecting Emesis: A Review (Part I) Drugs. 1992;43:295-315.
- [CrossRef] [Google Scholar]
- Methotrimeprazine Versus Haloperidol in Palliative Care Patients with Cancer-related Nausea: A Randomised, Double-blind Controlled Trial. BMJ Open. 2019;9:e029942.
- [CrossRef] [Google Scholar]
- The Efficacy of Haloperidol in the Management of Nausea and Vomiting in Patients with Cancer. J Pain Symptom Manage. 2010;40:111-6.
- [CrossRef] [Google Scholar]
- Antiemetic Efficacy of High-dose Metoclopramide: Randomized Trials with Placebo and Prochlorperazine in Patients with Chemotherapy-induced Nausea and Vomiting. N Engl J Med. 1981;305:905-9.
- [CrossRef] [Google Scholar]
- A Double-blind, Randomised, Parallel Group, Multinational, Multicentre Study Comparing a Single Dose of Ondansetron 24 mg p.o. with Placebo and Metoclopramide 10 mg t.d.s. p.o. in the Treatment of Opioid-induced Nausea and Emesis in Cancer Patients. Support Care Cancer. 2002;10:231-6.
- [CrossRef] [Google Scholar]







