Translate this page into:
Yoga Intervention and Inflammatory Homoeostasis in Breast Cancer Patients
*Corresponding author: Fatima Dsilva, Nitte Usha Institute of Nursing Sciences, NITTE (Deemed to be University), Mangaluru, Karnataka, India. ftds_1970@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaje KC, Dsilva F, Shetty PK, Mohan R, Kumar S, Dsouza N, et al. Yoga Intervention and Inflammatory Homoeostasis in Breast Cancer Patients. Indian J Palliat Care. 2025;31:1-7. doi: 10.25259/IJPC_181_2024
Abstract
Objectives:
Yoga, renowned for its ability to maintain physical, mental and spiritual well-being, has recently gained prominence as a supportive therapy during conventional breast cancer (BC) treatment. This paradigm shift reflects a growing trend of people embracing yoga to enhance their overall health and aid in managing BC. The objective of this study was to determine the yoga intervention and inflammatory homoeostasis in newly diagnosed BC patients.
Materials and Method:
This study recruited 44 newly diagnosed BC patients at stages II, III and IV (without distant metastasis or other inflammatory diseases), all admitted for neoadjuvant chemotherapy followed by surgery. A prospective non-randomised control design was employed. Baseline assessments were conducted before the first chemotherapy cycle, with follow-ups before the 2nd and 3rd chemotherapy cycles, before surgery, and 2 months post-surgery. The outcome was compared with the control group.
Results:
The study showed significant within-subject effects in the yoga intervention group on serum tumour necrosis factor-alpha, interleukin (IL)-1-beta and IL-6 levels, while no significant changes were observed in the control group. Although between groups did not show statistically significant, the mean values indicated a consistent downregulation of proinflammatory markers over time in the yoga group.
Conclusion:
Incorporating yoga as a complementary therapy alongside conventional BC treatment significantly improved the health outcomes of BC patients by modulating proinflammatory markers.
Keywords
Breast cancer
Inflammatory markers
Yoga intervention
INTRODUCTION
Chronic inflammation is a significant factor in breast cancer (BC) development and prognosis.[1] BC is the most prevalent cancer among women, accounting for a quarter of all cancer cases and leading in mortality rates.[2-4] In the cancer microenvironment, up-regulation of proinflammatory cytokines has a significant role in the progression and angiogenesis of the tumour by stimulating the stromal cells and modifying the cell cytology, accelerating the tumour growth and metastasis, while anti-inflammatory cytokines inhibit cancer cell proliferation and impede inflammation.[5,6] Elevated proinflammatory markers were found in the inactive lifestyle[7] and are associated with increased and persistent fatigue in BC survivors.[8]
Yoga, an ancient therapy of Indians, is known for maintaining physical, mental and spiritual sanity.[9] In recent years, yoga has been embraced to promote their well-being and as a supportive therapy during the conventional treatment of an illness. Yoga encompasses a spectrum of asanas (poses) that range from simple to complex poses and are inherently reflective.[10] In BC, yoga as a supportive therapy was a practical intervention, alleviating physical symptoms and psychophysiological distress and enhancing the quality of life.[11,12]
Tumour necrosis factor-alpha (TNF-α), interleukins (ILs)-6, and IL-1-beta (IL-1β) are proinflammatory markers and are potential risk markers of BC.[13] TNF-α activates nuclear factor kappa-B (NF-κB), which, in turn, releases genes-related inflammation,[14] contributing to tumorigenesis and metastasis of BC by degradation of the cell matrix, angiogenesis and invasion.[15] In animal models, elevated TNF-α showed poor prognosis and shorter lifespan[16] and is correlated with oxidative stress causing inflammation in the brain.[17] TNF-α is significantly upregulated in BC and showed a positive correlation of their role as prognostic biomarkers.[18] Higher levels of TNF-α are linked to the advancement of BC.[19]
IL-1β is released by the activated macrophages; exposure to IL-1β causes regional inflammation reaction while high levels cause extensive inflammation, coupled with tissue injury and invasion of the tumour.[20] IL-1β and vascular endothelial factor (VEGF) are pivotal in the initiation and maintenance of angiogenesis; IL-1β led to the maturation of endothelial precursor cells, tumour cell migration and formation of metastatic deposits. IL-1β, on binding to its receptor IL-1 receptor type 1, initiates downstream signalling, activating genes dependent on NF-Kb promoting cancer growth.[21] Its aggressiveness in the breast tumour is linked as a potential marker in the prediction of bone metastasis in BC.[22]
IL-6, a pro-inflammatory cytokine released by the lung epithelial cells, is crucial for tumour growth; it influences the synthesis of oestrogen in BC, regulating through cell receptors and participating in tumour progression through angiogenesis.[9] IL-6 directly communicates with tumour cells, initiating crucial intracellular signal pathways that promote cell migration and decrease apoptosis.[23] In mice, lower levels of IL-6 showed a notable effect on VEGF, causing a reduction in intra-tumour angiogenesis.[24]
Few studies carried out on the effect of yoga on inflammatory markers were reported among BC survivors who completed BC treatment except endocrine therapy. Tumour necrosis factor receptor II (TNF-RII) was significant (P = 0.03) at 12 weeks of Iyengar yoga but was not significant for IL-6 (P = 0.87).[25] Post 3 months of Hatha yoga, TNF-α, IL-6 and IL-1β have significantly reduced (P < 0.05) in BC survivors.[26] A study on the effect of therapeutic yoga and meditation for 04 months among the cancer survivors where the majority (65%) of the study population were BC showed significant reduction in IL-1β (P < 0.05).[27] A significant reduction of TNF-α (P < 0.05) was reported 4 weeks post-surgery in the integrated yoga group among BC who had undergone surgery.[28]
The fact chronic inflammation is deeply connected to the development and prognosis of BC, with proinflammatory cytokines playing pivotal roles in tumour progression, angiogenesis and metastasis. Investigating this area could yield valuable insights into the mechanisms by which yoga exerts its effects. Understanding how yoga influences the cancer microenvironment and inflammatory pathways may lead to more effective, integrative treatment strategies that enhance patient’s clinical outcomes. Therefore, it is crucial to prioritise research exploring the intersection of yoga, chronic inflammation in BC. This study aims to elucidate the role of yoga intervention in modulating the proinflammatory markers in newly diagnosed BC patients undergoing neoadjuvant chemotherapy followed by surgery.
MATERIALS AND METHODS
Study design and population
A prospective non-randomised control trial design with a purposive sampling technique was employed for this study. Forty-four newly diagnosed BC with stage II, III and IV without distant metastasis and other associated inflammatory conditions, and those who did not practice yoga in the control group, aged between 30 and 70 years, with the treatment plan regiment of neo-adjuvant chemotherapy, followed by surgery were recruited from Justice KS Hegde Charitable Hospital. The exclusion criteria were those not willing to participate in the study, debilitated patients, inflammatory disease conditions, those not able to perform yoga, any prior treatment of cancer and day-care BC patients. The control participants were recruited prospectively between August 2020 and March 2021; only after completing the data collection of the control group the yoga intervention participants were recruited between April 2022 and July 2023 [Figure 1].
- CONSORT flow chart of prospective non-randomised control trial.
Data collection
An information sheet was handed to the participants, and written consent was obtained from each participant who agreed to participate in the study. The confidentiality of the study participants was maintained by securely storing all data, anonymising personal identifiers and ensuring no unauthorised personnel had access to the information collected. The first 22 BC patients meeting the study criteria were assigned to the control group and the following 22 to the yoga intervention group. Data were obtained at 5 time points: Before the first, second and third cycles of chemotherapy, the day before the surgery, and 2 months post-surgery. The data collection time for each patient varied between 20 weeks and 21 weeks. Demographic details were obtained from the case file and interview. Blood samples were obtained using strict aseptic techniques. The separated serum was stored at −80°C in K S Hegde Medical Academy, Central research laboratory – enzyme-Linked Immunosorbent Assay (ELISA) kit from Elabscience, (TNF-α – Catalogue No. E-EL-H0109; IL-1β – Catalogue No. E-EL-H0149; IL-6 – Catalogue No.: E-EL-H6156), ELISA microplate reader, (Tecan Spark) at 450 nm, was used to determine the level of inflammatory markers.
Yoga intervention
Yoga was administered by a yoga shikshaka (yoga teacher) for 40-minute duration. The intervention consists of pranayama (alternate nostril breathing), Bhramari pranayama (deep inhalation and exhaling slowly while humming), hatha yoga asanas (Pranam mudra, Tadaasana, Hasthautanasana, Padahasasana, Ashwa sanchalanasana, Parvatasana, Shashankasana, Astangasana, Bhujangasana, Virabadra asana, Ardhachakra asana and Trikona asana Virabradra asana) coordinating with the breath, (three sets) and Shavasana (corpse pose), which was administered for 3 consecutive days to ensure that the techniques were mastered properly before the administration of first cycle chemotherapy. There was a weekly telephonic follow-up, and a diary was handed over to maintain their daily practices to ensure compliance during the interval until the next admission to the hospital. Yoga intervention was reinforced under supervision at subsequent admission to the hospital. The supervised reinforced sessions of yoga were continued until the day of surgery and resumed on the 5th and 6th day of post-surgery. Patients performed yoga in the morning between 7 am and 8 am or between 5 pm and 6 pm. The study participants were encouraged to continue performing yoga at home 6 days a week after hospital discharge.
Data analysis
Data were analysed using the Statistical Package for the Social Sciences 20. Fisher’s exact test and Chi-square test were employed for homogeneity between groups; Frequency and percentage described the demographic variables. A general linear model repeated measures analysis of variance (ANOVA) assessed the outcomes within the group. Independent t-test compared the outcome between the yoga intervention and the control group.
RESULTS
The mean ages of the study subjects were 51.36 ± 8.61 and 50.64 ± 9.21 years in the yoga intervention and control groups, respectively. The average monthly family income was Rs 19,000 ± 6761.23 in the yoga group and Rs 18,636.36 ± 7877.31 in the control group. Homemakers comprise 27.27% in the yoga group and 36.36% in the control group, with the least 9.09% employed in the yoga group and 4.54% in the control group. The majority were married, 38.63% in the yoga group and 36.36% in the control group. In both the groups, 09.09% each had completed higher secondary. The rest had completed their primary and secondary education. Distribution of the stages of BC is as follows: stage II (22.72% in each group), stage III (18.18% in the yoga group and 15.90% in the control group) and stage IV (9.09% in the yoga group and 11.36% in the control group) [Table 1]. Fisher’s exact test and Chi-square test confirmed homogeneity between groups (P > 0.05).
| Demographic variables | Yoga (n=22) | Control (n=22) | P | ||
|---|---|---|---|---|---|
| f | % | f | % | ||
| Age in years | |||||
| 31–40 | 4 | 9.09 | 4 | 9.09 | 0.921a |
| 41–50 | 5 | 11.36 | 8 | 18.18 | |
| 51–60 | 10 | 22.72 | 8 | 18.18 | |
| Above 61 | 3 | 6.81 | 2 | 4.54 | |
| Family income/month | |||||
| <10000 | 5 | 11.36 | 6 | 13.63 | 0.991b |
| 10001–20000 | 8 | 18.18 | 8 | 18.18 | |
| >20000 | 9 | 20.45 | 8 | 18.18 | |
| Occupation | |||||
| Homemaker | 11 | 27.27 | 16 | 36.36 | 0.374a |
| Agriculturist | 7 | 15.9 | 4 | 9.09 | |
| Employed | 4 | 9.09 | 2 | 4.54 | |
| Marital status | |||||
| Married | 17 | 38.63 | 16 | 36.36 | 1.000b |
| Widow | 5 | 11.36 | 6 | 13.63 | |
| Education | |||||
| Primary | 9 | 20.45 | 10 | 22.72 | 0.921a |
| Secondary | 9 | 20.45 | 8 | 18.18 | |
| Higher Secondary | 4 | 9.09 | 4 | 9.09 | |
| Stage of BC | |||||
| II | 10 | 22.72 | 10 | 22.72 | 1.000a |
| II | 8 | 18.18 | 7 | 15.9 | |
| IV | 4 | 9.09 | 5 | 11.36 | |
aFisher’s exact test, bChi-square, BC: Breast cancer
Inflammatory markers
In an ANOVA with Greenhouse-Geisser correction, sphericity not assumed, showed statistically significant within-subjects of the yoga intervention group, while no significance was found in the control group. The result depicted were as follows: Serum TNF-α in yoga intervention group (F [4] = 7.11, P = 0.007), control group (F [4] = 0.36, P = 0.674), serum IL-1β, yoga intervention (F [2] = 5.421, P = 0.013), control group (F [2] =1.814, P = 0.177) and serum IL-6 yoga intervention group (F [2] = 5.581, P = 0.004), control group, F (3) =6.13, P = 0.595 [Table 2]. However, the independent t-test comparing the effect of yoga intervention with the control group showed no statistically significant difference (P > 0.05). The mean values showed a consistent downregulation of proinflammatory markers over time in the yoga intervention group. TNF-α at 2 months post-surgery showed that the mean value was 93.21 ± 43.31 pg/mL, which is lower than all previous assessments, whereas, in control, it was upregulated to 99.10 ± 38.81 pg/mL, which is higher than before the 1st chemotherapy 98.04 ± 37.56 pg/mL. A reduction in serum IL-1β and IL-6 was observed in both groups; however, a significant attenuation at 2 months post-surgery in the yoga intervention group is noted compared to the control group. This highlights the beneficial impact of yoga intervention on clinical outcomes by modulating proinflammatory markers in the cancer microenvironment of BC patients. The comparison of the inflammatory marker’s attenuation between groups is projected in Figure 2.
| Proinflammatory markers assessments | Yoga intervention | Control group | ||||
|---|---|---|---|---|---|---|
| Mean±SD | F | P | Mean±SD | F | P | |
| TNF-α | ||||||
| Before 1st CT | 97.00±9.79 | 7.11 | 0.007* | 98.04±37.55 | 0.36 | 0.674 |
| Before 2nd CT | 97.59±0.62 | 99.17±38.70 | ||||
| Before 3rd CT | 98.51±0.67 | 99.35±38.67 | ||||
| Before Surgery | 98.40±1.01 | 99.18±39.26 | ||||
| 2 months post-surgery | 93.21±4.93 | 99.10±38.80 | ||||
| IL-1β | ||||||
| Before 1st CT | 98.67±2.22 | 5.42 | 0.013* | 98.61±43.58 | 1.81 | 0.177 |
| Before 2nd CT | 96.82±3.99 | 98.14±42.24 | ||||
| Before 3rd CT | 95.85±5.56 | 96.20±41.86 | ||||
| Before Surgery | 94.86±5.13 | 96.47±41.87 | ||||
| 2 months post-surgery | 92.97±5.10 | 95.73±42.74 | ||||
| IL-6 | ||||||
| Before 1st CT | 118.06±1.75 | 5.58 | 0.004* | 117.84±23.10 | 6.13 | 0.595 |
| Before 2nd CT | 116.14±2.16 | 117.22±24.82 | ||||
| Before 3rd CT | 116.94±2.22 | 117.35±25.53 | ||||
| Before Surgery | 115.61±1.74 | 116.73±23.87 | ||||
| 2 months post-surgery | 110.48±0.69 | 114.94±24.52 | ||||
CT: Chemotherapy; TNF-α: Tumour necrosis factor-alpha, IL-1β: Interleukin 1-beta, IL-6: Interleukin-6, SD: Standard deviation; “*”: Significant
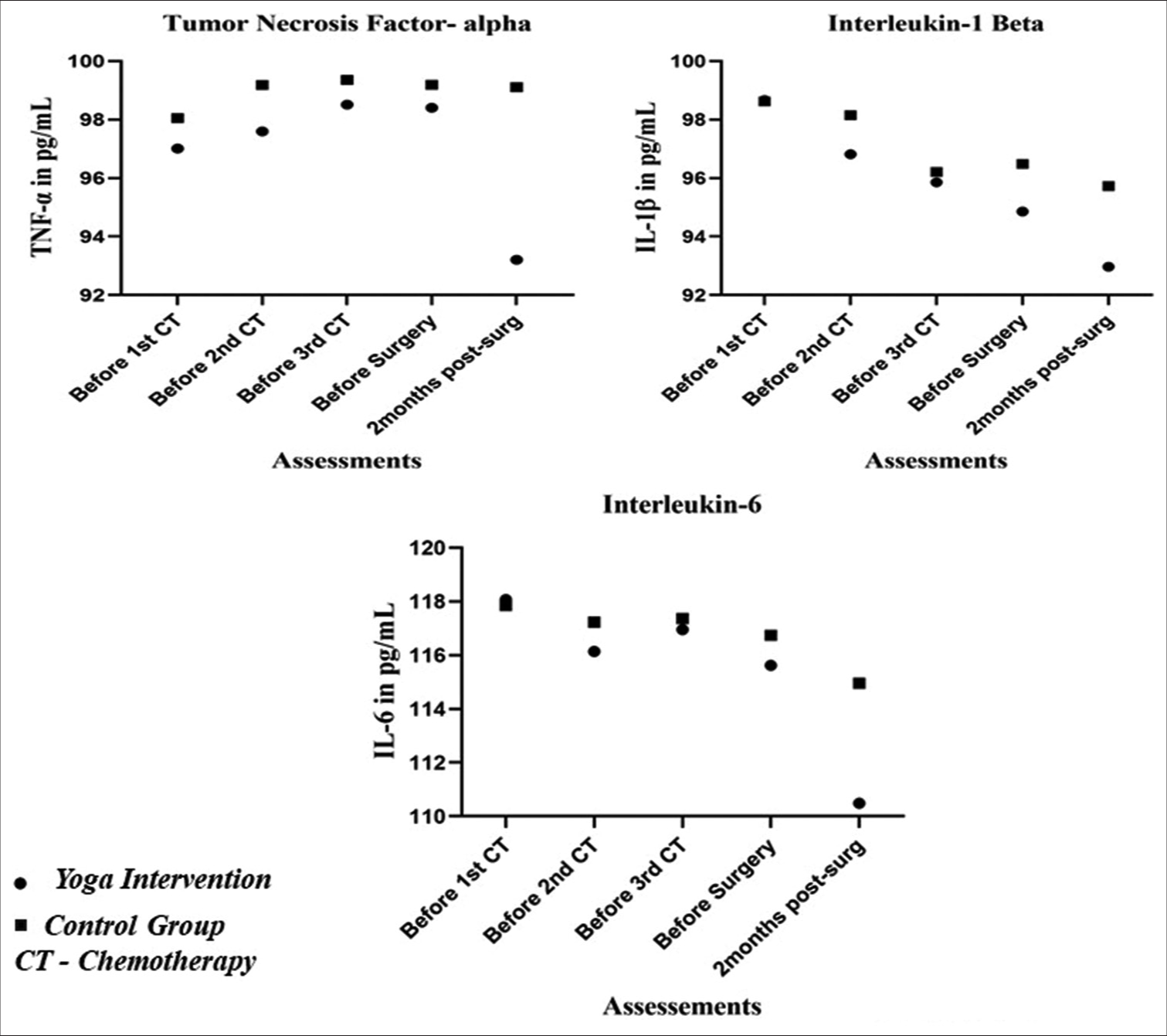
- Graph showing the attenuation of serum tumour necrosis factor-alpha, interleukin (IL)-1-beta and IL-6 at various time points between the groups. TNF: Tumour necrosis factor, CT: Chemotherapy.
DISCUSSION
While the evidence on yoga intervention in BC survivors is encouraging, there is limited information on yoga intervention’s effects on inflammatory homoeostasis in patients undergoing BC treatment. In the present study, though no statistical significance between the yoga intervention and the control groups (P > 0.05) was found, a significant reduction of the inflammatory markers within subjects of the yoga intervention was observed (P < 0.05), and the mean values across various time points suggested that yoga intervention downregulated proinflammatory markers (TNF-α, IL-1β and IL-6) in BC patients undergoing conventional treatment of BC. Kaje et al. and Estevoa, in their systematic review, reported a favourable effect of yoga on markers of inflammation in BC survivors.[9,29]
A randomised controlled trial by Kiecolt-Glaser et al. reported significant decreases in TNF-α, immediately to post-12 weeks of hatha yoga intervention and at 3 months follow-up (P < 0.05) in BC survivors,[26] Long Parma et al., on the effect of 6 months of yoga-based exercise in BC survivors, showed a decreased level of TNF-α when compared to comprehensive exercise and exercise of choice, the mean post-score minus pre-score and standard deviation were 184.61 (750.28), 860.74 (1620.38), −212.13 (756.10), respectively.[30] Bower et al. reported that Iyengar yoga in BC survivors was found to maintain the stability of soluble TNF-RII when compared to the health education group.[25] A study by Rao et al. on the effects of yoga on post-operative outcomes and wound healing in BC patients found that serum TNF-α was downregulated in the integrated yoga group, whereas in the control group, it was upregulated, though statistically, it was not significant.[31] These study results corroborated our research finding where TNF-α was attenuated in the yoga intervention group, and in the control group, it was up-regulated. These findings provided evidence of the impact of yoga on modulating the TNF-α and improving the clinical outcomes of BC patients. Consistent with the findings of our study result where IL-1β within the yoga intervention group was significantly downregulated (P = 0.013), Patel et al., in their pre-experimental study, found a significant decrease of plasma IL-1β (P = 0.003) post-therapeutic yoga intervention among cancer survivors where BC constitute 65%.[27] In our study between the yoga intervention and the control groups, it was not significant; however, Kiecolt-Glaser et al. reported a significant difference between the hath yoga and waitlist in BC survivors in IL-1β immediately to intervention (P = 0.037) and during follow-up at 3 months (P = 0.01).[26] The contrasting findings between the groups warrant further studies to be explored for the therapeutic effect of yoga in reducing IL-1β levels in BC patients, despite the significant differences within the yoga group in our study.
A randomised controlled trial by Kiecolt-Glaser et al. reported significant decreases in IL-6 immediately and at 3 months follow-ups (P = 0.05) of Hatha yoga among BC survivors,[26] However, Parma et al. in their three-arm randomised control yoga exercise, comprehensive exercise and exercise of choice found no significant difference between the groups (P > 0.05).[30] In our current study, a significant decrease in IL-6 was observed within subjects of yoga intervention but no statistical difference between the groups was noted. Further, investigation with larger samples on the yoga intervention effect in modulating the IL-6 in BC patients may shed more light.
Limitation and strength
This study faced several limitations. It was not a randomised controlled trial and was conducted at a single institution, employing a prospective non-randomised control trial. The counselling and the regular visits by social workers during the hospital stay and the social support received by the patients in the control group could have a placebo effect, which was not under the control of the researcher. In addition, the study participants were not blinded. Despite these limitations, the study has a striking strength. Notably, there were no dropouts among the participants, and the sample population was homogeneous, all receiving the same conventional BC treatment regimen. To prevent sample contamination, the control group was recruited first, followed by the yoga intervention group. To the best of our knowledge, this study is pioneering in its integration of conventional BC treatment with yoga intervention from the very beginning of the cancer treatment process.
CONCLUSION
Yoga helped downregulate proinflammatory markers (TNF-α, IL-1β and IL-6) in BC patients undergoing conventional treatment; this indicates incorporation of yoga as a complementary therapy alongside conventional BC treatment has the potential to modulate inflammation and improve clinical outcomes of BC patients.
Acknowledgment
The authors are grateful to the research directorate of Nitte (Deemed to be University) for the extended support and guidance throughout the study.
Ethical approval
The research/study was approved by the Institutional Review Board at the Central Ethics Committee, Nitte (Deemed to be University), number Ref. NU/CEC/2020/0288, dated 16th March 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers (Basel). 2021;13:3918.
- [CrossRef] [Google Scholar]
- Breast Cancer Epidemiology and Risk Factors. Clin Obstet Gynecol. 2016;59:651-72.
- [CrossRef] [Google Scholar]
- Breast Cancer. Available from: https://www.iarc.who.int/cancer-type/breast-cancer [Last accessed on 2024 Jun 23]
- [Google Scholar]
- Barriers for Early Detection of Breast Cancer among South Indian Women. Indian J Community Med. 2021;46:706-9.
- [CrossRef] [Google Scholar]
- Chronic Inflammation and Breast Cancer Recurrence. J Clin Oncol. 2009;27:3418-9.
- [CrossRef] [Google Scholar]
- The Role of Cytokines in Breast Cancer Development and Progression. J Interf Cytokine Res. 2015;35:1-16.
- [CrossRef] [Google Scholar]
- Epidemiological and Molecular Mechanisms Aspects Linking Obesity and Cancer. Arq Bras Endocrinol Metabol. 2009;53:213-26.
- [CrossRef] [Google Scholar]
- The Role of IL-1B in Breast Cancer Bone Metastasis. Endocr Relat Cancer. 2018;25:R421-34.
- [CrossRef] [Google Scholar]
- Effect of Yoga Intervention on Inflammatory Biomarkers among Women with Breast Cancer-A Systematic Review. Indian J Palliat Care. 2023;29:223-33.
- [CrossRef] [Google Scholar]
- Yoga: What You Need to Know. Available from: https://www.nccih.nih.gov/health/yoga-what-you-need-to-know [Last accessed on 2024 Jun 23]
- [Google Scholar]
- Physical and Psychosocial Benefits of Yoga in Cancer Patients and Survivors, a Systematic Review and Meta-analysis of Randomized Controlled Trials. BMC Cancer. 2012;12:559.
- [CrossRef] [Google Scholar]
- Yoga Breathing for Cancer Chemotherapy-associated Symptoms and Quality of Life: Results of a Pilot Randomized Controlled Trial. J Altern Complement Med. 2012;18:473-9.
- [CrossRef] [Google Scholar]
- Low-Grade Inflammation, Oxidative Stress and Risk of Invasive Post-menopausal Breast Cancer-A Nested Case-control Study from the Malmö Diet and Cancer Cohort. PLoS One. 2016;11:e0158959.
- [CrossRef] [Google Scholar]
- Role of Obesity-associated Dysfunctional Adipose Tissue in Cancer: A Molecular Nutrition Approach. Biochim Biophys Acta. 2011;1807:664-78.
- [CrossRef] [Google Scholar]
- The Dual Role of Tumor Necrosis Factor-alpha (TNF-α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell Onco. 2020;43:1-18.
- [CrossRef] [Google Scholar]
- Proinflammatory and Anti-Inflammatory Cytokines Mediated by NF-κB Factor as Prognostic Markers in Mammary Tumors. Mediators Inflamm. 2016;2016:9512743.
- [CrossRef] [Google Scholar]
- Breast Cancer Survivors, Common Markers of Inflammation, and Exercise: A Narrative Review. Breast Cancer. 2017;11:1178223417743976.
- [CrossRef] [Google Scholar]
- Transcriptional Elucidation of Tumor Necrosis Factor-α-mediated Nuclear Factor-?B1 Activation in Breast Cancer Cohort of Pakistan. J Cancer Res Ther. 2020;16:1443-8.
- [CrossRef] [Google Scholar]
- Association between Serum TNF-α Level with the Incidence of Metastases in Women with Breast Cancer in Dr. Soetomo General Hospital, Indonesia. Bali Med J. 2022;11:1548-52.
- [CrossRef] [Google Scholar]
- Interleukin-1 (IL-1alpha and IL-1beta) and Its Receptors (IL-1RI, IL-1RII, and IL-1Ra) in Prostate Carcinoma. Cancer. 2004;100:1388-96.
- [CrossRef] [Google Scholar]
- Exploring Immune Interactions in Triple Negative Breast Cancer: IL-1 β Inhibition and Its Therapeutic Potential. Front Genet. 2023;14:1086163.
- [CrossRef] [Google Scholar]
- Expression of Interleukin-1beta in Human Breast Carcinoma. Cancer. 1997;80:421-34.
- [CrossRef] [Google Scholar]
- Host Genetic Variants in the Interleukin-6 Promoter Predict Poor Outcome in Patients with Estrogen Receptor-Positive, Node-Positive Breast Cancer. Cancer Res. 2009;69:4184-91.
- [CrossRef] [Google Scholar]
- The Effect of Exercise Training on the Level of Tissue IL-6 and Vascular Endothelial Growth Factor in Breast Cancer Bearing Mice. Iran J Basic Med Sci. 2014;17:231-58.
- [Google Scholar]
- Yoga Reduces Inflammatory Signaling in Fatigued Breast Cancer Survivors: A Randomized Controlled Trial. Psychoneuroendocrinology. 2014;43:20-9.
- [CrossRef] [Google Scholar]
- Yoga's Impact on Inflammation, Mood, and Fatigue in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol. 2014;32:1040-9.
- [CrossRef] [Google Scholar]
- Therapeutic Yoga Reduces Pro-tumorigenic Cytokines in Cancer Survivors. Support Care Cancer. 2022;31:33.
- [CrossRef] [Google Scholar]
- Influence of Yoga on Postoperative Outcomes and Wound Healing in Early Operable Breast Cancer Patients Undergoing Surgery. Int J Yoga. 2008;1:33-41.
- [CrossRef] [Google Scholar]
- The Role of Yoga in Inflammatory Markers. Brain Behav Immun Health. 2022;20:100421.
- [CrossRef] [Google Scholar]
- Effects of Six Months of Yoga on Inflammatory Serum Markers Prognostic of Recurrence Risk in Breast Cancer Survivors. Springerplus. 2015;4:143.
- [CrossRef] [Google Scholar]
- Influence of Yoga on Postoperative Outcomes and Wound Healing in Early Operable Breast Cancer Patients Undergoing Surgery Influence of Yoga on Postoperative Outcomes and Wound Healing in Early Operable Breast Cancer Patients Undergoing. Int J Yoga. 2008;1:33-41.
- [CrossRef] [Google Scholar]






