Translate this page into:
A Randomized Placebo-Controlled Trial Evaluating the Analgesic Effect of Salmon Calcitonin in Refractory Bone Metastasis Pain
Address for correspondence: Dr. Parmanand N Jain, Division of Pain, Tata Memorial Centre and Homi Bhabha National Institute, Mumbai, Maharashtra, India. E-mail: pnj5@hotmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Injection calcitonin is a natural hormone inhibiting osteoclastic bone resorption have been used as an analgesic to control bone metastasis pain or pain due to osteoporosis or fracture. This randomized double blind placebo controlled trial was undertaken to determine the role of injection Salmon Calcitonin therapy to control refractory pain caused due to bone metastasis arising from cancer breast, lung, prostate or kidney. All patients had received palliative radiotherapy and were suffering unsatisfactory pain relief on NSAIDs and tab morphine. Fourteen days inj. calcitonin or placebo injections were administered in 23 patients initially as high dose induction dose (800 IU per day SC) followed 200 IU subcutaneous (SC) once a day. Patients were assessed for pain intensity and quality of life on EORTC QLQ-30 questionnaire 6 hourly for 2 days and on 7th and 30th day. Any incidence of hypercalcemia, bone fracture, nerve root and bone marrow compression were also noted. This study found a significant reduction in pain after SC calcitonin injection therapy at 14 and 30 days' assessment. No patients in the study group required rescue analgesia after 18 hrs. There was a statistically significant difference in rescue analgesics required between the groups during two days hospitalization. Global health as well as physical and social wellbeing was better at 30 and 90 days in the study group as compared to control group, however it could not reach a statistical significance which may be attributed to the small sample size of the study.
Keywords
Bone metastasis pain
randomized trial
salmon calcitonin
INTRODUCTION
Bone metastasis is a major cause of morbidity causing pain in cancer patients, especially of breast, lung, and genitourinary (prostate and kidney) origin. Pharmacotherapy is the mainstay for the control of metastatic cancer pain as recommended by the WHO pain guidelines. However, some patients may not respond to analgesics used concomitantly even with palliative radiotherapy (RT) and intravenous (IV) bisphosphonates.
Salmon calcitonin has been postulated to reduce bone resorption, to increase circulating endogenous opioids, and to have endorphin receptor agonist activity (Gennari, 1989; Spigset, 1993; and Mystakidou, 1999). Hence, injection calcitonin may be an option to control refractory bone pain. Calcitonin is also postulated to reduce bone complications, for example, hypercalcemia, fractures, and nerve compression.
This study was undertaken to assess the effectiveness of injection salmon calcitonin for controlling metastatic bone pain in breast, lung, and prostate cancers where routine analgesics (paracetamol, nonsteroidal anti-inflammatory drugs, and opioids) and bisphosphonates have failed and chemotherapy and RT have shown poor response.
MATERIALS AND METHODS
This randomized trial was undertaken consequent to the approval of institutional ethics and review board at a tertiary care cancer center. All patients referred to pain clinic who had moderate-to-severe refractory pain with bone metastasis having received RT were screened for inclusion. Those who fitted into the laid down criteria were recruited. Informed consents were obtained from patients explaining the entire trial details in the language best understood by them. Proof of evidence for bone metastasis was identified by X-ray, bone scan, or magnetic resonance imaging. All patients reported to the cancer pain clinic with bone metastasis were screened for possible inclusion into the trial.
Inclusion criteria
-
Moderate-to-severe bone metastasis pain
-
Uncontrollable spontaneous bone metastasis pain on routine analgesic during rest or movement
-
Multifocal bone metastasis pain with hypercalcemia
-
Recurrence of pain after failed bisphosphonate therapy or RT
-
Residual significant pain after RT.
Exclusion criteria
-
Patients on bisphosphonates or RT or any radioneucleotide for analgesic purpose
-
Patients allergic to calcitonin.
The patients were randomized according to a computer-generated random number table into two groups: Group A received subcutaneous injection of the study drug (salmon calcitonin 100 IU: 1 ml) and Group B received placebo similarly (1-ml normal saline), both of which were provided as preformed 1-ml ampoules by Shreya Life Sciences Pvt. Ltd., Mumbai, Maharashtra, India. All patients were admitted for 2 days either receiving the study drug (Group A) or placebo (Group B). Drugs were administered at a dose of 2 ml subcutaneously (SC) every 6 hourly for the first 48 h. Thereafter, 2-ml drug was administered once daily SC as a maintenance dose for 12 days on a domiciliary basis consequent to negative prior intradermal sensitivity test.
Tablet calcium carbonate 500 mg with Vitamin D3 250 IU was supplemented during 14 days of injection calcitonin therapy to prevent any incidence of hypocalcemia. Tablet ondansetron 8 mg was given during the calcitonin therapy as an antiemetic. Pain assessment was done using a Numerical Rating Scale (NRS, 0–10) at baseline; during the treatment 6 hourly for 2 days; and then on 7th, 14th, and 30th days. Serum calcium levels before and after the therapy were estimated.
All patients received analgesics (diclofenac 50 mg TDS with omeprazole 20 mg OD, oral morphine 30 mg controlled release preparation twice a day, and oral morphine 10 mg for breakthrough pain). The target pain score was <4/10 on movement. All patients received laxatives at night.
The patients were also assessed for bone metastases-related complications (hypercalcemia, bone fracture, nerve root, and bone marrow compression) during the 3-month observational period.
The European Organization for Research and Treatment of Cancer, Quality of life questionnaire (EORTC QLQ-C 30) was employed to assess the quality of life, and the scores were compared using repeated measures analysis between the groups. Statistical analysis was done using SPSS software version 24 (IBM, Armonk, NY, USA). Data were presented as mean and median (interquartile range [IQR]). Continuous variables were analyzed by Mann–Whitney test, and categorical data were analyzed by Chi-square test. P < 0.05 was considered statistically significant.
RESULTS
This randomized study included 11 females and 12 males. Age ranged from 32 to 79 years. Group A (study group) included 12 patients and Group B (placebo) included 11 patients. This study included patients with moderate-to-severe pain having lung (10), breast (11), and prostate (2) cancers [Table 1].
| Study group (drug A) (n=12) | Control group (drug B) (n=11) | |
|---|---|---|
| Age, median (range) | 53 (32-62) | 56 (45-79) |
| Gender | ||
| Male | 6 | 6 |
| Female | 6 | 5 |
| Diagnosis | ||
| Lung | 5 | 5 |
| Breast | 6 | 5 |
| Prostate | 1 | 1 |
| Serum calcium levels (meq/l) | ||
| At admission | 8.7 (6.5-9.6) | 9.2 (8.2-10.2) |
| At 48 h | 8.7 (7.0-9.4) | 9.2 (7.8-9.8) |
| Skeletal metastasis sites (% patients) | ||
| Skull | 1 (8.3) | 4 (36.4) |
| Spine | 10 (83.3) | 9 (81.8) |
| Ribs | 8 (66.7) | 8 (72.7) |
| Pelvis | 7 (58.3) | 8 (72.70 |
| Long bones | 5 (41.7) | 6 (54.5) |
| Treatment taken (% patients) | ||
| Surgery | 7 (58.3) | 5 (45.5) |
| Chemotherapy | 10 (83.3) | 7 (63.6) |
| Radiation therapy | 12 (100) | 11 (100) |
| Bisphosphonates | 10 (83.3) | 11 (100) |
| Investigations (% patients) | ||
| X-ray | 7 (58.3) | 10 (90.9) |
| MRI | 7 (58.3) | 4 (36.4) |
| CT | 4 (33.3) | 7 (63.6) |
| Bone scan | 9 (75) | 9 (81.8) |
| Site of pain | ||
| Spine | 8 (66.7) | 9 (81.8) |
| Upper limb | 4 (33.3) | 3 (27.3) |
| Lower limb | 9 (75) | 10 (90.9) |
| Pelvis | 6 (50) | 9 (81.8) |
| Ribs | 5 (41.7) | 4 (36.4) |
MRI: Magnetic resonance imaging, CT: Computed tomography
Pain severity
On admission, prior to randomization, all patients had moderate-to-severe pain, with a median pain score (at rest) of 5.0 (IQR: 4–6.25) and on movement of 6.0 (IQR: 6–7). Within 48 h after commencement of therapy, pain was found to be lower though not significantly in Group A [Figure 1], with reduced requirement of rescue doses of morphine [Figure 2]. On the 14th day after completion of drug therapy, in the study group, one patient had no pain, 7 had mild, 2 had moderate pain, 2 were lost to follow-up, and one patient did not complete the full study drug regimen. In the control group, 7/11 had moderate and 4 had mild pain. At 30 days, only one patient had moderate pain in the study group, but in the control group, 7/11 had moderate pain, 2 had mild pain, and 2 were lost to follow-up. No patients in the study group required rescue analgesia except one. On movement, difference was found to be statistically significant at 14 (P = 0.046) and 30 days (P = 0.016), but no difference was seen at 90 days [Figure 3].
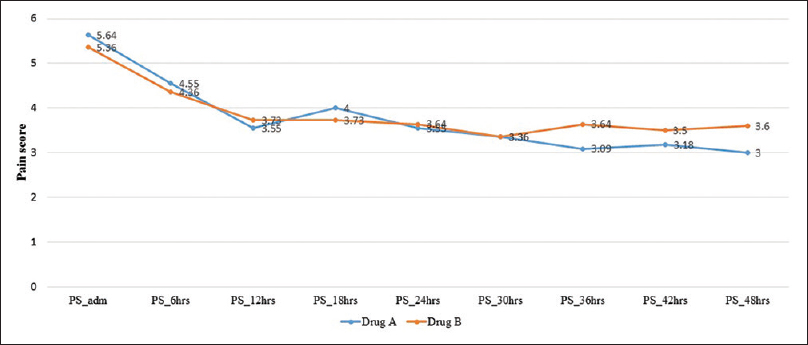
- Mean pain score in the first 24 h
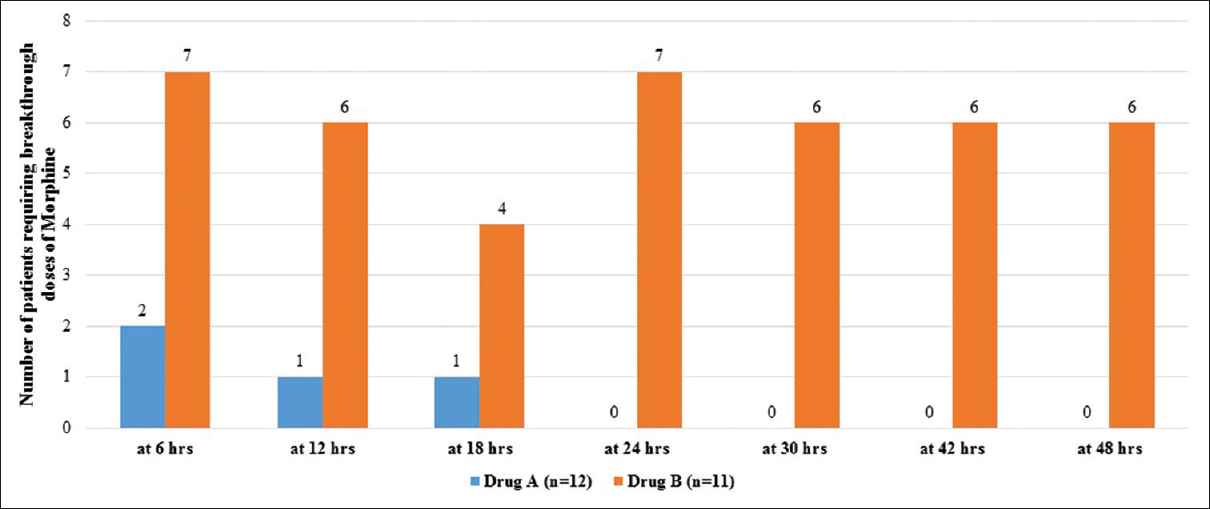
- Rescue analgesia in the first 24-h stay
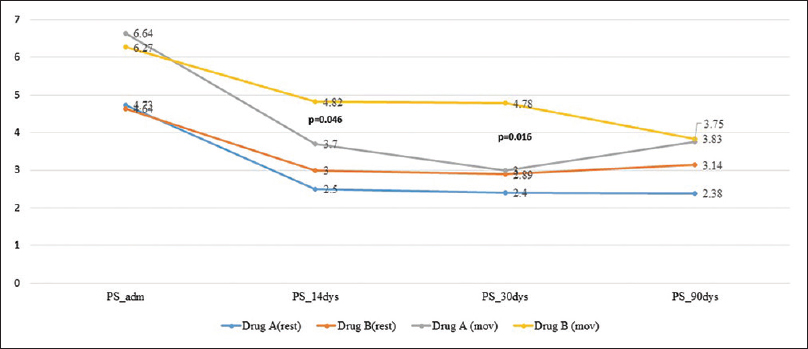
- Mean pain scores till 90 days
Quality of life
Physical function [Figure 4] and global health [Figure 5] were found to be better in the study group but not statistically significant at 14, 30, and 90 days. Fatigue was lower in the study group at 30 and 90 days. One patient developed hypocalcemia after injection calcitonin. Three patients in the study group and four in the control group died during the 90 days' period due to disease progression. Pain was lower as assessed on the symptom scale of EORTC QLQ 30 in the study group on days 14, 30, and 90 days [Figure 6].
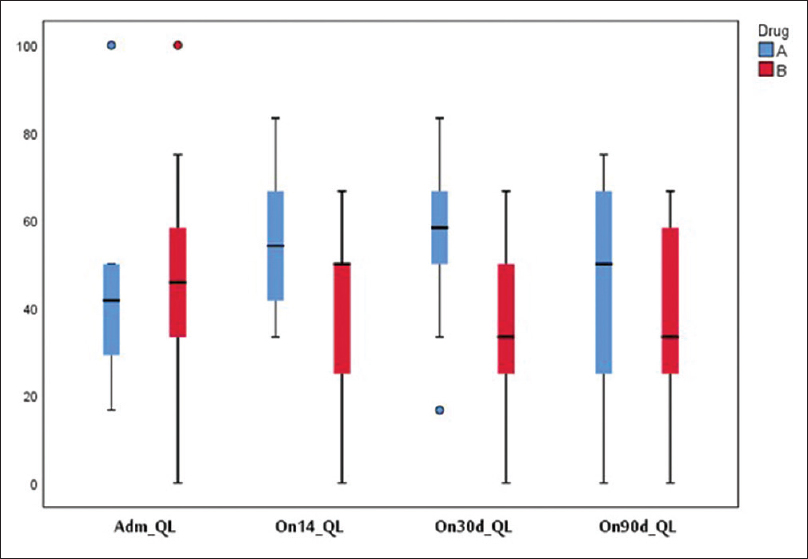
- Quality of life (global health status)
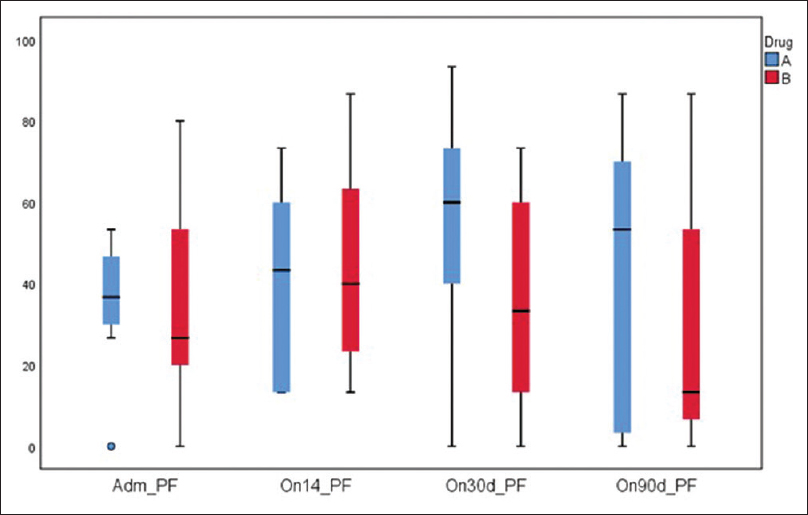
- Quality of life (EORTC) physical functioning
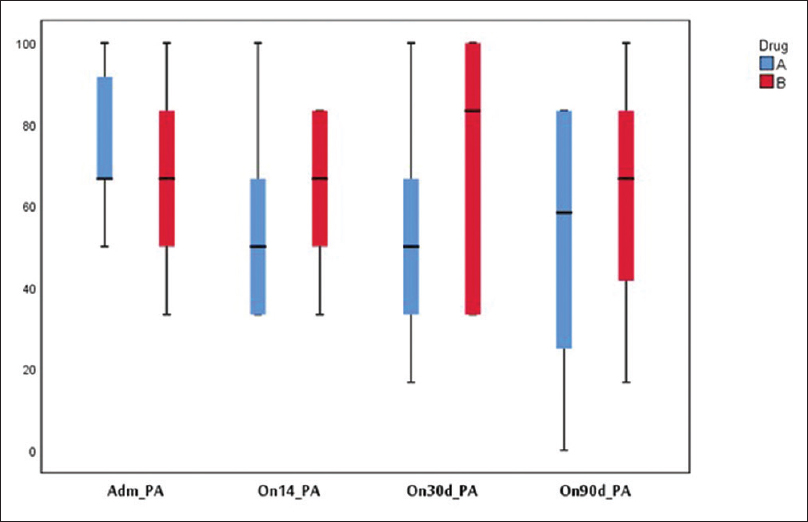
- Quality of life EORTC (pain)
Side effects
In the study group, three patients had nausea and three had vomiting, and in the control group, one patient had nausea and no one had vomiting. No allergic reaction to drugs was observed in either group; however, one patient in the study group developed tetany during high-dose calcitonin therapy. One patient in the study group and two in control group developed bone fracture. Nerve compression was observed in three patients in the control group and one in the study group.
DISCUSSION
Salmon calcitonin has been used in bone metastases pain, and its pain-relieving activity is due to its action of inhibition of osteoclysis. Its central analgesic property has been supported by its action through subarachnoid administration and generation of beta endorphin-like activity in serum. Encouraging results were shown in hormone-sensitive tumors such as breast and prostate; however, an appropriate size randomized trial has never been conducted to determine its exact role in bone metastases pain. Therefore, the analgesic action of salmon calcitonin remains incompletely understood.
In the present study, injection salmon calcitonin was administered in sufficient loading dose to induce the inhibitory osteoclastic activity with a low-dose maintenance daily injectable for 14 days to elucidate its action in different cancer-related bone metastasis conditions with refractory pain in previously RT-treated patients.
This study found a significant reduction in pain after injection salmon calcitonin after completion of injection therapy at 14 and 30 days' assessment. No patients in the study group required rescue analgesia after 18 h on injection. There was a statistically significant difference in rescue analgesics required between the groups during 2 days' hospitalization. On evaluation of quality-of-life indicators, global health and physical and social well-being were better at 30 and 90 days in the study group as compared to that of the control group; however, it could not reach a statistical significance, which may be attributed to the small sample size of the study. Calcitonin has been postulated to reduce bone resorption, to increase circulating endogenous opioids, and to have endorphin receptor agonist activity.[123] Currently, the use of calcitonin for the relief of metastatic bone pain is not very common, and little information is available about its use in this context. It has potential efficacy in lowering pain and improving bone density, thereby lowering the risk of fractures is under investigation. First, randomized controlled trial (RCT) using injection calcitonin 200 IU QID only for 48 h showed good pain relief up to 1–2 weeks after the commencement of therapy in 22 patients.[3] A similar study conducted a nonrandomized trial on 22 patients with SC infusion of calcitonin and reported reduction of pain intensity from the mean baseline value of 4.43–1.17 (Visual Analog Scale), which was observed in 85% (18/22) of the patients. The study suggested that calcitonin in high doses may be a useful adjuvant analgesic when combined with low doses of morphine in continuous SC administration for the management of metastatic bone pain.[4] Our study results showed similar results in agreement with Hindley et al. and Mystakidou et al.[34] Unfortunately, the Cochrane Database of Systematic Reviews, 2003,[5] concluded that due to limited available evidence, use of calcitonin may not be supported for controlling pain arising out of bone metastases. Previous two studies by Roth, 1986,[6] including forty women with carcinoma breast and by Blomqvist, 1988,[7] with fifty breast cancer women did not provide quantifiable data for pain reduction. During the same period, an open study of 34 lung patients by Schiraldi et al.[8] reported excellent pain relief with 400 IU dose/day. An open-label study (2006) showed that high-dose IV calcitonin with steroids may reduce pain and analgesic requirement in 10% of patients.[9] This randomized, double-blind trial should be considered as a pilot study for conducting a large RCT on calcitonin for bone pain, which may show its exact effectiveness to control pain in refractory clinical settings.
CONCLUSION
Bone metastasis pain was reduced for 1 month after the commencement of high-dose subcutaneous salmon calcitonin therapy. A large randomized, double-blind trial on injection calcitonin is required to further evaluate its analgesic effect in metastatic bone pain.
Financial support and sponsorship
Nil.
Conflicts of interest
The company provided blinded ampoules of the drugs.
Acknowledgment
We acknowledge with thanks to Shreya Life Sciences (P) Ltd. for providing blinded ampoules of the drugs for the study.
REFERENCES
- A double-blind controlled trial of salmon calcitonin in pain due to malignancy. Cancer Chemother Pharmacol. 1982;9:71-4.
- [Google Scholar]
- Continuous subcutaneous administration of high-dose salmon calcitonin in bone metastasis: Pain control and beta-endorphin plasma levels. J Pain Symptom Manage. 1999;18:323-30.
- [Google Scholar]
- Analgetic activity of calcitonin in patients with painful osteolytic metastases of breast cancer. Results of a controlled randomized study. Oncology. 1986;43:283-7.
- [Google Scholar]
- Evaluation of salmon calcitonin treatment in bone metastases from breast cancer – a controlled trial. Bone. 1988;9:45-51.
- [Google Scholar]
- Salmon calcitonin in cancer pain: Comparison between two different treatment schedules. Int J Clin Pharmacol Ther Toxicol. 1987;25:229-32.
- [Google Scholar]
- Analgesic activity of high-dose intravenous calcitonin in cancer patients with bone metastases. Oncol Rep. 2006;16:871-5.
- [Google Scholar]






