Translate this page into:
Adaptation of a Quality of Life Questionnaire for Iranian Patients with Esophageal Cancer
Address for correspondence: Dr. Morteza Tabatabaeefar; E-mail: tabatabaeefar@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Evaluation of quality of life is very important in cancer patients. Esophagus-specific quality of life questionnaire (QLQ-OES18) is a disease-specific questionnaire for assessing quality of life in esophageal cancer (EC). So we aimed to translate and evaluate the reliability and validity of the QLQ-OES18 when applied to Iranian patients.
Materials and Methods:
This study was designed as cross-sectional study on 62 newly confirmed EC in two referral hospital in Tehran, Iran. Reliability of the subscales was evaluated by intraclass correlation coefficients. Pearson's correlations of an item with its own scale and other scales were calculated to assess convergent and discriminant validity. Clinical validity was also evaluated by known-group comparisons.
Results:
Cronbach's alpha was higher than 0.7 in most subscales. All subscales met the standards of convergent and discriminant validity. Also QLQ-OES18 had discriminatory power for differentiation between patient's groups with different clinical status.
Conclusion:
Our results provide evidences that Persian version of QLQ-OES18 is a valid and reliable questionnaire when applied to a sample of Iranian patients with EC and is recommended for use in clinical research.
Keywords
Esophageal cancer
QLQ-OES18
Quality of life
Reliability
Validity
INTRODUCTION
Esophageal cancer (EC) is one of the most common cancers worldwide.[12] The majority of patients are diagnosed with advanced-stage disease, and therefore, prognosis is poor.[23] Treatment methods including surgery, chemotherapy, and radiation may increase survival of patients, but have some negative impact on patient's quality of life (QOL).[4] Most of EC patients suffered from dysphagia and pain. They have also encountered difficulties in eating, dry mouth, and trouble with taste.[4]
Among the palliative procedures, surgical approach is not desirable due to high mortality. Endoluminal stenting, external beam radiation, brachytherapy, chemotherapy, chemoradiotherapy, laser treatment, photodynamic therapy, or ablations using injection of alcohol or chemotherapeutic agents are selected method in patients who are not suitable for curative method.[5] Self-expandable esophageal stents is a new method in palliative treatment which is applied to reduce dysphagia in patients with neoplastic esophageal obstruction.[6] Studies have shown that QOL score in EC patients improved significantly after stent replacement.[7]
In the past decades, a number of general and specific measurement tools have been explored in order to assess the health-related QOL in EC patients.[89] Evaluation of QOL in EC patients is emphasized on patient-based outcome assessment. Most studies have measured impact of different methods of treatment on QOL in EC patient using generic QOL questionnaires,[1011] but use of disease-specific questionnaire can demonstrate benefits of treatment such as relief of dysphagia after endoscopic palliation.[3] One of these questionnaires is esophagus-specific QOL questionnaire (QLQ-OES18) that was developed by European Organization for Research and Treatment of Cancer (EORTC). Since there is no specific questionnaire for assessing QOL in EC patients in Iran, we decided to translate and evaluate the reliability and validity of the QLQ-OES18 when applied to two groups of EC patients: Under chemotherapy and under self-expanding metallic stenting.
MATERIALS AND METHODS
Patients
In this cross-sectional study, 62 patients with newly confirmed EC who were referred to Taleghani and Imam Hossein Hospital were reviewed from September 2009 to March 2011. These hospitals are referral centers for EC diagnosis and treatment in Tehran, capital of Iran. The diagnosis of EC was made according to pathological report. Patients were categorized into two groups including: A) Patient's candidate for stenting who had following conditions: nonoperative EC and dysphagia ≥3, unsuitable patients for chemotherapy and dysphagia ≥3, high risk patients for surgery and dysphagia ≥3, tumor recurrence after surgery and dysphagia ≥3, dysphagia during or after chemotherapy and dysphagia ≥3, and fistula. B) patient's candidate for chemotherapy with following conditions: No history of surgical treatment, no endoscopic treatment; prediction of survival more than 6 month; normal liver, kidney, and bone marrow tests; no other concurrent cancer; lack of pregnancy or lactation; and lack of allergy to chemotherapy. The exclusion criteria were: Diagnosis in less than 3 months, cognitive impairment, and other previous or concurrent malignancies. Questionnaire was completed before and 3 months after treatment for each patients. The individuals were informed that attending in study was not compulsory. Informed consent for enrolment was obtained and patient anonymity was preserved.
Questionnaire
The EORTC QLQ-OES18 is a multidimensional module addressing esophageal-specific symptoms during the last week.[3] It is made up of 18 items and 10 symptom scales: Four multi-item scales including: Dysphagia, eating, reflux, and pain domain and six single-item scales consisting of trouble swallowing saliva, choked when swallowing, dry mouth, trouble with taste, trouble with coughing, and trouble in talking. All of the scales and single-item measures range in score from 0 to 100. A high symptom scale score represents a higher level of symptomatology or problems.
Standardization procedures
To test the reliability, internal consistency of the questionnaire was measured with Cronbach's alpha coefficient. Internal consistency refers to the interrelation of items within a scale. Cronbach's alpha coefficient above 0.7 is regarded as an acceptable reliability estimate.[12]
For evaluation of ability of questionnaire to cover all relevant aspects of the phenomena of interest (face validity); expert's opinions were used.[13] Two gastroenterologists and one psychologist were investigated for face validity of the instrument.
Construct validity is composed of two parts: Convergent validity and discriminant validity. Convergent validity is referred to a moderately to high correlation between an item and its own subscale.[14]A desirable convergent validity is characterized by correlation coefficient of 0.4 or higher. Discriminant validity shows a poor correlation between an item and any of the other subscales. Each items should be correlated with own subscales significantly equal or higher than two standard errors than correlations with other subscales. Since the standard error value is strongly influenced by sample size and given the small sample size in the present study, one standard error was used as a criterion for assessment of discriminant validity.[15] Pearson's correlation coefficient is used to evaluate the convergent and discriminant validity.
Ability of the questionnaire in differentiation of patients with various clinical statuses was assessed by clinical validity. For analyzing the comparison made between known groups, t-test was used.
Interscale correlations indicate that each subscale only measures a single trait. Correlation coefficients between different subscales should be lower than the internal consistency estimates of each subscale separately.
Sensitivity to changes shows the power of questionnaire to distinguish any important changes over time, if it seems to be small.[16] Sensitivity to change was assessed by comparing the mean of QLQ-OES18 score in patients who reported a change in symptoms between the two completions and over time using a paired t-test.
All tests were two-sided and P values less than 0.05 were considered as statistically significant. Calculations were performed using Statistical Package for Social Sciences (SPSS) V.13 software (Chicago, IL, USA).
RESULTS
A total of 62 EC patients were recruited in the study. Half of the patients were female (n = 31). The mean ± standard deviation (SD) age was 65.6 ± 11.1 years. Sixty percent of patients (n = 37) underwent chemotherapy and other patients were undergoing stenting.
Cronbach's alpha was lower than 0.7 in eating subscale in follow-up assessment and the highest Cronbach's alpha was seen in dysphagia subscale [Table 1].
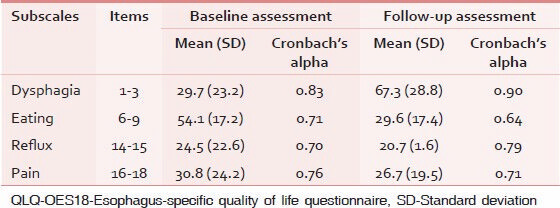
As can be seen in Table 2, all interscale correlations were in the expected direction. The estimated correlation between the subscales was lower than the internal consistency of each of them. It indicates that each subscale of QLQ-OES18 had an ability to measure only a single concept.
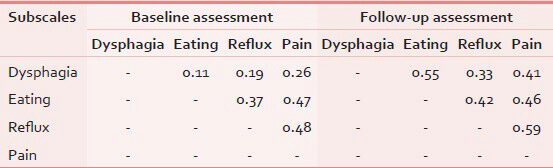
Ability of questionnaire for covering of all relevant aspects of the phenomena of interest was confirmed by experts.
As shown in Table 3, convergent and discriminant validity for all subscales was satisfactory.
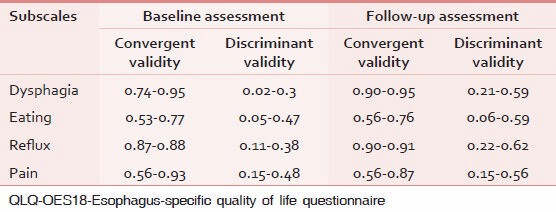
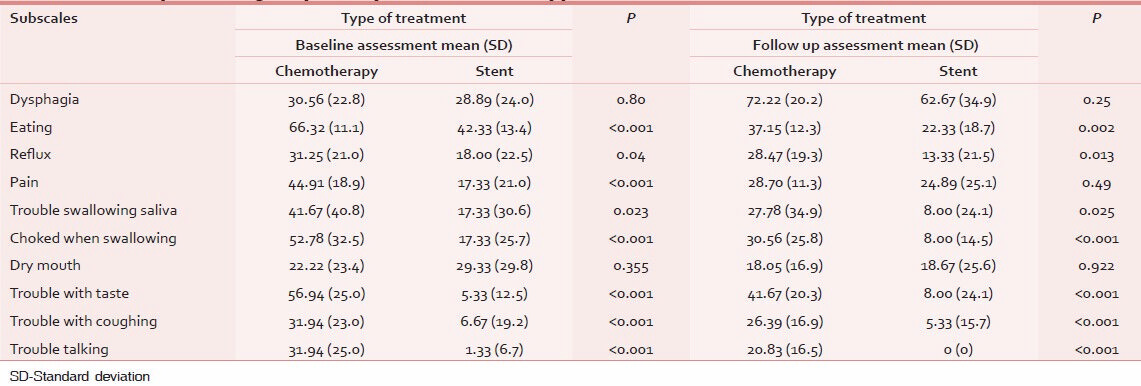
Table 4 shows the discriminatory power of QLQ-OES18 in differentiation of patients according to type of treatment. As seen in baseline assessment, the questionnaire is able to separate between two known groups regarding eating, reflux, and pain subscales. But in follow-up assessment, only eating and reflux subscales were significantly different between known groups under evaluation.
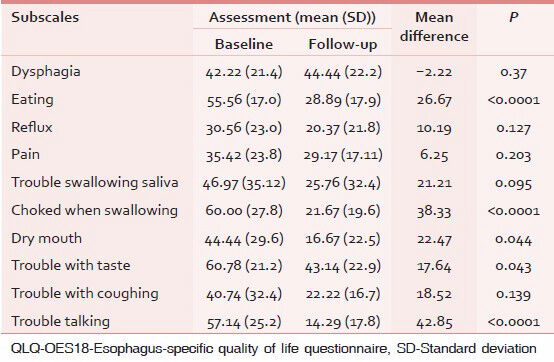
Sensitivity to change was analyzed in patients who showed a change between two assessments. The results of paired t-test are shown in Table 5.
DISCUSSION
This study aimed to translate and validate the QOL-OES18 questionnaire as a specific measurement tool in order to assess the QOL in ES cases for use in Iran and other countries with Persian language. Our findings indicate that the Persian version of QLQ-OES18 is a valid and reliable questionnaire for use in clinical researches.
Internal consistency higher than 0.7 in all of the multi-item scales of QLQ-OES18 provide evidence that each scale is measuring a distinct construct and is suitable for inter group comparisons.
Low to moderate correlation of all subscales of QLQ-OES18 questionnaire with the other subscales in interscale correlation analysis showed that these areas are related, but demonstrate various aspects of QOL.
Standards of convergent and discriminant validity was observed in all subscales so that correlation between items within each subscale was higher than 0.4 and correlation of each item with its constitutive dimension was higher than with the others.
According to known group comparisons, only dysphagia and dry mouth subscales were significantly different between two treatment groups. It indicates that QLQ-OES18 is a powerful measurement tool for differentiation among subgroups of patients according to their clinical status.
QLQ-OES18 was sensitive to clinical changes in health over time in most of subscales and was able to discriminate between clinically distinct groups of patients.
In the present study, for the first time, a disease-specific questionnaire was standardized for assessment of QOL of EC patients in Iran. However, this study also has some limitations.
First, our sample size was relatively small. Second, study setting was limited to only two treatment centers. A multicentric study with a larger sample of patients under different treatment methods is necessary.
In conclusion, our results provide evidences that Persian version of QLQ-OES18 is a valid and reliable questionnaire when applied to a sample of Iranian patients with EC and is recommended for use in future studies.
ACKNOWLEDGMENT
We would like to thank all patients who participated in this study for their valuable collaborations.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Epidemiology and histopathological features of esophageal cancer. East Afr J Public Health. 2012;9:7-9.
- [Google Scholar]
- Increased oesophageal cancer mortality rate in Iran. Arab J Gastroenterol. 2012;13:82-4.
- [Google Scholar]
- European Organisation for Research and Treatement of Cancer Gastrointestinal and Quality of Life Groups. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
- [Google Scholar]
- Quality of life of patients with oesophageal cancer in Taiwan: Validation and application of the Taiwan Chinese (Mandarin) version of the EORTC QLQ-OES18: A brief communication. Qual Life Res. 2010;19:1127-31.
- [Google Scholar]
- Radiotherapy versus stenting in treating malignant dysphagia. J Gastrointest Oncol. 2012;3:322-5.
- [Google Scholar]
- The evaluation of esophageal stenting complications in palliative treatment of dysphagia related to esophageal cancer. Medical science monitor. Int Med J Exp Clin Res. 2012;18:CR323-9.
- [Google Scholar]
- Improvement in dysphagia and quality of life with self-expanding metallic stents in malignant esophageal strictures. Indian J Gastroenterol. 2006;25:62-5.
- [Google Scholar]
- Validation of the Italian translation of the inflammatory bowel disease questionnaire. Dig Liver Dis. 2011;43:535-41.
- [Google Scholar]
- ×. Validation of the mainland Chinese version of the Inflammatory Bowel Disease Questionnaire (IBDQ) for ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2007;13:903-10.
- [Google Scholar]
- A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer. 2000;88:1781-7.
- [Google Scholar]
- Evaluation of quality of life in patients with malignant dysphagia. Tumori. 2000;86:134-8.
- [Google Scholar]
- Validation study of a quality of life (QOL) questionnaire for use in Iran. Asian Pac J Cancer Prev. 2007;8:543-6.
- [Google Scholar]
- Development and validation of a quality of life questionnaire for patients with colostomy or ileostomy. Health Quality Life Outcomes. 2005;3:62.
- [Google Scholar]
- Quality of life outcomes in 599 cancer and non-cancer patients with colostomies. J Surg Res. 2007;138:79-87.
- [Google Scholar]
- MAP-R for windows: Multitrait multi-item analysis program – revised user's guide. Boston, MA: Health Assessment Lab; 1997.
- Validation of the dutch translation of the inflammatory bowel disease questionnaire (IBDQ): A health-related quality of life questionnaire in inflammatory bowel disease. Digestion. 1997;58:282-8.
- [Google Scholar]






