Translate this page into:
Bleeding Control in Advanced Gastric Cancer; Role of Radiotherapy
*Corresponding author: Asifa Andleeb, Department of Radiation Oncology, Sheri-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India. asifaandleeb29@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Andleeb A, Fatima K, Nasreen S, Sofi MA, Najmi AM, Qadri S, et al. Bleeding control in advanced gastric cancer; the role of radiotherapy. Indian J Palliat Care 2023;29:279-84.
Abstract
Objectives:
The aim of our study is to see the efficacy of palliative radiotherapy (RT) for bleeding control in patients with advanced gastric cancer (AGC).
Materials and Methods:
It is a retrospective review based on observations of 74 AGC patients with a median age of 60 years (range 50–82 years) who had active tumour bleeding and were treated with palliative RT. Treatment response was assessed by both subjective symptom relief and objective change in parameters. Objective response to RT was defined by an increase in the median haemoglobin (Hb) level of patients and a decrease in number of packed red blood cell (RBC) units needed by patients after RT.
Results:
Response to haemostatic RT was observed in 52 patients out of 74 patients (70.27%). We observed a significant increase in mean Hb level after palliative RT. Pre-RT mean Hb was 6.14 ± 1.01 and post-RT mean Hb was 7.19 ± 1.75 (P < 0.05). Response to RT was also evident in a significant decrease in the number of packed RBC units post-haemostatic RT. The mean number of pre-RT transfused packed RBC units was 8.28 ± 3.76 and post-RT, it was 4.34 ± 2.91 (P < 0.05). The median overall survival was 90 days and the median transfusion-free survival was 40 days.
Conclusion:
RT may be an effective treatment option for bleeding control in AGC. In our study, we observed fair and reasonably durable haemostasis. A success rate of 70.24% was documented with clinical palliation, a higher Hb level and fewer transfusions after RT. This modality for bleeding control is more important and reliable in situations where alternative modalities are not feasible.
Keywords
Advanced gastric cancer
Bleeding
Palliative
Haemostatic
Radiotherapy
INTRODUCTION
Gastric cancer is one of the most common cancers across the world.[1] In the United States, about 22,220 patients are diagnosed every year out of which 10,990 are expected to die.[2] In India, it is one of the most common cancers and is placed at 7th rank in GLOBOCON 2020. As per the same in India, the estimated number of new cases of stomach cancer in both sexes in 2020 is 60222 and among them, 53,253 cases are expected to die.[3] Treating such a huge number of patients is a big challenge for healthcare providers, especially in developing countries where most of the patients present in an advanced stage of the disease. The standard of treatment for advanced gastric cancers (AGCs) is a multimodal approach, which includes surgery followed by chemotherapy or chemoradiotherapy (CRT) or perioperative chemotherapy or CRT.[4-8]
AGC often presents with symptoms such as obstruction, pain, anorexia, bleeding and anaemia. These symptoms are very distressing to the patient and also have a negative impact on disease management. Anaemia, in particular, can even interrupt the continuation of chemotherapy and hence control of bleeding to improve the quality of life is a must.[9] Severe anaemia with very low haemoglobin (Hb) levels can be life-threatening and in such a situation, blood transfusion is the only option for immediate correction of anaemia. A variety of treatment modalities (both surgical and non-surgical) are available for the control of tumour-induced bleeding in patients of AGC; however, due to a lack of controlled comparative studies, their relative efficacy over one another is still a matter of debate. These modalities include palliative gastric resection, endoscopic interventions and radiotherapy (RT). Although palliative gastric resection provides immediate control of bleeding, it is appropriate only for a small subset of patients who have a severe haemorrhage, are refractory to conservative management and are not fit to undergo a surgical procedure. Haemostatic endoscopic interventions like the use of Laser photocoagulation can be effective, especially for large tumours with diffuse bleeding; however, the cost of equipment and its availability in developing countries is a limiting factor.[10,11] Thermal probes or epinephrine injections provide temporary control in limited patients.[12] Argon plasma coagulation is another endoscopic haemostatic intervention, which has shown its efficacy in 67% of gastroduodenal tumour bleeding but is associated with a high recurrence rate and severe complications like perforation reported in 5–15% of patients.[13]
Endoscopic application of a haemostatic nanopowder (Hemospray) is another alternative modality available in Canada, Europe, most of Asia and the United States.[14] Some clinicians have used arterial embolisation safely and effectively to achieve haemostasis in limited patients with AGC.[15,16] The role of RT for the control of bleeding in patients of AGC who cannot undergo surgery or other haemostatic interventions is well defined.[17-20] In a developing country like ours where these aforementioned modern-day haemostatic technologies are not easily and widely available, so for bleeding control from AGC, we largely rely on palliative surgery and palliative RT, along with supportive measures such as fluid replacement and blood transfusion. Palliative surgery is mainly an option in patients with good performance status and a long life expectancy. Therapeutic endoscopy can also be performed but because of the high rebreeding rate and need for repetitive procedures, we use them mainly in non-malignant bleeding conditions of the stomach and some cancerous patients as well.
Like many other institutes, we have been using RT for bleeding control in various malignancies for long and our experience is reasonably good.[21] Bleeding gastric cancers constitute a major portion of referrals to our department for haemostatic RT. Considering the paucity of other haemostatic facilities in our institute, palliative RT for bleeding control in AGC is the only option for the majority of patients and we here intend to report the results of a retrospective study on palliative RT for bleeding control from AGC and evaluated its efficacy in relieving symptoms of bleeding.
MATERIAL AND METHODS
In the present study, we retrospectively reviewed the clinical data of patients with AGC who received palliative RT for control of bleeding from a primary lesion in our institute between January 2016 and July 2022. After taking clearance from the Ethical committee of the Institute, data were collected by reviewing the records entered in patient files. Inclusion criteria were pathologically confirmed gastric cancer, subjective or objective evidence of bleeding from gastric cancer lesions and the use of palliative haemostatic RT. In many patients, gastric bleeding was confirmed on endoscopic examination while in others, there was clinical evidence (history of hematemesis and/or melena) of upper gastrointestinal bleeding. Exclusion criteria were patients with early-stage disease, patients who had received gastric RT previously and patients on an anticoagulant. All patients in the study group received external beam RT (EBRT) using photons from either cobalt 60 or a high-energy (6 MV) linear accelerator. Patients were treated in the supine position using two (anterior/posterior) parallel-opposed pairs of fields. The RT schedules given were 30Gy in ten fractions over 2 weeks, 20Gy in five daily fractions over 1 week and 15Gy in three daily fractions.
Information regarding patient’s gender, age, histopathology, stage of disease, dose and fractionation regimen of haemostatic RT received, pre-RT chemotherapy if any, side effects of radiation observed, lowest Hb level noted in the month before the start of RT and Hb level attained 1 month after completion of RT and number of packed red blood cell (RBC) units transfused before and after RT were collected from the patient records.
Response assessment
Patients were defined as responders if they had achieved any of the following criteria; (1) subjective improvement of symptoms that is, complete disappearance of bleeding with no episode of hematemesis or melena during 1 month after the completion of RT, (2) patients were alive 1 month after the last fraction of RT and did not require any blood transfusion during that period and (3) patients whose Hb level 1 month after completion of RT was more than the lowest Hb level noted in 1-month period before RT.
Transfusion-free survival (TFS) was calculated from the last day of RT to the first post-RT transfusion or the date of the last follow-up or death. Overall survival (OS) was calculated as the interval from the 1st day of RT to death or last follow-up. The survival curve was estimated by the Kaplan–Meier method statistical analysis was done using SPSS V: 25 software (IBM SPSS, Chicago, USA).
RESULTS
In our study, 134 patients received palliative EBRT to stomach over 6 years i.e. from January 1, 2016 to July 31, 2022. Out of the 134 patients, 42 patients received palliative EBRT to the stomach for obstructive symptoms and 14 patients for pain palliation. In the remaining 78 patients, RT was planned for bleeding control, but only 74 patients could complete the prescribed dose of haemostatic RT as in two patients, RT was stopped due to worsening of performance status and two patients refused to continue RT. Patient characteristics are listed in [Table 1]. The median patient age at the start of haemostatic RT was 60 years. The majority of our patients were not in a good state of health at the time of starting haemostatic RT and more than 50% had Eastern Cooperative Oncology Group (ECOG) performance status of more than 2 at the initiation of RT. ECOG performance status of 3 and 4 was noted in 45.94% and 27.02% of patients, respectively. The proximal stomach (cardia and fundus) was the most common site of involvement. All the 74 patients studied had locally AGC and 21 (28.37%) patients had distant spread also. Among the various histologies, we observed adenocarcinoma as the commonest (67.56% of patients) variant. Various EBRT regimens were used for the control of bleeding and 30 Gy in 10 fractions was the most common (54.05%) regimen used, followed by 20 Gy in 5 fractions (29.72%). None of our patients had received any RT to the stomach in the past; however, there were 64.68% of patients had received chemotherapy before they were started on homeostatic RT. We observed a response rate of 70.27% (52 patients out of 74) to haemostatic RT as per the set criteria defined for response assessment already mentioned in the materials and methods section. The failure rate was 29.73% is explained by; (1) persistent bleeding (hematemesis) in one patient (1.35%) in the 1st month after the completion of RT, (2) 12 patients (16.21%) died within 1 month after starting RT and (3) nine patients (12.16%) did not show any rise in Hb when their lowest Hb level 1 month before the start of RT was compared with the Hb level attained 1 month after the completion of RT. Individual differences in Hb level of all 74 patients 1 month before and 1 month after the haemostatic RT is shown as a bar diagram in [Figure 1]. The overall increment observed in mean Hb level was 1.09 ± 1.33g/dL (Range: −1.5 g/dL– 5.0 g/dL) P < 0.05. As a well-known fact, Hb level varies with sex and comorbidity, so a single Hb level may not reflect the acute gastric bleeding directly. To overcome this bias instead of taking a single Hb level as an endpoint for response evaluation, we compared the mean Hb level of the patient group before and after RT. We observed pre-RT mean Hb of 6.14 ± 1.01 and a post-RT mean Hb of 7.19 ± 1.75 (P < 0.001). All our patients needed transfusion of packed RBC units at some point during their treatment course and follow-up. We compared the number of transfused packed RBC units during the month before RT and 1 month after the completion of RT. Response to RT was also evident in a significant decrease in the number of packed RBC units post-haemostatic RT. The mean number of pre-RT transfused packed RBC units was 8.28 ± 3.76 and post-RT it was 4.34 ± 2.91 (P < 0.001). To see the added advantage of chemotherapy, which was received by 48 patients (64.86%) before start of RT, a sub group analysis was done and comparative survival curves for TFS and OS were calculated for RT group and CRT group [Figures 2 and 3]. There was a statistically significant survival benefit in patients who received chemotherapy in addition to haemostatic RT. The mean TFS in CRT group was 86 days (95% Confidence Interval [CI]: 67.300–105.048) and in RT group was 44 days (95% CI: 29.751–59.095); with a significant P = 0.002. The mean OS in CRT group was 101 days (95% CI: 84.144–117.939) and in RT group was 78 days (95% CI: 62.068–94.855); with a significant P = 0.047.
| Characteristics | Number (n=74) | Percentage |
|---|---|---|
| Gender | ||
| Male | 44 | 59.45 |
| Female | 30 | 40.54 |
| Age (years) | ||
| 50–59 | 24 | 32.43 |
| 60–69 years | 40 | 54.05 |
| ≥70 years | 10 | 13.51 |
| Median (Range) 60 (50–82) | ||
| Performance status (ECOG) | ||
| 1 | 8 | 10.81 |
| 2 | 12 | 16.21 |
| 3 | 34 | 45.94 |
| 4 | 20 | 27.02 |
| Location | ||
| Cardia | 25 | 33.78 |
| Fundus | 29 | 39.18 |
| Body | 15 | 20.27 |
| Pylorus | 5 | 6.75 |
| Disease status | ||
| Locally Advanced | 53 | 71.62 |
| Locally Advanced with metastases | 21 | 28.37 |
| Histopathology | ||
| Adenocarcinoma | 50 | 67.56 |
| Squamous cell carcinoma | 4 | 5.40 |
| Undifferentiated carcinoma | 10 | 13.51 |
| Signet ring cell carcinoma | 8 | 10.81 |
| Mucinous adenocarcinoma | 2 | 2.70 |
| EBRT regimen given | ||
| 15Gy in 3 fractions | 12 | 16.21 |
| 20Gy in 5 fractions | 22 | 29.72 |
| 30Gy in 10 fractions | 40 | 54.05 |
| Chemotherapy received | ||
| Yes | 48 | 64.68 |
| No | 26 | 35.13 |
EBRT: External beam palliative radiotherapy, ECOG: Eastern cooperative oncology group
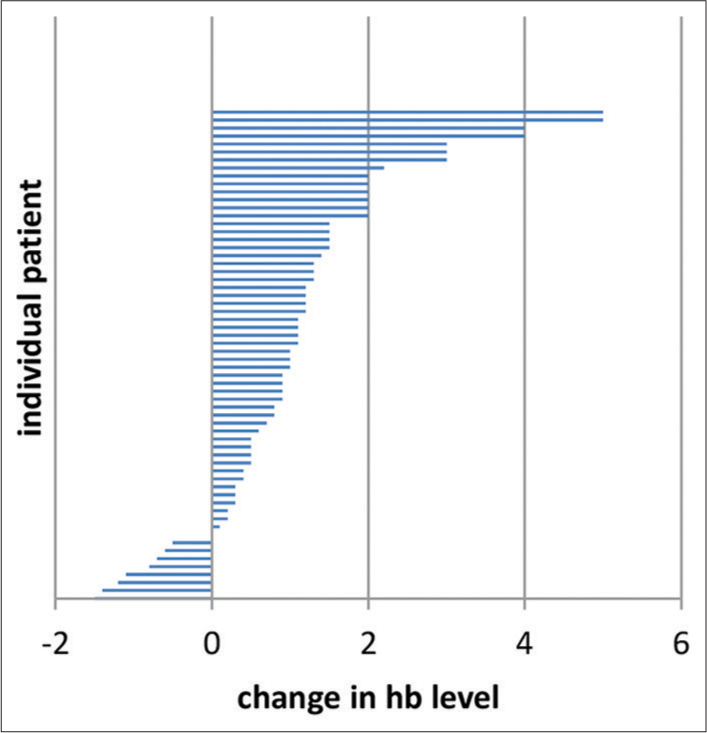
- Individual differences in haemoglobin level before and 1 month after the haemostatic radiotherapy in 74 patients. The overall increment in mean haemoglobin level was 1.09 ± 1.33 g/dL (Range: −1.5–5.0 g/dL) P < 0.05.
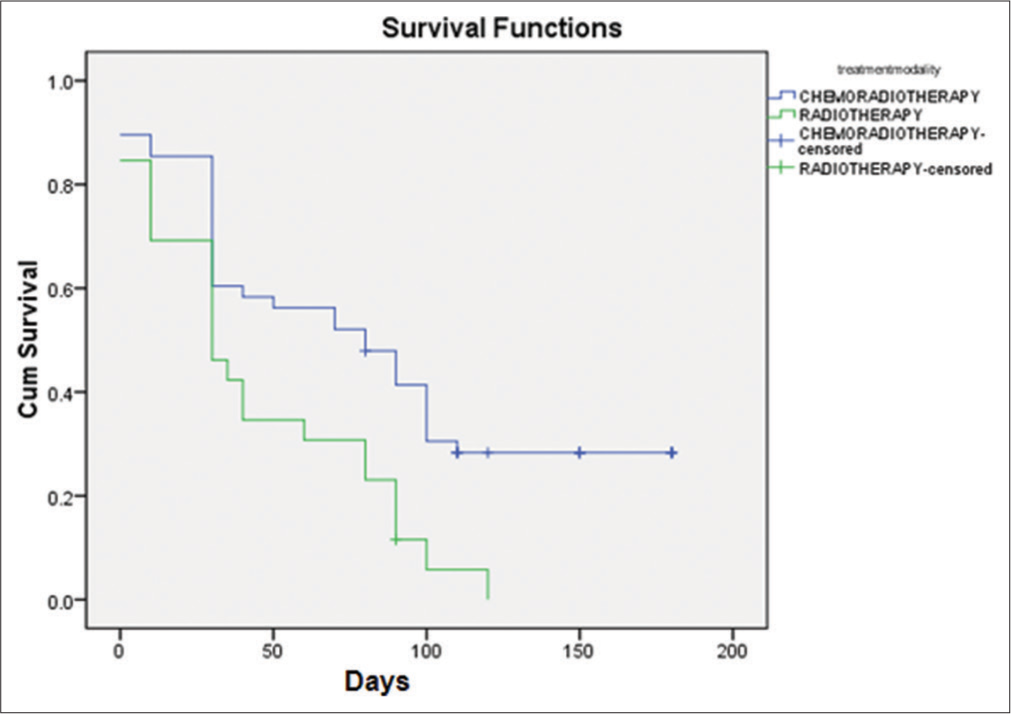
- Kaplan–Meier curve for transfusion free survival.
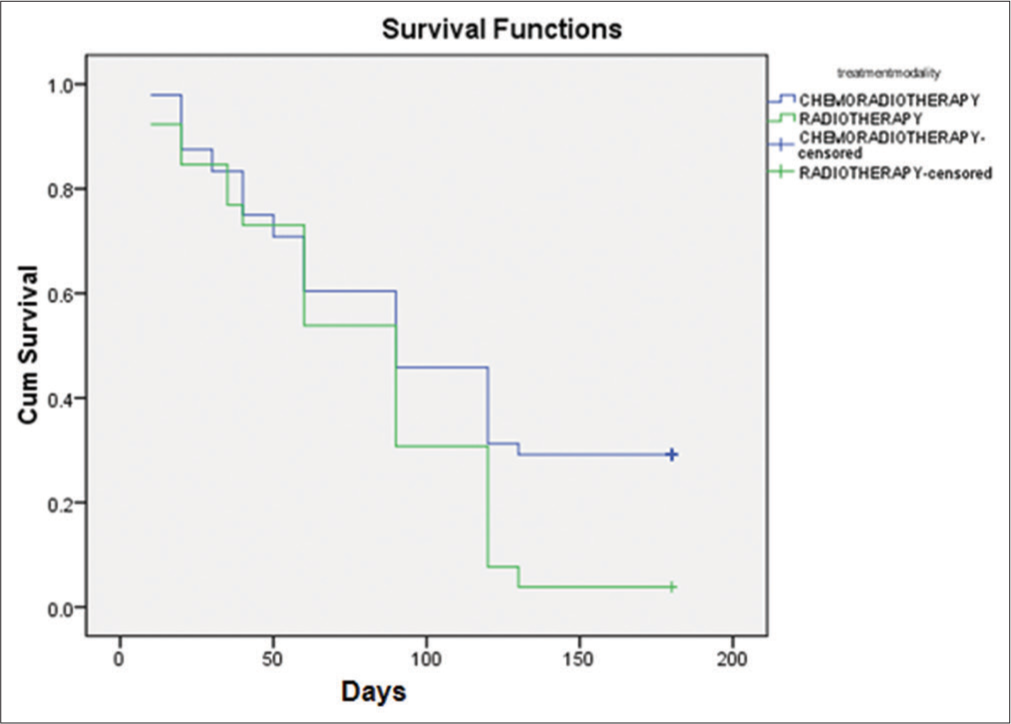
- Kaplan–Meier curve for overall survival.
Most of our patients tolerated RT without any high-grade toxicity. The toxicity profile of patients as per the Radiation Therapy Oncology Group grading system is shown in [Table 2]. We did not observe any Grade 3 or 4 toxicity during RT The median follow-up of our study was 90 days (range 10– 180 days). Kaplan–Meier survival curves for OS and TFS are shown in [Figure 4]. The median OS was 90 days and median TFS was 40 days.

- Kaplan–Meier curve for curve for transfusion free survival and overall survival in 74 patients.
| Symptom (n=74) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Nausea | 60 | 10 | 0 | 0 |
| Vomiting | 40 | 8 | 0 | 0 |
| Abdominal pain | 12 | 0 | 0 | 0 |
| Diarrhoea | 10 | 0 | 0 | 0 |
| Fatigue | 50 | 20 | 0 | 0 |
DISCUSSION
Multiple studies have confirmed the efficacy of RT in bleeding control in various malignancies such as cervical cancer, rectal cancer, bladder cancer, lung cancers and also in gastric cancers. Rasool et al. recommended from the review of 25 patients who presented with bleeding (urinary bladder cancers: 12, lung cancer: 5, cervical cancer: 4, uterine cancer: 1, rectal cancer: 2 and 1 case of schwannoma) that radiation should be considered in reducing bleeding from cancers that are directly connected to tumour invasion. He further added that shorter fraction regimens appear to be equally effective in controlling bleeding as multiple fractions.[21] RT has an established role for control of vaginal bleeding in cervical and endometrial cancers. Halle et al. used 1000 cGy in a single fraction to the pelvis repeated once or twice at monthly intervals in incurable gynaecological malignancies and achieved bleeding control in 60% of patients.[22] Biswal et al. evaluated efficacy of RT (either EBRT or Brachytherapy) in bleeding cervical cancers and achieved 100% haemostasis control of haemorrhage within 12–48 h after RT. EBRT dosage schedule given by them varied from 5 Gy in a single fraction to 20 Gy in five fractions and Brachytherapy were given a 30Gy low dose rate.[23] David et al. reviewed 99 symptomatic locally advanced rectal cancer patients, who were treated with palliative radiation. Dose-fractionation regimen ranged from 18 Gy in six fractions to 54 Gy in 30 fractions. The most prevalent fractionation scheme was 30 Gy in 10 fractions delivered at 3 Gy per fraction, five fractions/week. Out of 99 patients 83 patients presented with bleeding (with or without pain or obstruction). About 86.7% of these patients (72/83) responded to radiation therapy.[24] RT has also been effectively used in bleeding control in Gastric Cancer and reported a success rate of 50–91%.[17-20,25-27] In the present study, success rate was 70.27%. Although the haemostatic mechanism of radiation is not clearly understood, it is widely believed that radiation-induced platelet aggregation and vascular endothelial cell damage are responsible for bleeding control. Radiation leads to vascular embolisation has been shown both in vivo and in vitro.[28-30] In previously published papers, different dose fractionation schedules of RT ranging from 8 Gy in a single fraction to 50 Gy in 25 fractions have been used for bleeding control in AGC.[17-20,25-27] Asakura et al. achieved a response rate of 73% (12 of 30 patients) for bleeding from AGC with RT at a dose rate of 30 Gy in 10 fractions. They reported that 12 patients received concurrent CRT and had a significantly lower rebleeding rate than those who received RT alone (P = 0.001).[20] Our study also showed that patients who had received chemotherapy before RT have significantly prolonged TFS and OS as compared to those who received RT alone, though none of our patients received concurrent CRT. Although concurrent CRT shows better clinical outcomes than RT alone, but being more aggressive than monotherapy, it should be cautiously used in patients with good performance status only. Hashimoto et al. and Kim et al. suggested that the use of RT doses with BED (biologically effective dose) of more than 39 Gy would provide better local control and OS.[19,25] Tey et al. also favoured the use of higher doses, which tended to confer longer survival although the difference in median survival between lower and higher BED was not statistically significant.[17] A meta-analysis conducted by GA Viani et al. for gastric cancer patients, has shown that an RT dose with BED of 30 Gy or higher was associated with better haemostatic response.[31] Hence, it seems if the patient can tolerate it, a higher dose of RT is more beneficial to achieve haemostasis in gastric cancer. However, the efficacy of low-dose – short course RT has been proven by randomised and control trials in patients with inoperable non-small cell carcinoma lung and patients with carcinoma bladder.[32,33] On the other hand, Kawabata et al. showed in their study that RT given at a dose of 6 Gy in three fractions either a single course or multiple courses was safe and effective for bleeding control in patients of gastric cancer.[34] Considering these reports it seems reasonable to consider a short course of RT for some patients with a limited prognosis. In our study, the radiation fractionation schedule received by patients was at the discretion of the treating radiation oncologist, who had given due consideration to patient prognosis and life expectancy; taking into account patients’ age, performance status, disease stage and expected toxicity. However, further studies are needed to validate such discretion. In the present study, we have used RT dose schedules with BED10 (assuming the alpha/beta ratio was 10) ranging from 22.5 Gy (15 Gy in three fractions) to 39 Gy (30 Gy in 10 fractions). Various studies have reported the response to RT by reviewing the pre- and post-RT Hb levels and also by the decrease in the number of transfusion units or the amount of blood transfused after RT. The cutoff point of 4 weeks used by these authors for response assessment seems arbitrary.[19,20,30] In our study, response to RT was evaluated by the change in Hb levels observed 4 weeks before and 4 weeks after completion of RT. To assess the efficacy of RT for bleeding control, we adopted a similar approach. We observed a statistically significant (P < 0.05) increase in the level of haemoglobin and a decrease in the need for transfusions post-RT following published data.[19,30]
Our study has some limitations: First, there is a chance of bias because of the retrospective nature of the study with a small number of patients; second, the RT dose schedule given was the physicians’ choice, so we could not do any subgroup analysis to comment on the superiority of one regimen over other. Selection criteria used by physicians for particular dose schedule need further studies for their validation.
CONCLUSION
Our study results suggest that palliative RT may be an effective and safe treatment option for controlling bleeding in patients with AGC who are inoperable or unfit for surgery, especially in countries where other haemostatic procedures are not an easy option because of cost and availability factor. Further studies (randomised and control trials) are necessary to establish an ideal RT dose regimen and to choose patients most appropriately.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. Erratum in: CA Cancer J Clin 2011; 61:134
- [CrossRef] [PubMed] [Google Scholar]
- Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. Erratum in: CA Cancer J Clin 2014;64:364
- [CrossRef] [PubMed] [Google Scholar]
- GLOBOCAN 2020 Global Cancer Observatory. 2022. France: International Agency for Research on Cancer; Available from: https://www.gco.fr [Last accessed on 2022 Nov 15]
- [Google Scholar]
- Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-30.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-20. Erratum in: N Engl J Med, 2008;358:1977
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-21.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-84.
- [CrossRef] [PubMed] [Google Scholar]
- Management of bleeding in patients with advanced cancer. Oncologist. 2004;9:561-70.
- [CrossRef] [PubMed] [Google Scholar]
- Interstitial laser photocoagulation for treating bleeding gastric cancer. BMJ. 1989;299:659-60.
- [CrossRef] [PubMed] [Google Scholar]
- Laser photocoagulation in the palliative treatment of upper digestive tract tumors. Cancer. 1986;57:396-9.
- [CrossRef] [PubMed] [Google Scholar]
- Severe upper gastrointestinal tumor bleeding: Endoscopic findings, treatment, and outcome. Endoscopy. 1996;28:244-8.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic treatment of major bleeding from advanced gastroduodenal malignant lesions. Mayo Clin Proc. 1994;69:736-40.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors affecting outcomes in patients with malignant GI bleeding treated with a novel endoscopically delivered hemostatic powder. Gastrointest Endosc. 2018;87:994-1002.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal bleeding: Treatment with gastrointestinal arterial embolization. Radiology. 1992;183:505-8.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal hemorrhage in hepatocellular carcinoma: Management with transheptic arterioembolization. Abdom Imaging. 2000;25:380-4.
- [CrossRef] [PubMed] [Google Scholar]
- The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:385-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore). 2014;93:e118.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative radiation therapy for hemorrhage of unresectable gastric cancer: A single institute experience. J Cancer Res Clin Oncol. 2009;135:1117-23.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative radiotherapy for bleeding from advanced gastric cancer: Is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011;137:125-30.
- [CrossRef] [PubMed] [Google Scholar]
- Hypofractionated radiotherapy as local hemostatic agent in advanced cancer. Indian J Palliat Care. 2011;17:219-21.
- [CrossRef] [PubMed] [Google Scholar]
- 1000 cGy single dose palliation for advanced carcinoma of the cervix or endometrium. Int J Radiat Oncol Biol Phys. 1986;12:1947-50.
- [CrossRef] [PubMed] [Google Scholar]
- Hemostatic radiotherapy in carcinoma of the uterine cervix. Int J Gynaecol Obstet. 1995;50:281-5.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of palliative radiation therapy for symptomatic rectal cancer. Radiother Oncol. 2016;121:258-61.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008;47:421-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: A single-institution experience. Ecancermedicalscience. 2014;8:384.
- [Google Scholar]
- Radiation therapy for gastric cancer bleeding. Tumori. 2009;95:726-30.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular-bed--specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555-64.
- [CrossRef] [PubMed] [Google Scholar]
- The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746-60.
- [CrossRef] [PubMed] [Google Scholar]
- Experimental thrombosis model induced by free radicals. Application to aspirin and other different substances. Thromb Res. 1995;79:109-23.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative radiotherapy for gastric cancer: Is there a dose relationship between bleeding response and radiotherapy? Clinics (Sao Paulo). 2020;75:e1644.
- [CrossRef] [PubMed] [Google Scholar]
- Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer. 1991;63:265-70.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: Results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47:379-88.
- [CrossRef] [PubMed] [Google Scholar]
- Experience of low-dose, short-course palliative radiotherapy for bleeding from unresectable gastric cancer. J Palliat Med. 2017;20:177-80.
- [CrossRef] [PubMed] [Google Scholar]






