Translate this page into:
A Multicentric Field Test to Study the Validity and Feasibility of the SHS-tool to Screen for Serious Health-related Suffering in Adult Patients with Cancer
*Corresponding author: Nandini Vallath, Department of Pain and Palliative Medicine, St Johns Medical College Hospital, Bengaluru, Karnataka, India. aanandini@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vallath N, Paul A, Ghoshal A, Sundararaj JJ, Balakrishnan K, SHS Field Test Working Group of the National Cancer Grid – India. A Multicentric Field Test to Study the Validity and Feasibility of the SHS tool to Screen for Serious Health-related Suffering in Adult Patients with Cancer. Indian J Palliat Care 2024;30:239-51. doi: 10.25259/IJPC_13_2024
Abstract
Objectives:
The 2017 Lancet Commission reports ‘Serious Health-related Suffering’ (SHS) as an abyss in healthcare services. It lists 20 common health conditions and 15 symptoms as commonly associated with SHS. In 2015, 80% of SHS prevalence, an estimated 61 million, was noted as from low-middle-income countries. Acknowledging the high prevalence of SHS in cancer patients and aligning with global efforts to address and alleviate the suffering, the National Cancer Grid of India developed and evaluated the SHS screening tool (SHS-tool). The SHS tool was developed during phase 1 of the study through a systematic consensus-building methodology. During phase 2, the validity and feasibility study of the SHS tool was completed through a multicentric field test, which is described here.
Materials and Methods:
The SHS tool developed during phase 1 was field-tested across nine tertiary cancer care centres (TCC sites) selected from different healthcare sectors and regions of India. The study utilised a purposive sample of 254 cancer patients to evaluate the validity of the SHS screening tool at selected sites and additionally recorded the feasibility, relevance, acceptability and feedback comments from patients (n = 121), research associates (n = 11) and principal investigators (PIs) (n = 9). A documented interview of the patient within the same timeframe by experienced personnel selected by the PI served as the standard.
Results:
The field-test TCC-sites represented government academic institutions, non-government and private sectors. The sites used patient waiting areas and inpatient/daycare wards for conducting field tests. The Cronbach’s alpha of the SHS-tool questionnaire showed an internal consistency of 0.728. The tool detected SHS in 137/254 patients, compared to 116/254 through the interview method. The outcomes concurred with that of the interview in 64.17% of instances. The tool exhibited a sensitivity of 70% and specificity of 59%. 66.67% of patients might not have reached the interviewers if not for the field test processes. The feasibility questionnaire responses from patients (n = 121) indicated ease of understanding (91.74%), ease of use (92.56%) and relevance (89.26%). The selected settings were found suitable by 96.69%. Feedback responses from research associates indicated ease of administration (10/11) and relevance (8/11) and found no reasons preventing its use (8/11). The feedback comments from the stakeholders were thematically grouped for insights.
Conclusion:
The SHS tool is validated for screening SHS where none exists. It has been found to be a feasible, relevant and acceptable tool for use in adult cancer patients attending TCCs across India. Insights from analysing the feedback comments from the stakeholders have been integrated as ‘instruction for use’ for refined implementation of the SHS tool. The SHS tool may be utilised to recognise and trigger an in-depth evaluation and expedited access to essential palliative care packages towards alleviating it, as recommended by the Lancet Commission. Future studies using the SHS tool in other disease conditions with a high burden of SHS can assess its wider applicability.
Keywords
Serious health-related suffering
Screening
Essential palliative care package
Tool validation
INTRODUCTION
The relief of suffering and access to palliative care are neglected dimensions of global healthcare. The report of the Lancet Commission on Global Access to Palliative Care and Pain Relief (GAPCPR) -2017[1] (report) highlights the concept of ‘Serious Health-related Suffering’ (SHS) at physical, psychological, social and spiritual domains notable at end-of-life; and during acute or chronic life-limiting, life-threatening illnesses, or injury. It states the suffering to be ‘health-related’ when it is associated with illness or injury of any kind and as ‘serious’ when it cannot be relieved without professional intervention and when it compromises the functioning of the individual. The report lists fifteen symptoms from twenty life-limiting and life-threatening conditions, as most associated with SHS.[1] It estimates that 61 million persons experienced SHS in 2015 alone, of whom 80% resided in low- and middle-income countries (LMICs).[2] The burden of SHS is expected to double by 2060, with the fastest increases occurring in low-income countries.[3] Around 7.2 million Indians were noted to have SHS.[3]
The Lancet Report recommends an ‘essential palliative care package’ to alleviate SHS. The consensus-based definition of palliative care by the International Association for Hospice and Palliative Care integrates and emphasises the critical need for assessment and management of SHS.[4]
To alleviate suffering, it is essential first to identify it. The framework developed by the GAPCPR attempts to measure the SHS burden from a population perspective.[2] However, perceptions of suffering, particularly when health-related, are subjective. Identifying and responding to the suffering endured by individuals requires a suitable screening tool. An appraisal of tools measuring suffering in health conditions yielded seven instruments. They measure certain domains that contribute to suffering but not those emphasised in the Lancet Report [Supplementary Table 1].
Cancer has emerged as a significant contributor to SHS, with an estimated 19.3 million new cases worldwide in 2020.[5] National Cancer Grid (NCG) of India is a robust network of over three hundred cancer-care institutions across India, which supports the establishment of uniform standards and best practices in cancer care.[6] NCG covers over 90% of cancer care in India. The NCG envisaged developing a tool to facilitate early recognition and address of SHS amongst cancer patients in busy cancer-care settings in India. For satisfactory screening, the SHS screening tool, hereafter referred to as ‘the tool’ or ‘SHS tool,’ is required to be sensitive, easy and quick to administer to identify those with possible SHS who would then need in-depth evaluation and suitable care.
The tool was developed and evaluated in two phases. Phase 1 of developing the SHS tool describes the concept clarification and the consensus-building processes (Delphi, Nominal Group Technique, Transparent Expert Consultation and online poll).[7] The questionnaire framework of the tool that emerged during Phase 1 has two sections. Section 1 screens for the presence of ‘health-related suffering’ using questions framed along five domains of suffering: Physical, psychological, social and spiritual domains, as articulated in the Lancet Report, along with a fifth domain of financial suffering. This additional domain emerged during the consensus-building process of phase 1 as quintessential for the population served in LMICs like India, where the cost of healthcare is out-of-pocket.[7] Section 2 of the SHS tool screens for the ‘seriousness’ of the health-related suffering in that patient through (i) its impact on daily functions and (ii) the expressed need for professional help to address the suffering [Appendix 1]. The Phase-2 study describes the multicentric field test of the SHS tool that evaluated its validity, reliability, feasibility, relevance and acceptability. Figure 1 provides an overview of the flow of events during Phase 1 and Phase 2 of the study on the SHS screening tool.
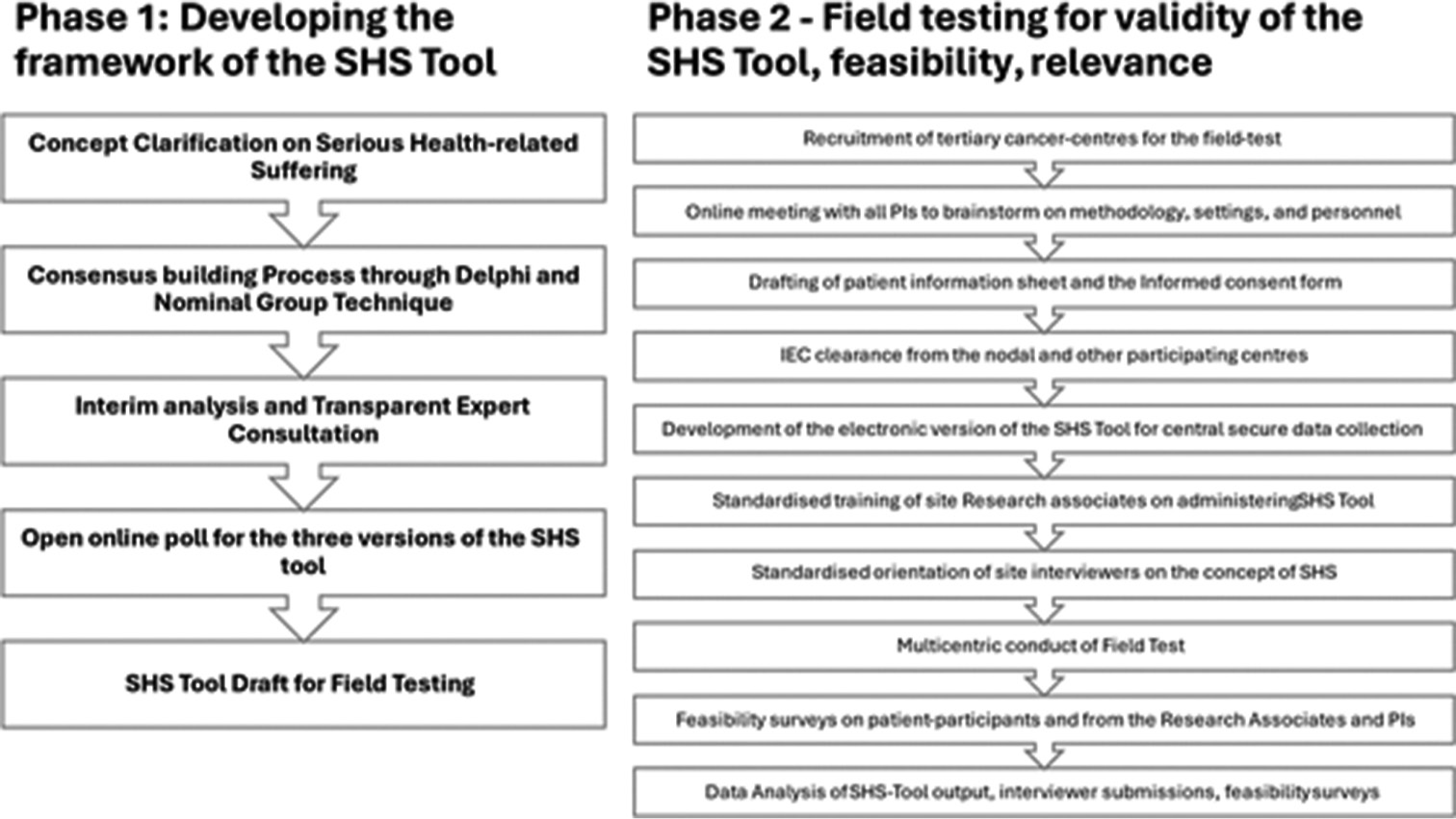
- The phase 1 and phase 2 processes of development and validation of the serious health-related suffering (SHS)-screening tool.
MATERIALS AND METHODS
The SHS screening tool developed through phase 1 [Appendix 1] was field-tested for validity and feasibility across nine tertiary cancer-care centres (TCC sites) selected from different healthcare sectors and regions of India [Figure 2]. The study utilised a purposive sample of 254 cancer patients attending the TCC sites. Selecting the prevalence of suffering as 50%[8,9] and a tool sensitivity of 70% (10% precision), this sample size returned power of 95% on power analysis.
- Names and locations of participating tertiary cancer centre sites.
The field test process began by identifying the nine TCC sites and principal investigators (PIs) from across the country to participate. Online interactions helped introduce the SHS tool to the PIs, finalise the standard procedure for field testing and support the submission of study proposals to respective institutional ethics committees (IECs). Personnel at each site included the PI, co-investigators and a research associate (RA) nominated by the PI, who administered the electronic version of the SHS tool using smartphone technology. An interview of the same patient to elicit SHS within the same period served as the standard to validate the tool output, as eliciting SHS was a de novo activity without established practices to compare within the settings. In addition, the study used survey questionnaires to ascertain the feasibility, relevance and acceptability of patient respondents (n = 121) and all RAs (n = 11). Feedback comments from PIs were recorded at the end of the field test study.
PIs selected the personnel to engage with the study (the RA and the professional to interview the patient) and setting(s) suitable for conducting the field test: patient waiting area and in-patient/daycare ward. All RAs received standardised training and the interviewers received an orientation on the concept of SHS. To reduce bias, interviewers were kept blinded to the design and contents of the SHS tool.
RAs at the selected setting identified the potential participants who fulfilled the inclusion criteria: (1) adult patient (age more than 18 years); (2) with a cancer diagnosis; (3) on follow-up/review visit; and (4) able to comprehend English. After eliciting their inclination with a brief overview of the study, the RA, under the supervision of PIs, shared the Participant Information Sheet, clarified their questions and obtained written consent. They then administered the electronic version of the SHS tool through a secure link. (https://efficiency365.com/shs). The interviewer interacted with the patient-respondent, built rapport and elicited their concerns related to their health condition without time delay to avoid variance in the SHS status due to fresh interventions. Interviewers assessed for the presence of health-related-suffering using conversation/interview/observation methods. Notes of the interviewer, with their impression on the presence or absence of SHS and their responses to additional questions, were uploaded using the Google form designed for this purpose. The question ‘Did you get the impression that this patient currently has SHS?’ directed the interviewer to focus on the ongoing status of suffering. They also responded, ‘Would the patient have reached a professional like you for evaluation as per the usual processes in your setting, outside of this study?’ When interviews could not be initiated or completed due to lapse in time, withdrawal or absenteeism, the corresponding tool-related data was discarded.
Google-based survey questionnaires on the feasibility, acceptability, relevance and ease of using/administering the SHS tool were used to elicit perceptions of patients and RAs. The RAs administered a feasibility questionnaire to a convenience sample of patient respondents (n = 121/254), and PIs conducted the survey for all RAs (n = 11/11). Patients and RAs who recorded <7/10 on the feasibility survey (0 indicating extreme dissatisfaction and 10 extreme satisfaction) were asked to post their comments and suggestions. In addition, the PIs of each site shared their feedback comments at the end of their field test. Data output of the SHS-tool, notes uploaded by the interviewers, as well as feasibility survey outcomes were captured centrally in real-time by the research team. Data collection across nine TCCs-sites took place between September 2021 and December 2022, with successive waves of COVID-19 contributing to significant delays. Analyses were done using Microsoft Excel. The sites followed their own institutional policy to care for those patients screened as having SHS by either the SHS tool or the interview.
This study was approved by the IEC of NCG-India and by the IECs of all sites. The registration number with the Clinical Trials Registry-India (CTRI) is CTRI/2021/09/036681. There were no financial incentives involved. PIs ensured that care-related processes of patients, for which they visited the hospital, were left undisturbed. The de-identified data was stored on password protected devices accessible only to the research team, to protect confidentiality.
RESULTS
The field test was conducted across nine sites in India, situated in the south, east and west zones [Figure 2 and Table 1] and yielded complete data from 254 respondents for analysis using descriptive statistics. Of the field-test TCC sites, three were stand-alone government-authorised TCC sites, three were academic institutions, two belonged to the non-government sector, and one was privately funded. Three sites used a patient waiting area, one used an in-patient/daycare ward and five sites used both settings for conducting the field test. The profile of interviewers and RAs is listed in Supplementary Tables 2 and 3.
| Patients (n=254) | Contribution in % | |
|---|---|---|
| Demographics of TCC-sites | ||
| The federal government authorised Standalone TCC (n=2) | 43 | 16.93 |
| State govt authorised Standalone TCC (n=1) | 28 | 11.02 |
| TCC part of a Medical College Hospital (n=3) | 91 | 35.83 |
| Non-govt authorised Standalone TCC (n=2) | 57 | 22.44 |
| Privately authorised Stand-alone TCC (n=1) | 35 | 13.78 |
| Demographics of Patient-respondents | ||
| Gender | ||
| Female | 119 | 46.85 |
| Male | 135 | 53.15 |
| Age | ||
| 18–30 | 50 | 20.87 |
| 31–50 | 81 | 31.89 |
| 51–70 | 96 | 37.80 |
| 71–90 | 24 | 9.44 |
| Education | ||
| Graduate/PG | 194 | 76.38 |
| High-School | 54 | 21.26 |
| Literate | 6 | 2.36 |
| Employment | ||
| Business/Professional | 69 | 27.17 |
| Daily wages | 8 | 3.15 |
| Government job/service | 61 | 24.01 |
| Nil/retired | 116 | 45.67 |
| Primary treating team of the patient-participants | ||
| Medical Oncology | 164 | 64.57 |
| Radiation Oncology | 39 | 15.35 |
| Surgical Oncology | 51 | 20.08 |
| Settings | ||
| In-patient/Daycare Ward | 128 | 50.39 |
| Outpatient waiting area | 126 | 49.61 |
TCC: Tertiary cancer centres, SHS: Serious health-related suffering, PG: Postgraduate
Table 1 describes the demographics of patient-respondents; with 53.15% male and 69.69% in the age-group of 31–70 years. Furthermore, 76.38% were graduates or postgraduates, 54.33% were employed and 64.57% were registered under Medical Oncology. Three TCC-sites selected patient waiting area, one selected in-patient/daycare ward and five sites tested the tool in both settings.
Applying Cronbach’s alpha, the internal consistency of the section 1 questionnaire of the SHS tool was 0.728. The tool detected SHS in 137 instances, while the interviewer recorded SHS in 116 instances. The SHS-tool outcomes concurred with the interview outcomes in 64.17% (163/254) instances. Of the 137 patients screened as having serious suffering by the tool, 51 (37.23%) were based on the impact on functions, 37 (27 %) on seeking professional help, and 49 (35.77%) responded with ‘yes’ to both. The tool exhibited a sensitivity of 70%, specificity of 59%, positive predictive value of 59% and negative predictive value of 70%, all at 95% confidence intervals [Table 2].
| SHS-tool output | Output from direct interview with the patient | Concurrence in 163/254 | ||
|---|---|---|---|---|
| SHS present | SHS Not present | Total | ||
| SHS Present | 81 | 56 | 137 | |
| SHS Not present | 35 | 82 | 117 | |
| Total | 116 | 138 | 254 | |
| Measure | Estimate (95% CI) | |||
| Concurrence | 64.17 (58, 70) | |||
| Sensitivity | 70 (61, 78) | |||
| Specificity | 59 (51, 68) | |||
| Positive predictive value | 59 (50, 67) | |||
| Negative predictive value | 70 (61, 78) | |||
SHS: Serious health-related suffering; CI: Confidence interval
Analysis of the patient feasibility questionnaire responses (n = 121) showed that 91.74% of respondents found the SHS tool easy to understand, 92.56% found it easy to use, 89.26% felt the questions were relevant to their condition, and 96.69% of patients found the settings selected for screening to be suitable [Table 3]. Analysis of feasibility questionnaire responses from RAs (n = 11) showed that 10/11 found the tool easy to administer, 8/11 found it relevant to the patient’s situation, and 8/11 found no reasons preventing its regular use [Table 3]. The interviewers reported that, in 66.67% of instances, patients would not have reached them if not for the field test. The PIs shared their feedback and observations on administering the SHS tool within their settings. Analysis of comments in the feasibility surveys of patients and RAs yielded insights, as shown in Supplementary Table 2.
| Likert Scale 0 = extreme dissatisfaction; 10 = extreme satisfaction | ||
|---|---|---|
| Question | Likert Scale | |
| % scoring >7 | % scoring < 6 | |
| Results of patient feasibility survey questionnaire (n=121) | ||
| Q1. How easy or difficult was it to understand the SHS tool? | 91.74 | 8.26 |
| Q2. How easy or difficult was it to use the SHS tool to complete and submit it? | 92.56 | 7.44 |
| Q3. How relevant (connected) were the questions to your current situation? | 89.26 | 10.74 |
| Q4. Is there anything in this tool that may prevent other patients from using it? | 88.43 | 11.57 |
| Results of research associate feasibility survey questionnaire (n=11) | ||
| Q1. How easy or difficult was it to administer the SHS tool? | 90.91 | 9.09 |
| Q2. How relevant did you find the SHS tool to the patient’s situation? | 72.73 | 27.27 |
| Q3. Is there anything about this tool that may prevent you from using it? | 72.73 | 27.27 |
SHS: Serious health-related suffering
DISCUSSION
Suffering is a subjective experience that can be difficult to measure, even more so within the complex settings of health care, and a questionnaire-based screening tool to identify a complex phenomenon like suffering might appear simplistic. The purpose of this study was to develop and evaluate a brief, user-friendly screening tool for frontline staff in oncology settings to screen patients for SHS where none exists. There is a dearth of validated tools with the right combination of psychometric properties to screen for the presence and S HS.[3,10-20] The purpose of this study was to develop and validate a useful tool to screen to recognize SHS in adult cancer patients at busy tertiary-care centres. The SHS tool designed and developed during phase 1[10] was validated and found feasible to use through this phase 2 field-test study.
The TCC-sites from government, academic institutions, non-government and private sectors participated in the field-test, representing diverse population of patients from geographically different regions of India, with various socio-cultural-linguistic and economic backgrounds.
An interview conducted by a skilled professional (health-care professionals or post-graduates with counselling skills [Supplementary Table 3] allows for a detailed bio-psychosocial formulation to screen and identify suffering and can elicit several contributing factors. Interviews hence served as the ‘relevant standard’ in this study, against which the SHS tool was compared, although the logistical limitations may preclude it from routine usage in time- and resource-constrained cancer-care settings such as seen in India. The field test, along with the feedback observations from PIs, indicate that the tool may be administered satisfactorily by trained RAs with modest educational backgrounds [Supplementary Table 4].
The Cronbach’s alpha of 0.728 of the SHS-tool contents, although not ideal, indicates acceptable internal consistency for screening at 95% Confidence Intervals. The SHS tool’s sensitivity of 70% and specificity of 59% suggest a moderate level of accuracy with the possibility of false positives and negatives. There was a moderate concordance rate of 64.17% between the tool output with that of the interview. This may be considered reasonable, as an interview to elicit SHS is not part of routine cancer-care processes and was introduced as the relevant standard for the purpose of this study. The feasibility survey of patient-respondents and RAs indicates the SHS tool to be relevant, easy to understand and use [Table 3]. The above findings support the applicability of the field-tested SHS tool mainly as a screening tool in busy cancer settings in India, where none exists [Table 2]. The screen-positive patients will need to undergo in-depth evaluation by the primary or palliative care team, as per the institutional protocol, to confirm and address the SHS.
An interesting observation was that 46.06% of patient-respondents in the interview sought professional help to reduce their suffering, much higher than the 33.86% self-reported on the SHS tool. Analysis of interviewer notes indicates influencers; for example, patients were made aware of the potential avenues of support available to them during the interview; some of them experiencing SHS preferred to ‘be strong’ and ‘did not want help from anyone as of now;’ which may have contributed to the fewer instances of seeking help while responding on the-tool.
A survey on the suitability of the waiting areas versus in-patient settings for administering the SHS tool did not favour a particular setting. From the overall comments, it may be inferred that an unhurried environment with sufficient rapport-building may be of greater significance than the actual venue.
PIs reported comfort with conducting the field test on the SHS tool and noted that it did not impact or cause a delay in the clinical processes of their setting. The key feedback comments posted by patients and RAs who recorded <7/10 satisfaction on any one of the feasibility survey questions were thematically grouped and analysed [Supplementary Table 2]. The table also provides corresponding insights, responses and actions taken by the research team. Certain comments indicate that patients, as well as RAs, differentiated concerns stemming from treatment from those due to the disease. This attitude may be linked with the hopefulness accompanying treatment, which can impact the meaning and experience of suffering.[21] The post-field-test version of the tool includes an ‘Instructions for use’ section to support the appropriate administration of the tool. It also encourages the person using the tool to reflect upon all health-related suffering experienced over the previous 2 weeks, irrespective of whether it is due to cancer or treatment. The revised, post-field-test version of the SHS tool, with instructions and context for routine use, is depicted in Appendix 2. It emphasises the need for institutional policies, for in-depth evaluation of the screened-positive patients and for aligning them with the essential palliative care package.
Strengths
The methodology used in developing and testing the SHS-tool, including the multi-stage consensus-building processes (of phase 1), and the multi-centric nature of the field-test across institutions from different sectors and regions of India reflect the diversity inherent to the demographics, disease, treatment, settings and health-care sectors in India (phase 2). This has likely contributed to the authenticity of the study-output for the population studied. The uniformity in training and orientation provided to site personnel, and centralised submission of output enabled consistency in data collection across settings. Successful completion of field-test with trained RAs with modest educational background is a notable strength.
Limitations
The field-testing faced challenges during its execution. Contribution of data from the nine sites was non-uniform, influenced by the footfall and available resources. Response bias, which is usually evident during staff-administered surveys, may have been operational during this study. A general lack of awareness surrounding the concept of SHS, as evidenced in the Lancet report, may have influenced the approach and attitude of stakeholders. Overall, the interviewers’ notes reveal nuanced aspects of comprehending suffering, which can indeed be challenging to capture through device-based inquiries about sensitive deep-seated concerns using digital interface. The research team acknowledges these fundamental characteristics of human behaviour and recommends administration of the SHS-tool during review-visits, by trained personnel with the best rapport with the patient.
The purposive sampling allows results to be applicable only for the population studied. As the Tool was in English, there was a high percentage of graduates/postgraduates in the study population, which may limit generalisation the result. However, the study corroborates the main objective; that a selected set of questions is able to elicit unexpressed suffering, pro-actively. Whether the same set of questions in other languages may allow the respective population to reflect upon, and score their domains of suffering, is yet to be explored. Time efficiency of screening with them tool in real-time although a central objective of the study, was not achieved due to internet disruptions at few sites that delayed submissions, occasionally by more than 24 h. However, analysis of partial data that was uploaded in real time, suggests time advantage in using the SHS-tool. Future studies using print-version of the SHS-tool or in settings with robust internet facility can authenticate this central objective.
The successive waves of COVID-19 led to disruptions in the study with staff redeployment, illness and attrition. Administrative hurdles, technical glitches and inconsistent internet connectivity at field sites affected the analysable data and prolonged the duration of the field test. A non-negotiable focus of healthcare services, to elicit and treat SHS towards alleviating their suffering, is likely to find practical ways to overcome barriers as they arise.
The way forwards
SHS tool can be developed as a paper-based tool or may be built as an online web-based application for institutions to consider within their care processes. Since the field test included adult patients with cancer who comprehended English, further studies to check the generalisability of findings to other sets of populations across the country and across other LMICs or its relevance in patients with nononcological conditions can enhance the value of this tool. It may also be evaluated across a wider population once validated in regional languages.
CONCLUSION
The development of the SHS tool was in response to the evident need emphasised by the Lancet Report (2017) to recognise and alleviate SHS and to improve access to essential palliative care packages as a global priority. The SHS tool is likely to provide an opportunity to identify patients with SHS who remain unrecognised hitherto to communicate their suffering and seek help and, thus, influence subsequent clinical encounters. The SHS tool that was designed and developed for the highly subjective domain of SHS has completed the field test to substantiate its validity, feasibility, relevance and usefulness for screening SHS in adult cancer patients attending Indian cancer-care settings. Those screened as having SHS may be evaluated in-depth, with expedited access to essential palliative care packages. The effectiveness of the tool outside of field-test conditions is yet to emerge. This study responds to the fundamental need for routine screening for SHS in vulnerable patient populations and aligns with the recommendations of the Lancet Report (2017) to alleviate the access bias to essential care.
Acknowledgments
The authors would like to thank the PIs, RAs and Interviewers at each of the nine NCG Tertiary Cancer Centre sites for their wholehearted and enthusiastic participation in this study. Thanks also to each of the 254 patients who gave their time and input towards fine-tuning the SHS screening tool. Gratitude is due to Dr Nitin Paranjape, CEO of Max Office, and Raj Chaudhuri, CEO of Rajware, for converting the SHS screening tool (English) into an online format for the study and for providing backend support. We thank Dr Santu Ghosh, Associate Professor, Department of Biostatistics, St Johns Medical College, for his inputs on statistical analysis, and Dr Hari Menon, professor of medical Oncology at St John Medical College Hospital, for extending wholehearted support to this study. We are grateful to the NCG -India for providing the funds required for conducting the field test and for the constant support that helped the completion of this bi-phasic study.
Ethical approval
The research/study approved by the Institutional Review Board at Amritha Institute of Medical Sciences, number ECASM-AIMS-2021-042, dated 19/01/2021 followed by the Institutional Review Boards of all eight other participating centres.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Support Grant by the National Cancer Grid.
References
- Alleviating the Access Abyss in Palliative Care and Pain Relief-an Imperative of Universal Health Coverage: The Lancet Commission Report. Lancet. 2018;391:1391-454.
- [CrossRef] [PubMed] [Google Scholar]
- Global Data Platform to Calculate SHS and Palliative Care Need-International Association for Hospice and Palliative Care. Available from: https://hospicecare.com/what-we-do/projects/global-data-platform-to-calculate-shs-and-palliative-care-need/database [Last accessed on 2023 Jul 12]
- [Google Scholar]
- The Escalating Global Burden of Serious Health-Related Suffering: Projections to 2060 by World Regions, Age Groups, and Health Conditions. Lancet Glob Health. 2019;7:e883-92.
- [CrossRef] [PubMed] [Google Scholar]
- Redefining Palliative Care-A New Consensus-Based Definition. J Pain Symptom Manage. 2020;60:754-764.
- [CrossRef] [PubMed] [Google Scholar]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- NCG Map-NCG. Available from: https://tmc.gov.in/ncg/index.php/overview/map [Last accessed on 2023 Jul 12]
- [Google Scholar]
- Developing a Screening Tool for Serious Health-related Suffering for Lowand Middle-Income Countries-Phase-1: Domain Identification and Item Generation. Indian J Palliat Care. 2022;28:51.
- [CrossRef] [PubMed] [Google Scholar]
- Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage. 2016;51:1070-90.e9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Pain in Patients with Cancer: A Systematic Review of the Past 40 Years. Ann Oncol. 2007;18:1437-49.
- [CrossRef] [PubMed] [Google Scholar]
- The Burden of Serious Health-Related Suffering among Cancer Decedents: Global Projections Study to 2060. Palliat Med. 2021;35:231-5.
- [CrossRef] [PubMed] [Google Scholar]
- Solving the Global Crisis in Access to Pain Relief: Lessons from Country Actions. Am J Public Health. 2019;109:58-60.
- [CrossRef] [PubMed] [Google Scholar]
- Bridging Gaps to Universal Palliative Care Access in Chile: Serious Health-Related Suffering and the Cost of Expanding the Package of Care Services. Lancet Reg Health Am. 2023;19:100425.
- [CrossRef] [PubMed] [Google Scholar]
- Serious Health-Related Suffering in Latin America: A Secondary Analysis. Lancet Oncol. 2022;23:S37.
- [CrossRef] [Google Scholar]
- 'Mini-Mental State' A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiatr Res. 1975;12:189-98.
- [CrossRef] [PubMed] [Google Scholar]
- The Suffering Pictogram: Measuring Suffering in Palliative Care. J Palliat Med. 2017;20:869-74.
- [CrossRef] [PubMed] [Google Scholar]
- PRISM: Pictorial Representation of Illness and Self Measure: A Brief Nonverbal Measure of Illness Impact and Therapeutic Aid in Psychosomatic Medicine. Psychosomatics. 1999;40:314-20.
- [CrossRef] [PubMed] [Google Scholar]
- An Initial Assessment of Suffering in Terminal Illness. Palliat Med. 1987;1:37-47.
- [CrossRef] [Google Scholar]
- Unbearability of Suffering at the End of Life: The Development of a New Measuring Device, the SOS-V. BMC Palliat Care. 2009;8:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Psychometric Properties of the Suffering Assessment Questionnaire in Adults with Chronic Diseases or Life-Threatening Illness. Scand J Caring Sci. 2018;32:1279-87.
- [CrossRef] [PubMed] [Google Scholar]
- Easier Said Than Done: Keys to Successful Implementation of the Distress Assessment and Response Tool (DART) Program. J Oncol Pract. 2016;12:e513-26.
- [CrossRef] [PubMed] [Google Scholar]
- Pain and Pleasure: Alternative Interpretations for Identical Stimulation. Eur J Soc Psychol. 1980;10:207-12.
- [CrossRef] [Google Scholar]
SUPPLEMENTARY
| Instrument | Domain screened | Domains missed |
|---|---|---|
| Mini-suffering state examination[1] | For dementia patients | Spiritual, Financial |
| Suffering pictogram[2] | Emotional | Physical, Social, Spiritual |
| Pictorial representation of illness and self-measure[3] | A 2D visual representation based on other scales like WHOQOL-BREF and BDI | Spiritual, Financial |
| Initial assessment of suffering[4] | Physical (persistent pain) with some mention of social and emotional consequences | Spiritual, Financial |
| State of Suffering-Five (SOS-V)[5] | Physical, Social, Emotional | Spiritual, Financial |
| Suffering assessment questionnaire in adults with chronic diseases or life-threatening illnesses [6] | Psychological, Social, Spiritual | Physical, Financial |
| The DART[7] | Physical symptoms, emotional burden, and practical | Spiritual, Financial |
DART: Distress assessment and response tool, WHOQOL-BREF: World Health Organization quality of life questionnaire-Brief Version, BDI: Beck depression inventory
REFERENCES FOR SUPPLEMENTARY TABLE 1
Folstein MF, Folstein SE, McHugh PR. “Mini-mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiatr Res 1975;12:189-98. Beng TS, Ann YH, Guan NC, Chin LE, Loong LC, Ying NT, et al. The Suffering Pictogram: Measuring Suffering in Palliative Care. J Palliat Med 2017;20:869-74. Büchi S, Sensky T. PRISM: Pictorial Representation of Illness and Self Measure: A Brief Nonverbal Measure of Illness Impact and Therapeutic Aid in Psychosomatic Medicine. Psychosomatics 1999;40:314-20. MacAdam D, Smith M. An Initial Assessment of Suffering in Terminal Illness. Palliat Med 1987;1:37-47. Ruijs KD, Onwuteaka-Philipsen BD, Van Der Wal G, Kerkhof AJ. Unbearability of Suffering at the End of Life: The Development of a New Measuring Device, the SOS-V. BMC Palliat Care 2009;8:16. Encarnação P, Oliveira CC, Martins T. Psychometric Properties of the Suffering Assessment Questionnaire in Adults with Chronic Diseases or Life-threatening Illness. Scand J Caring Sci 2018;32:1279-87. Li M, Macedo A, Crawford S, Bagha S, Leung YW, Zimmermann C, et al. Easier Said Than Done: Keys to Successful Implementation of the Distress Assessment and Response Tool (DART) Program. J Oncol Pract 2016;12:e513-26.
| Feedback, comments, and suggestions from Patients, RAs who rated <7/10 on the feasibility questionnaire | Insights, responses, and actions by the Research team |
|---|---|
| Terminology related | |
| ‘It was difficult to understand a few terms like constipation and feeding.’ – Patient. ‘Some terms were difficult to understand’ – Patient. ‘The questions included most general symptoms, and my symptom of poor appetite was missing as it was a major concern for me and my family’ – Patient. ‘Language (medical terminology) used here was not always clear to all the patient-participants.’ – RA. ‘Many patients who interacted with me were in daycare receiving chemotherapy, and they had appetite issues. This symptom is not listed’ - RA. |
• As the suggestions served to simplify items, the first domain of Section 1 of the SHS-tool has been modified with difficult bowel movements and poor eating- instead of constipation, feeding. • This change also responds to ‘lack of appetite’ not being featured as an item under the physical domain. |
| Formatting of the Tool | |
| ‘The questions are elaborate/long, mentioning many symptoms in one. Instead, we can have one short and crisp question which asks for the presence of a physical complaint. An additional question may be added to explain the complaint. The same applies to other questions like spiritual/emotional.’ – RA. ‘The question on the physical complaint is exceedingly long; patients tend to miss out on symptoms when reading the question. It might be made into two shorter questions.’ – RA. |
• The tool was meant to be administered step-by-step, beginning with the domain question and to use items (symptoms) only to suggest what the domain represents. The item list itself was never intended to be comprehensive. • This is a gap in the training and will have to be addressed with specific attention. |
| Meanings and interpretations of suffering | |
| ‘…There must be clarity before treatment or afterwards.’ ‘What stage of treatment or state of specific disease can be included, as that can change the response’ – Patient. ‘Patients who are on chemotherapy have multiple persistent complaints. What should they do? RA ‘I am suffering from pain related to surgery for my breast, which is normal and will take time to heal. Other questions were not relevant to me’ – Patient ‘…. it’s difficult to differentiate between cancer-related symptoms (e.g., pain and vomiting) versus treatment (e.g., chemotherapy/radiation) related symptoms.’ – RA |
• The comments may be linked with organised beliefs surrounding the symptom, which can impact the subjective component of suffering for patients and poor clarity of RAs. • This entails additional training of RAs to encourage patients to disclose all suffering irrespective of their cause. |
| Relevance to specific patient populations | |
| ‘Patients on follow-up after completion of cancer treatment find it irrelevant to their current condition.’ – RA. ‘Some patients thought this was not relevant for them’ – RA. |
• For those who may find it not relevant, the SHS tool has been modified with the option of ‘Not applicable’ to the section-1 response column of ‘Not at all.’ • ‘Do not wish to respond’ may be considered as an exit option from the SHS tool. These patients may require further conversations to elicit suffering (if any). |
| Ambiguity related to Section 2 of the tool | |
| ‘Functional limitation of 14–30 days; …there must be clarity’ – RA The section 2 questions seem very nonspecific, and often they are unable to understand/answer it.’ RA |
• To improve clarity, the SHStool has been simplified and asks to reflect on suffering ‘over the past 2 weeks.’ |
| Expectations from patients | |
| ‘Do you seek additional help….’ Patients often interpret this question as an option to seek financial assistance from the hospital.’ – RA ‘Most patients seek help for physical problems and expect that to be cured.’ – RA ‘Administration of this tool should be backed up by relevant resources for appropriate treatment.’ – RA |
• The question will be modified to ‘Do you seek professional help for these concerns?’ • Training of Research Associates will need to ensure their ability to clarify this aspect when it arises. • Institutions utilising the SHS tool will need to activate processes to follow up on the presence of SHS and to provide access to the Essential Palliative Care Package. An instruction to this effect will be added to the SHS-tool details. |
| Concerns related to non-physical domains | |
| ‘My issues are not about hospital related, but about family, mind and spiritual. ’ ‘Patient doesn’t want to open up and answer the questions’ – RA. ‘Psychological factors or questions might not be clearly answered. ’ ‘During the interview, I noticed that some patients did not want us to be privy to their emotional, social, psychological, and financial problems. There were four questions addressing these aspects, which seem to form the major chunk of the tool. Some cancer patients, especially those on treatment, feel vulnerable and do not want to open up about this aspect of their health. ’ - RA |
• Patients, PIs, and RAs highlighted the requirement of rapport and support while administering the SHS tool. • Training of RAs will need to emphasise that the SHS tool is attempting to elicit general suffering in the domain without having the patient clarify details of their exact concerns in any of the domains, be it physical, emotional, social, financial, or spiritual. |
| Logistics related | |
| ‘Constant internet issues’ – 4 patients ‘Time taken was long due to net disruptions’ - 5 patients. ‘I was not comfortable handing over my phone to patients for the study. Paper-based questionnaire would be more suited for this purpose. ’ - RA |
• The print version of the SHS tool is an option for centres with poor net connectivity. |
| Excerpts of comments from Principal Investigators | |
| Easy to use and effective tool. ‘Tool was easy to administer. ’ ‘The training of RAs and checking their comprehension was essential before and while conducting the field test.’ ‘Simple and easy tool once the RAs got oriented. Some words need more clarity, as indicated in comments by the RA of my setting. Caregivers try to dominate to help answer the questions. Needed to reiterate that it is about the patient. ’ ‘Will need translation in other languages if this proves to be a valid, feasible tool. ’ ‘Recruiting English-speaking patients was at times a challenge.’ |
• The need for orientation and training of RAs are emphasised before integrating the SHS-tool within a clinical setting. • The PIs emphasise the need to align the caregivers with the purpose of the tool so they allow patients to respond directly. This is an important step within the Indian setting, where one patient is usually accompanied by more than one family member. • Further studies are needed to translate and validate the SHS tool. |
RA: Research associate, SHS: Serious health-related suffering
| Educational qualifications | Number of years of work experience in the current setting | Total number of years of work experience |
|---|---|---|
| Master’s in psychology | 12 | 22 |
| Master’s in social work | 3 | 13 |
| BPT, MSW | 0.5 | 2.5 |
| MD | 5 | 17 |
| M.Phil., Ph.D. Psychology (Psycho-oncology) | 16 | 20 |
| MSW, MSc Psychology | 7 | 7 |
| MSW, MBA | 7 | 13 |
| MA in Clinical Psychology. Spl. Ed, Dip. in Guidance and Counselling | 2 | 9 |
| MSc Health Psychology | 4 | 4.5 |
| MPhil Clinical Psychology | 3 | 5 |
| Educational qualifications | No. of persons | Range of years of work experience in the current setting | Range of total years of work experience |
|---|---|---|---|
| Non-graduates | 3 | 2–2.5 | 2.5–19 |
| Graduates | 3 | 1.5–5 | 2.5–20 |
| Post-graduates | 5 | 2–29 | 6–29 |
| The SHS-tool framework used for the Field Test | |||||
|
Section-1 Domains: P- Physical; E – Emotional; S – Relations/Social; Sp – Spiritual; F- Financial |
|||||
| Domains of Health-related Suffering | Not at all Score 0 | A little Score 1 | A lot Score 2 | Domain score | |
| Associated with your health, do you suffer physically? With pain/breathing difficulty/vomiting/constipation/weakness/feeding/loose motion/bleeding/itching/wounds/difficulty with senses (see, hear, smell, touch, taste)/difficulty moving/other issues | P = | ||||
| Associated with your health, do you suffer emotionally? Feeling sad/unloved/worried/angry/lonely/difficulty sleeping/confused/poor memory/other issues | E = | ||||
| Associated with your health, do you suffer due to issues with family/relationships/friends/community/feeling isolated/difficulty at work/difficulty with hospital visits/difficulty communicating/other issues | S = | ||||
| Associated with your health, do you suffer due to feeling punished/fearful/shame/guilty/angry with God/no meaning in life/disconnected/other issues | Sp = | ||||
| Associated with your health, do you suffer due to lost job/stopped studies/stopped working/loan/debt/sold property/sold assets/migrated out/other issues | F = | ||||
| Is there Presence of Health-related Suffering? P+E+S+Sp+F |
Total score >2 |
Total score <2 |
|||
| Total Score <2 ➔ No SHS The screening for SHS is continued at pre-decided intervals, as per the Institutional policy |
|||||
| Total Score >2 ➔ Screen further through Section 2 Check for Seriousness of the health-related suffering by asking the 2nd level questions |
|||||
| Section-2 | |||||
| Has this suffering limited you from doing what you need to do, for >14 days over the last 30 days? e.g., self-care (feed, bathe, dress, walk, toilet); care for others; communicate; learn /think/perform duties; sleep/rest? Yes/No | Ask the patient – Do you seek additional help for your concerns? Yes/No | ||||
| Responses to Section 2 | Screening Outcomes with suggested actions | ||||
| ☐ YES, to both A and B | SHS ☑ | Notify the treating team so the patient may be evaluated in-depth and aligned with essential care-pathways as per the policy of the department/administration. | |||
| ☐ NO to A and YES to B | SHS☑ | ||||
| ☐ YES, to A and NO to B | SHS ☑ | Educate patient/family about availability of support and encourage to seek support when they need; empower with information. | |||
| ☐ NO to both A and B | SHS ☑ | The screening for SHS is continued at pre-decided intervals, as per the Institutional policy. | |||
SHS: Serious health-related suffering
| Background | ||
| • The Lancet Commission Report (2017) on ‘Alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage,’ emphasises the huge burden of Serious Health-related Suffering across the world, with maximum incidence in low- and middle-income countries (LMICs). • Suffering is health-related when it is associated with illness or injury of any kind. Suffering is serious when it cannot be relieved without professional intervention and/or when it compromises physical, social, spiritual, and/or emotional functioning. • Patients with life-threatening and life-limiting conditions such as cancer, endure ‘serious health-related suffering’ (SHS), which remain unrecognised and unaddressed. • Identifying the SHS of patients at physical, psychological, social, spiritual, and/or financial, is the first active step towards alleviating and addressing them. • The SHS screening tool (English) has been developed by the research group, under the aegis of the National Cancer Grid -India. The group used a systematic consensus building process during the Phase 1 of the study (Delphi methodology, Nominal Group Technique, Transparent Expert Consultation, and public polling) and proceeded to complete a multi-centric field-test across nine major cancer-care institutions in India during the Phase 2. The results show acceptable validity and feasibility. • The SHS-tool presented below, is validated to screen for Serious Health-related Suffering in adult patients with cancer, who reach cancer-care settings. • Institutions planning to utilise the SHS- tool must have processes in place to activate an in-depth evaluation and enable access to an Essential Palliative Care Package for patients identified as having SHS. |
||
| Instructions for use | ||
| 1. Use the Tool to screen for Serious Health-related Suffering, be it due to the cancer or its treatment. 2. The Tool is better suited for screening patients during their review visits, to be administered by trained personnel sharing rapport with them. 3. Identify and approach patients with cancer (adult, English comprehending) at clinical settings selected by the institution: waiting are/in-patient ward/day care. 4. Respectfully introduce yourself and build rapport with patient and family, e.g., enquire how they are doing, how long they have been on treatment, their travel to hospital etc. 5. Enquire about their interest to screen for concerns related to their health-condition. 6. Explain that the aim is to note their suffering over the previous 2 weeks, irrespective of whether it is due to the cancer or the treatment. 7. Encourage the family-member, bystander to allow and support the patient to respond directly. 8. Once they indicate willingness, note the patient’s demographic details, and administer section 1 of post-field-test SHS Screening Tool, • Begin with reading out the domain question asking for the presence of any concern in that domain, pause…. then, read out the items listed under it. • Clarify that the items listed are examples only, of concerns commonly seen under that domain; encourage the patient to share any other cause for suffering, which may not have been listed. • Give time and allow the patient to comprehend and consider their response for each domain. • Note the severity of suffering under each domain, by using the scoring system. • If the patient shares concern other than those listed as items, score its severity, under the appropriate domain. 9. Maintain an unhurried attitude right through; clarify questions and respond to their doubts. 10. Total score of 0 or 1 from section 1 of the-Tool indicates no SHS. Here, it may be appropriate to continue screening for SHS at pre-decided intervals, as per the Institutional policy. 11. If section-1 yields total score >2, ask the two questions under Section 2 of the-Tool. Note the Yes/No response. 12. Use the Interpretation table to mark the presence or absence of SHS. 13. Thank the patient for their time and proceed further based on the output of the Tool. 14. Patients who do not wish to engage or respond may exit at any stage. They may be encouraged to have a one-on-one conversation with their treating clinician regarding their concerns (if any). |
||
| The Revised SHS Screening Tool post-Field Test | ||
| Section 1 (When answering each question, consider your suffering over the last 2 weeks) | ||
| 1. Associated with your health, do you suffer physically? Due to pain/breathing difficulty/vomiting/difficult bowel movements/loose motion/bleeding/weakness/difficulty in eating/loss of appetite/itching/wounds/difficulty with seeing, hearing, smelling, touch, taste/difficulty moving/any other issues? Not suffering at all/Not Applicable ☐ Score 0 A little suffering ☐ Score 1 A lot of suffering ☐ Score 2 2. Associated with your health, do you suffer emotionally? Due to feeling sad/unloved/worried/angry/lonely/difficulty sleeping/difficulty sleeping/confused/poor memory/any other issues? |
||
| Not suffering at all/Not Applicable ☐ Score 0 A little suffering ☐ Score 1 A lot of suffering ☐ Score 2 3. Associated with your health, do you suffer socially? Due to concerns with family/relationships/friends/community/feeling isolated/difficulty at work/difficulty with hospital visits/difficulty communicating/any other issues? Not suffering at all/Not Applicable ☐ Score 0 A little suffering ☐ Score 1 A lot of suffering ☐ Score 2 4. Associated with your health, do you suffer spiritually? Due to feeling punished/fearful/shame/guilty/angry with God/no meaning in life/disconnected/any other issues? Not suffering at all/Not Applicable ☐ Score 0 A little suffering ☐ Score 1 A lot of suffering ☐ Score 2 5. Associated with your health, do you suffer financially? Due to lost job/stopped studies/stopped working/loan/debt/sold property/sold assets/migrated out/any other issues? Not suffering at all/Not Applicable ☐ Score 0 A little suffering ☐ Score 1 A lot of suffering ☐ Score 2 |
||
| Total Score < 2: The suffering is NOT serious ➔ continue screening at pre-decided intervals, as per Institutional policy. Total Score > 2: There is significant Health-related suffering ➔ move on and respond to Section 2 questions |
||
| Section 2 (When answering each question, consider your suffering over the last 2 weeks) |
||
| A: Has this suffering limited you from doing what you need to do? For example, self-care (feed, bathe, dress, walk, toilet); care for others; communicate with others; learn, think, do work, complete duties; sleep or rest? | ☐ Yes ☐ No | |
| B: Do you seek additional professional help for these concerns? | ☐ Yes ☐ No | |
| Interpretation of the SHS Tool Output | ||
| Responses to Section-2 questions, A & B | Screening Outcomes with suggested actions | |
| ☐ YES, to both A and B | SHS ☑ | Notify the treating team so the patient may be evaluated in-depth and aligned with essential care-pathways as per the policy of the department/administration. |
| ☐ NO to A and YES to B | SHS ☑ | |
| ☐ YES, to A and NO to B | SHS ☑ | Educate and empower the patient/family about availability of professional help and encourage them to seek support as and when they need. |
| ☐ NO to both A and B | SHS ☑ | The screening for SHS is continued at pre-decided intervals, as per the Institutional policy. |
| ☐ Patient exited the tool before completing the SHS-tool questionnaire | ||
SHS: Serious health-related suffering






