Translate this page into:
Cancer-Related Fatigue – Clinical Evaluation Scales and Interventions: A Systematic Review
*Corresponding author: Athar Javeth, Department of Medical Surgical Nursing, College of Nursing, All India Institute of Medical Science, Patna, Bihar, India. atharjaveth05@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: D’Silva F, Javeth A, Singh P. Cancer-related fatigue – Clinical evaluation scales and interventions: A systematic review. Indian J Palliat Care 2022;28:88-98.
Abstract
Background:
Cancer-related fatigue (CRF) is one of the most frequent and prevalent symptoms expressed by cancer patients and cancer survivors. It is a multifactorial phenomenon that causes a direct detrimental impact on quality of life.
Objectives:
This systematic review aims to identify different clinical evaluation scales and interventions available for fatigue associated with cancer.
Materials and Methods:
A methodology of the systematic literature review was carried out. Two separate databases PubMed and Google Scholar searches were performed using different MeSH terms.
Results:
A total of 2611 research articles were screened and identified 10 unidimensional scales (four with one item scales and six with numerous item scales) and 13 multidimensional scales which are available for the screening and clinical evaluation of fatigue. Reviews have also revealed non-pharmacological interventions such as exercise, complementary therapies, nutritional and psychoeducational interventions, sleep therapy, energy therapy, bright white light, restorative therapies upcoming anthroposophical medicine, and various pharmacological agents effective in managing CRF.
Conclusion:
Clinical evaluation of fatigue and its management is crucial for improving the quality of life. Yet, more rigorous research studies with higher statistical power need to be conducted on these interventions to generate adequate evidences for managing the CRF.
Keywords
Cancer
Clinical evaluation scales
Interventions
Fatigue
Systematic review
INTRODUCTION
Symptom clusters in cancer refer to multiple symptoms which appear in clusters or groups in patients with cancer undergoing treatment.[1] These can be categorised as the first symptom cluster or second symptom cluster.[2] The first symptom cluster comprises psychological and general symptoms. Psychological symptoms include anxiety and depression whereas general symptoms include loss of appetite, fatigue, dyspnoea, and insomnia. The second symptom cluster involves physical symptoms such as adverse effects, pain, and GI symptoms which involve nausea, vomiting, diarrhoea, and constipation.[2] Assessment of symptom cluster is of prime importance for the patients undergoing chemotherapy.[3] The severity of symptom cluster has an unfavourable impact on the quality of life and physical functioning of the cancer survivors.[3-5] The most frequent symptom prevalent in the symptom clusters is fatigue followed by depression and psychological distress among cancer survivors.[6]
Cancer-related fatigue (CRF) and its prevalence
As per the National Comprehensive Cancer Network, ‘Cancer-related fatigue is distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and it interferes with usual functioning.’[7] In the words of Cella et al. (1998), ‘Cancer- related fatigue is the subjective state of overwhelming, sustained exhaustion and decreased capacity for physical and mental work that is not relieved by rest.’[8] A patient’s level of fatigue varies during the day, according to their treatment, and follows a pattern related to the course of the day. Cancer patients and cancer survivors cite fatigue as the most disturbing and disabling of symptoms.[7]
Patients with cancer report frequent fatigue as a symptom of their disease.[8-10] Among patients undergoing chemotherapy or radiation therapy, fatigue ranked highest along with pain (48%) and nausea/vomiting (48%) as distressing symptoms.[10-12] Fatigue was identified as the top-rated; high ranked, and the most concerning symptom among patients with advanced cancers (10 out of 11 types of various cancers).[13] Thus, the variability in fatigue prevalence among cancer types is high, ranging from 25% to 100%.[14,15] Period before, during, and after treatment has also played a major role in the experience of fatigue by the patients.[16,17]
Although a most prevalent symptom, fatigue from cancer is one of the understated, underestimated, and undermanaged symptoms which are least taken into consideration by the health professionals while managing cancer patients. Fatigue associated with cancer has a detrimental effect on the quality of life, adherence to treatment, productivity, or efficiency. Hence, this review article highlights the importance of clinical evaluation and appropriate intervention for managing fatigue. The prime objective of this review was to explore the different scales utilised for the screening and assessment of fatigue associated with cancer. The second objective was to identify the various non- pharmacological and pharmacological interventions which aid in the mitigation of fatigue. Therefore, this systematic review included all types of research article to provide comprehensive evidence about the scales and multiple interventions options for CRF.
METHODS
Literature search as a systematic process was executed based on Preferred Reporting Items for the Systematic Reviews and Meta-Analysis guidelines published in 2009.[18] Ethical clearance was not needed for systematic reviews.
Data sources and search strategy
A broad systematic search of two separate databases, that is, PubMed and Google Scholar was carried out to recognise all the available and relevant full-text articles dating from 2000 to December 2020. The key words such as ‘Scales,’ ‘Tools,’ ‘Assessment,’ ‘Interventions,’ ‘Management’ and ‘Cancer-related Fatigue’ were merged using the Boolean operators (‘AND’ and ‘OR’). [Table 1] represents the search combination of keywords and Medical Subject Heading (MeSH) terms. Hand searches of books and grey literature of the relevant articles were also conducted. This review aimed to examine and summarise the evidences related to CRF scales and interventions.
| MeSH terms related to scales, tools and assessment | MeSH terms related to interventions and management | MeSH terms related to cancer | MeSH terms related to fatigue |
|---|---|---|---|
| Scales OR Measure OR tools OR Assess OR Assessment OR Measure | Interventions OR Interventional OR Methods OR Manage OR Management OR Administration Or Disease Management | Cancer OR Cancerous OR Neoplasm OR carcinoma | Fatigue Or Fatigability OR Fatigable |
MeSH: Medical subject heading
Eligibility criteria
Inclusion criteria for the articles are as follows:
Populations: Adult patients who were more than 18 years undergoing any treatment for cancer
The outcome of interest: Scales or tools for the evaluation and interventions for fatigue management
Type of research articles: All types of research articles including the grey literature
Articles published only in the English language.
Exclusion criteria
Research articles involving paediatric population
Research studies involving participants without cancer
Articles published in other languages other than English
Research protocols and case reports.
Methodological quality assessment of articles
The methodological quality and risk of bias of the included articles were determined by Joanna Briggs Institute critical appraisal tools, a quality assessment tool. Different critical appraisal tools were used for different types of articles such as tool development studies, RCTs, systematic reviews or reviews, or opinions. Ratings are made as ‘Yes,’ ‘No,’ ‘Unclear’ or ‘Not/Applicable’ for the domains of quality appraisal tools.[19]
RESULTS
The systematic search of the literature identified a total of 2611 records from two databases PubMed and Google Scholar and one from the additional source. The duplicate records were identified and excluded, 2203 articles were retained for the title and abstract screening. After the exclusion of irrelevant title and abstract and non-relevant articles, 231 articles were identified as potentially eligible articles. Full-text analysis of these 231 articles identified 38 articles finally met the inclusion criteria for the present review as depicted in [Figure 1].
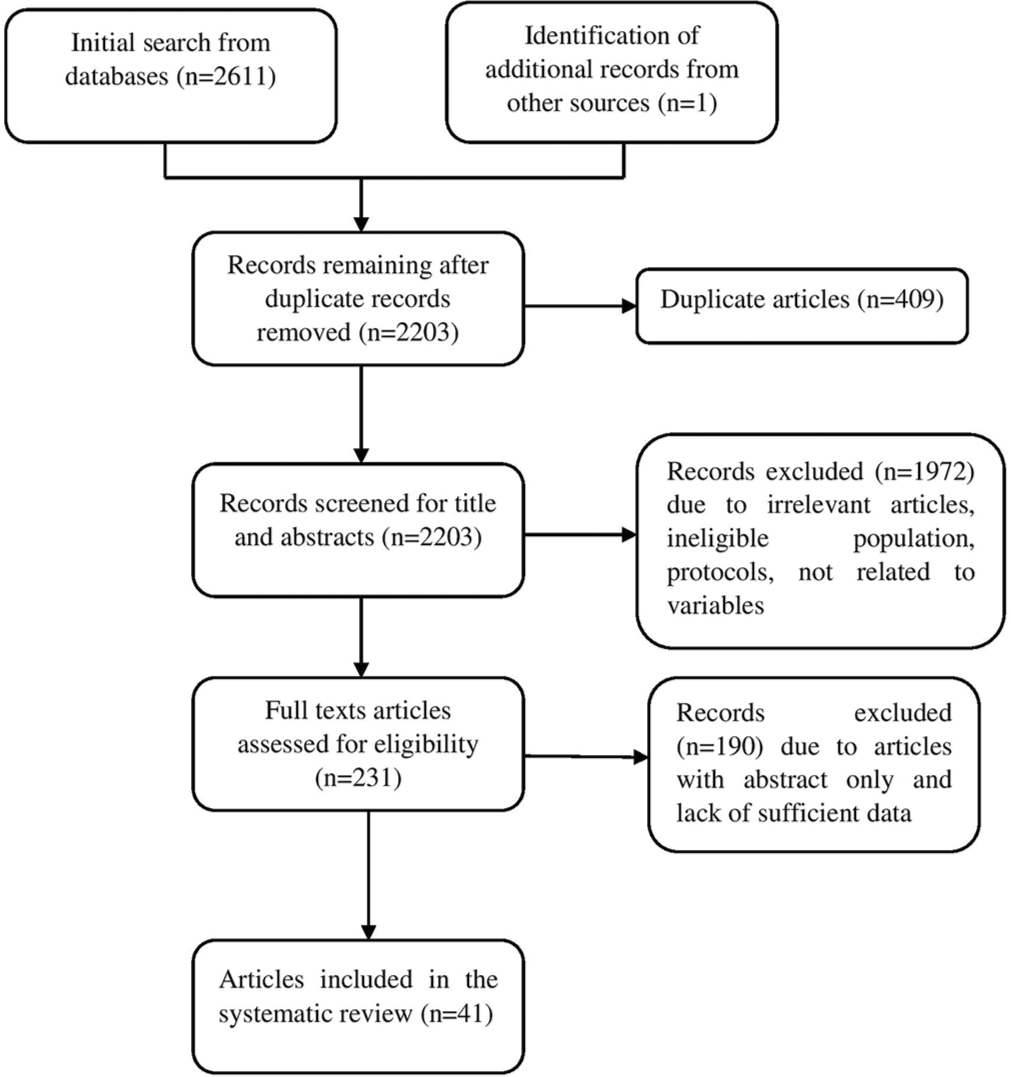
- Preferred Reporting Items for the Systematic Reviews and Meta-Analysis flowchart of literature search.
Data synthesis (characteristics of included articles) related to scales for the assessment of CRF
The extracted data were synthesised under the following headings such as:
Details of publication: Author’s last name and year of publication
Types of the research article
Sample characteristics: Type of cancer
Outcome measurement: Type of scale which assessed the CRF and the interventions.
A qualitative review was done by the reviewers based on the data extracted and presented the attributes of the included articles condensed in the form of narrative synthesis in [Tables 2 and 3].
| Author and year | Type of article | Subjects characteristics – type of cancer | Scales described | Major findings of the article |
|---|---|---|---|---|
| NCCN, 2020[7] | Review article –guidelines | Cancer population | Unidimensional scales with one item or several items and multidimensional scales | Psychometric properties of various unidimensional and multidimensional scales |
| Piper et al., 2008[20] | Review article | Cancer population | Unidimensional scales with one item or several items and multidimensional scales | Screening and measurement scales for fatigue validated in patients with cancer. Vital role of nurses for the assessment, documentation and on-going monitoring of CRF |
| Strebkova et al., 2017[21] | Review article | Cancer population | Unidimensional and multidimensional scales | Available and practical tools for the determination of fatigue |
| Minton and Stone, 2009[22] | Systematic review | Cancer population | Unidimensional and multidimensional scales | Recommendation for the utilisation of EORTC QLQ C30 subscale (fatigue) or the FACT fatigue scales. Fatigue questionnaire provides a multidimensional picture of fatigue. |
| Fisher et al., 2018[23] | Systematic review | Cancer population | Unidimensional and multidimensional scales | Numeric rating scale is best rating scale and multidimensional scale is more recommended tool |
| Ryan[24] | Grey literature | Cancer rehabilitation including other population | Fatigue severity scale | Evaluate fatigue severity and its impact on various aspects of life of patients with cancer and other neurological disorders. |
| Okuyama et al., 2000[26] | Diagnostic article – psychometric Properties | Cancer population | Cancer fatigue scale | Brief, valid and reliable tool to measure fatigue among cancer patients. |
| Borneman, 2013[25] | Review article | Cancer population | One item and several items unidimensional scales and multidimensional scales | Subjective nature of CRF and contributing factors should be incorporated along with the assessment of CRF |
| Beutel et al., 2006[27] | Diagnostic article – psychometric properties | German cancer population | Fatigue assessment questionnaire | Good reliability and validity of fatigue assessment questionnaire. Age and gender are the factors affecting fatigue |
| Al Maqbali et al., 2020[28] | Diagnostic article – psychometric properties | Arabian cancer patients | Functional assessment of chronic illnesses therapy (FACIT-F) fatigue subscale | FACIT F has good reliability and validity in assessing fatigue in cancer patients |
| Cella et al., 2008[29] | Diagnostic article –psychometric properties | Nonmyeloid malignancies patients and anaemia patients receiving chemotherapy |
Fatigue and functional impact scale | Evaluation of fatigue and its impact is possible due to its reliability and practicality |
| Jacobsen, 2004[59] | Review article | Cancer population | Multidimensional fatigue scales | Multidimensional scales provide the possibility of the assessment of clinical syndrome of fatigue. Factors affecting the appropriate selection of scales |
| Author and year | Type of article | Intervention | Type of participants | Main findings of the article |
|---|---|---|---|---|
| Kirshbaum, 2010[30] | Review article | Exercise, pharmacological approaches, adjustment strategies, complimentary therapies, psychological and nutritional education | Cancer population | Extensive review of various nursing interventions |
| Lavdaniti, 2019[31] | Review article | Nursing management of patient with cancer-related fatigue | Cancer population | Pharmacological and non-pharmacological management including the nursing interventions |
| Stefani et al., 2017[32] | Review article – guidelines | Different types of exercise and dietary interventions for cancerrelated fatigue | Cancer survivors | Evidence-based guidelines for the comprehensive post-cancer treatment rehabilitation programmes for the cancer survivors |
| Mustian et al., 2007[33] | Review article | Exercises, mindfulnessbased stress reduction MBSR, yoga, sleep therapy, nutritional therapy, restorative therapy and polarity therapy | Cancer population | Effectiveness of multiple nonpharmacological behaviouralinterventions |
| Cohen et al., 2004[34] | Randomised controlled trial | Tibetan yoga | Patients with lymphoma | Improvement of sleep quality but no significant betterment in anxiety, fatigue and depression. |
| Cassileth and Vickers, 2004[35] | Experimental design | Different massages – standard massage, light touch massage and foot massage | Cancer population | Significant bettermentin symptom scores such as pain, fatigue, stress/anxiety, nausea and depression |
| Stasi et al., 2003[36] | Review article | Pharmacological (erythropoietin, antidepressants, hypnotics and aerobic exercises) and non-pharmacological agents | Cancer population | Patient education, aerobic exercise and psychostimulants are effective in managing the cancerrelated fatigue. |
| Molassiotis et al., 2007[37] | Randomised controlled trial | Acupuncture and acupressure | Cancer patients with moderatetosevere fatigue | Significant betterment in general fatigue and physical fatigue in both acupuncture and acupressure groups |
| Vickers et al., 2004[38] | Randomised clinical trials | Acupuncture | Patient with cancer completed cytotoxic chemotherapy | Betterment in fatigue |
| Tsang et al., 2007[39] | Pilot crossover design | Reiki therapy versus rest | Mixed cancer population in Stages I–IV | Reiki group experienced significant decrease in fatigue, pain and anxiety |
| Ravasco et al., 2005[40] | Randomised controlled trial | Dietary counselling | Colorectal cancer patients | Significant betterment in quality of life, fatigue including the other symptoms. |
| Yarbro et al., 2010[41] | Book | Pharmacological and nonpharmacological interventions | Cancer population | Improvement in fatigue |
| Dirksen and Epstein, 2007[42] | Randomised controlled trial | Insomnia intervention cognitive behavioural therapy | Women with breast cancer | Significant betterment in fatigue, depression, anxiety and quality of life. |
| Mohandas et al., 2017[43] | Review article | Non-pharmacological (selfcare strategies) treatment and pharmacological management | Cancer population | Inadequate evidence related to effectiveness of self-care strategiesdue to methodological issues. |
| Roscoe et al., 2005[44] | Pilot study | Polarity therapy | Breast cancer women undergoing radiation therapy | Polarity therapy is effective, non- invasive and nonpharmacological measure for fatigue |
| Fu et al., 2020[48] | Systematic review | Anthroposophical medicine – art therapy | Women with gynaecological cancers | Insufficient evidence. Recommends for the more vigorous research |
| Agteresch et al., 2000[49] | Randomised clinical trials | Adenosine 5’triphosphate | Advanced nonsmallcell lung cancer patients | Improvement in physical and functional scores of quality of life |
| Salehifar et al., 2020[50] | Randomised clinical trial (doubleblind placebo) | Bupropion | Cancer patients with fatigue | Significant betterment in fatigue at 6 weeks |
| Ashrafi et al., 2018[51] | Randomised controlled trial (double blind placebo) | Bupropion sustained release | Patients with fatigue due to cancer | Significant improvement in fatigue |
| Shaw et al., 2006[52] | Randomised clinical trials Phase II | Donepezil drug | Brain tumour patients underwent irradiation | No significant improvement in physical score and functional score |
| Radbruch et al., 2008[53] | Review article | Methylphenidate, donepezil, modafinil and steroids | Cancer population | Pharmacological and non-pharmacological management of symptomatic fatigue. |
| Cruciani et al., 2006[54] | Phase I/II openlabel trial | Lcarnitine | Adults with advanced cancer | Significant improvement in fatigue and performance status |
| Yeom et al., 2007[55] | Prospective study | Vitamin C | Patients having terminal cancer | Significant betterment in fatigue, nausea/vomiting, pain and appetite |
| Bohlius et al., 2014[56] | Systematic review and metaanalysis | Erythropoietinstimulating agents | Cancer population | Promising benefits of drugs that stimulate erythropoietin for the betterment of fatigue and quality of life |
| Tomlinson et al., 2018[57] | Systematic review and metaanalysis | Various pharmacological agents | Cancer population | Erythropoietin and methylphenidate have significant impact on fatigue severity in cancer patients and recipients of stem cell transplant patients |
| Cella et al., 2003[58] | Review article | Erythropoietic agents | Cancerrelated anaemia patients | Improvement in energy level, level of activity and health-relatedquality of life |
Clinical evaluation scales used for measuring CRF
This systematic review revealed two types of scales, namely unidimensional and multidimensional scales that is, four single-item unidimensional scales, six multiple item unidimensional scales, and 13 multidimensional scales from different types of research articles. The research articles comprised five review articles, four research articles related to psychometric properties, two systematic reviews, and one grey literature. Before, during and after treatment, it is recommended that cancer-related fatigue be assessed at regular intervals.
Unidimensional scales deal with the examination of fatigue in terms of severity and its existence. Different single-item unidimensional scales are as follows:
Single-item unidimensional scales
NCCN fatigue intensity scale – National Comprehensive Cancer Network developed this fatigue severity screening tool (NCCN, 2018). It aids to screen the severity of fatigue by rating among cancer patients at regular intervals on a scale of 0–10. The score of ‘0’ represents an absence of fatigue and 10 represents worst fatigue. According to this scale, 0–3, 4–6, and 7–10 indicate no or mild fatigue, moderate fatigue, and severe fatigue, respectively[7]
The fatigue intensity scale – This scale is similar to NCCN fatigue intensity scale. The score ‘0’ depicts no fatigue and score 10 depicts overwhelming fatigue. It is a single-item and single dimension scale used for the screening of fatigue in cancer patients. It has strong criterion validity estimates and strong concurrent validity with the piper fatigue scale revised[20]
Rhoten fatigue scale – it can be recommended for screening purposes. The dimension of this scale is severity. A score of 0 represents not being tired or full of energy and 10 represents totally exhausted[20]
Visual analogue scale – Visual analogue scale for fatigue (VAS-F) is a 10 cm scale of the horizontal line (0–100 mm) for the assessment of the severity of fatigue. The score ‘0’ indicates ‘I don’t feel tired’ and score 10 indicates ‘I feel totally exhausted.’ This scale is predominantly tested in cancer patients of Switzerland and Germany. Similar to other VAS, it is also recommended to consider the measurement characteristics while using it for research screening and clinical practice.[20]
There are numerous multiple item unidimensional scales [Table 4] available for the assessment of fatigue.[20-25]
| Scale | No. of items | Characteristics | Reliability and validity | Population | Dimension |
|---|---|---|---|---|---|
| Brief Fatigue Inventory[20-23] | 9 | Examines fatigue in the previous 24 h Requires less than 5 min to respond to the questionnaire |
Internal consistency is 0.96 Construct and discriminant validity established |
Mixed cancers | Physical functioning |
| EORTCQLQ FS[20-23] | 3 | Part of quality of life scale. Takes 10 min to complete. Determines the fatigue over the past week. Produces ceiling effect in advanced cancers |
Internal consistency is 0.85 Convergent validity is established |
Patients with lung cancer, metastatic cancers and bone marrow transplant | Physical functioning |
| Functional assessment of chronic illness therapy (FACIT F) – Fatigue subscale[21-23] | 13 | Part of quality of life scale FACT G scale. Assess the fatigue over the past 7 days. Requires not more than 15 min to complete | Internal consistency is 0.95 Convergent validity is established |
Mixed cancers | Physical functioning |
| Fatigue severity scale[21-24] | 9 | Differentiates fatigue from depression. Determines the fatigue over the past 7 days. Takes not more than 5 min to finish | Internal consistency is 0.96 Convergent validity established |
Mixed cancers | Physical functioning |
| Wu cancer fatigue scale[21-23] | 9 | Utilised in both clinical and research settings. Examines the fatigue over the past 24 h. Need less than 5 min to finish | Internal consistency is 0.91 Concurrent and convergent validity established |
Breast cancer population | Physical and mental fatigue |
| Fatigue assessment scale[25] | 10 | Requires less than 5 min to complete. Assesses the present fatigue level | Internal consistency is 0.88 Content and construct validity |
Dutch cancer patients | Physical and mental fatigue |
Multidimensional scales are the scales which measure the impact of fatigue on various domains such as physical, psychological, emotional, social, and affective functioning of patients with cancer. Different multidimensional scales used for the evaluation of fatigue are depicted in [Table 5].[20-23,25-29]
| Scale | No. of items | Characteristics | Reliability and validity | Population | Dimension |
|---|---|---|---|---|---|
| Cancer fatigue scale (numerical rating scale)[20,23,26] | 15 | Describes the fatigue of current situation. Less than 2–3 min to complete | Internal consistency is 0.88 Construct and convergent validity established |
Mixed cancers in different stages | Physical, affectiveand cognitive |
| Cancerrelated fatigue distress scale (Likert’s scale)[20,23] |
20 | Determines the distress related to fatigue over the past 7 days. Ten minutes to complete | Internal consistency is 0.98 Concurrent and construct validity established CVI=0.6–1.00 |
Breast cancer population receiving chemotherapy | Physical, social, psychological and spiritual distress |
| Lee fatigue scale/VAS fatigue (numerical rating scale)[20,22] | 13 | Simple and easy administration and scoring Psychometric properties are minimal | Internal consistency is 0.91 Convergent validity is established |
Sleep disorder patients | Energy and fatigue |
| Chalder fatigue scale/fatigue questionnaire (Likert’s scale)[20-22] | 11 | Use of scale and scoring is easy. Bimodal scoring and high ceiling effect can be seen | Internal consistency is 0.88–0.9 Convergent validity established. |
General population | Physical and mental |
| Multidimensional fatigue inventory MFI (Likert’s scale)[20,22,23] |
20 | Examines fatigue over the previous 24 h. Need not more than 10 min to finish | Internal consistency is 0.84 Convergent and discriminant validity is established |
Mixed cancer population | Cognitive, physical and emotional |
| Multidimensional fatigue symptom inventory MFSI (Likert’s scale)[22,23] | 30 | Ease of use and scoring are of moderate level. Requires 10 min to complete the inventory | Internal consistency is 0.87–0.96. Convergent, concurrent and discriminant validity established | Breast cancer population | Cognitive, physical and mental |
| Fatigue symptom inventory FSI (numerical rating scale)[22,23] | 13 | Frequency, severity and disturbance in life associated with fatigue are identified. Five minutes to complete the inventory | Internal consistency is 0.94 Convergent, concurrent and discriminant validity is established |
Patients undergoing treatment for breast cancer | Physical and mental |
| Piper fatigue scale revised PFR (Likert’s scale)[20,22,23] | 22 | Examines the current state of fatigue. Takes 5 min to administer it. Most commonly used scale in cancer as well as healthy individuals | Internal consistency is 0.97 Convergent validity, content validity and construct validity is established |
Patients with breast cancer | Behavioural/intensity, cognitive, affective and sensory |
| Schwartz cancer fatigue scale (Likert’s scale)[20,22,23] | 28 | Evaluate the fatigue over the past 2–3 days. Four minutes to complete the scale |
Internal consistency is 0.96 Convergent and discriminant validity established |
People with mixed cancers | Physical, cognitive and emotional |
| Fatigue assessment questionnaire (4point rating scale)[20,26,27] | 20 | Measure the fatigue over the past week. Unknown time to finish it | Internal consistency is 0.95 Validity established with correlation |
People with mixed cancer | Physical, cognitive and affective |
| Patient Reported Outcome Measure Information System Cancer Fatigue Short Form 3 (Likert’s scale)[23] | 7 | Examines the fatigue over the past 7 days. Undetermined time to complete the test Easy to administer and score |
Internal consistency is 0.87–0.88 Convergent and discriminant validity is established |
Population with haematological malignancies and prostate cancer | Fatigue intensity and severity including the disruption in daily activities |
| Multidimensional assessment of fatigue (numerical rating scale)[20,22] | 16 | Prepared from the piper fatigue scale (original version). Used for population with various disease including cancer. Fifteen items are used to assess global fatigue index. Five minutes to complete | Internal consistency is 0.88 Content validity is established. No appropriate construct validity established |
Initially done in arthritis patients later done in mixed cancer population | Severity, distress, disruption in ADLs and frequency |
| Fatigue and functional impact scale (Likert’s scale)[28,29] |
8 | Easy scoring and administration. 2–3 min to complete | Internal consistency is 0.9 Content and construct validity established |
Mixed cancer people receiving chemotherapy | Fatigue and its impact |
Interventions for CRF
Regarding the interventions, this systematic review identified various non-pharmacological and pharmacological approaches for the treatment of fatigue. Out of 29 research articles related to interventions, there were seven review articles, four systematic reviews and meta-analysis, 10 RCTs, one experimental research, two pilot study, one book, one prospective research study and three grey literature. Interventions for fatigue can be grouped into non-pharmacological and pharmacological measures. The non-pharmacological interventions are as follows:
Exercise
Exercise intervention has a beneficial impact on physiological process as well as quality of life. Exercise has a direct impact on cardiorespiratory status, promotes the well-being and reduces chances of mortality related to cancer. It is recommended to undergo 150 min of moderate-intensity exercise or 75 min of vigorous intensity exercise in a week. Exercise can be walking, cycling, running, bowing or any aerobic exercises. It should be planned, spread throughout the week for short intervals of 10 min.[30-32]
Complementary therapies
The present-day yoga form involves the combination of physical activity, yoga asanas and mindfulness. Patients with cancer are able to manage cancer-related fatigue more effectively through yoga and enhance their quality of lives.[33,34] ‘Yoga is considered as the viable therapeutic intervention for CRF.’[33,34] Mindfulness-based stress reduction programme is a form of complementary therapy that helps to improve the health and well-being of the individuals. In the 1970s, Kabat-Zinn developed MBSR programme.[33] Majority of complementary therapies are found to have a beneficial influence on fatigue and the patients’ quality of life. Complementary therapies such as aromatherapy, acupressure and acupuncture, foot soak with reflexology, massage and Reiki therapy are providing promising results in treating CRF, but limited studies are available for developing the frame of evidence with greater significant results.[35-39]
Nutritional intervention
One of the contributing factors for the CRF is malnutrition. Anorexia cachexia syndrome is usually experienced by the cancer patients and it predisposes to malnutrition in them. Adverse effects associated with treatments include nausea, vomiting, stomatitis, diarrhoea and mucositis which contribute to malnutrition and aggravate the fatigue associated with cancer. Dietary counselling aided in the reduction of fatigue rather than providing the protein supplements to cancer patients as stated by Ravasco et al.[40] It not only increased the nutritional status of patients but also improved the CRF.[35,40]
Educational interventions (psychoeducation)
Education intervention combined with psychological support is termed as psychoeducation. It has a positive influence on fatigue, its pattern, self-management, supportive positive coping (counselling) and coordinated care. This intervention aids in enhancing the motivation and empowering the patients for self-care, positive coping and provides opportunity to improve the self-efficacy and emotional control. Supportive counselling promotes social support with assistance in coping.[33,41]
Cognitive behavioural therapy
Fatigue associated with cancer can be managed effectively by cognitive behavioural therapy by indirectly acting on the concurrent symptoms such as sleep problems, pain and depression. Randomised controlled trials in metastatic breast cancer population demonstrated that CBT administered for depression significantly resulted in the reduction of CRF.[33,41,42]
Sleep therapy
One of the persistent causes of fatigue is sleep disorders which can be hypersomnia or insomnia. It results in reduced nocturnal quality of sleep and ultimately leads to fatigue throughout the day. Providing guidance on sleep hygiene such as consistent time of sleep and rise in every morning, reduced time spent in the bed and limited day time naps is the aspects of sleep hygiene. The sleep therapy incorporates sleep hygiene measures and contributes in minimising fatigue.[41,43]
Polarity therapy/energy therapy
Dr. Randolph Stone developed an innovative energy therapy commonly known as Polarity therapy in the year 1947. It incorporates the balance of energy field within the organisms and enhances the health and well-being of the individual. Researchers evaluated the significance of polarity therapy in alleviating fatigue and found it to be useful among survivors of breast cancer. More number of RCTs are needed to demonstrate the effectiveness of energy therapy.[41,44]
Bright white light therapy
Bright white light therapy is a therapeutic intervention in which very high fluorescent light is emitted from light box and patients are exposed to it. It is available for purchase and can be used at home. It is commonly deployed for the treatment of sleep and mood disorders in elderly group and in the general public. Breast cancer patients undergoing chemotherapy are exposed to bright white light therapy and a favourable outcome was seen in CRF. Ideal time of administering BWLT is early morning for the duration of 30–90 min. It is recommended for the patients experiencing fatigue during active treatments.[7]
Restorative therapy
Restorative therapy is the use of restorative activity aids in managing the mental or attentional fatigue. It was developed by Kaplan. Engagement in activities such as music therapy and natural environment helps in the restoration of feeling of mental peace. Enjoying the endeavour from the initiation and focus on new assignment and challenges is also a part of restorative therapy.[33] More research studies are recommended to identify the effectiveness of restorative therapy for fatigue management with higher statistical power.
Anthroposophical medicine
Anthroposophical medicine is integrative medicine which is a type of alternative system of medicine. It was originated in the early 1920s by Rudolf Steiner and Ita Wegman. It comprises art therapy (painting or drawing), music therapy, sculptures, therapeutic speech and eurythmy therapy.[45-47]
Eurythmy therapy is an expressive art movement which attempts to reintegrate the components body, soul and spirit and thereby enhance the health-related life functions.[48] Therapeutic speech also claims to improve the fatigue, as there is no sufficient research evidence to support eurythmy therapy and therapeutic speech for its utilisation in clinical practice.[47]
Pharmacological management for CRF
There are several pharmacological interventions for CRF. Different pharmacological agents which are found to be effective through clinical trials include ATP infusion,[49] bupropion sustained release,[50-52] donepezil,[53] methylphenidate, modafinil, steroids,[53] L-carnitine,[54] high dose Vitamin C,[55] paroxetine,[56] and recombinant human erythropoietin.[57,58]
DISCUSSION
The systematic literature search identified a wide range and a number of scales are available for the clinical evaluation of fatigue associated with cancer. A total of 23 types of scales were identified including single item unidimensional, multiple items unidimensional, and multidimensional scales exclusively used for the patients with cancer. Single-item unidimensional scales are commonly used for determining fatigue.[7] The most extensively used scales for the clinical assessment of CRF were FACIT F scale and EORTC QLQ C30 fatigue subscale. These scales are mostly recommended for experimental research studies for the management of fatigue.[22] The multidimensional fatigue symptom inventory (short form) is the only most recommended tool for the comprehensive examination of fatigue. It includes the various aspects of fatigue such as physical, emotional, and mental dimensions in somatic, cognitive, behavioural, and global contexts.[23]
The main advantages of using the unidimensional scales are strong psychometric properties, concise one (no of items 3–13), ease to administer, and ability to detect effects of intervention by significant changes in scores of fatigue. Regarding the disadvantages, it incorporates physical fatigue only and it provides the subjective nature of fatigue only.[21,22] The multiple dimensions of fatigue are taken into consideration in multidimensional scales results in the comprehensive assessment of fatigue. However, they have limited scope in their usage. Moreover, it requires more time to collect data because of more items, and the majority of the scales were tested only in patients with breast cancer.[21,22]
It is really challenging to identify an appropriate scale to measure CRF. In clinical as well as research settings, the important factors must be taken into consideration for appropriate selection of scales are time frame in which patient experiences the fatigue, Psychometric properties, impact of fatigue and research problem. The nature of impact must also be taken into consideration while selecting the scale.[59]
Impact and distress associated with CRF are not appreciated by the healthcare team members due to various reasons such as concealed nature of fatigue; fatigue is neither life threatening nor leads to mortality; assumption of patient and physician that CRF is an unavoidable outcome of the disease process and its treatment, hence underestimated. Clinical evaluation and identification of fatigue is trivial among cancer patients.[41,60] A study was conducted to identify the prime barriers in diagnosing and managing the CRF was as follows.
Clinician failure to provide appropriate intervention (47%)
Inadequate information regarding the management of fatigue (43%)
Patient’s inclination toward managing fatigue without medication (40%)
Patients did not want them to be labelled as complainers (28%).[61]
Hence, healthcare team members should be vigilant to patient and family reports of fatigue, concurrent symptoms and disruption in activities of daily living. They must be accountable for the delivery of quality patient care and impart the knowledge to the patients and significant others regarding fatigue, factors affecting fatigue, impact, and different management techniques.
The comprehensive literature search recognised a wide variety of non-pharmacological interventions and pharmacological agents for the treatment of fatigue. Pharmacological agents have been found to be safe and effective in treating fatigue in numerous research articles. Exercises are the most beneficial and cost-effective intervention for reducing the severity of fatigue among patients with cancer.[32-34] Complimentary therapies, nutritional interventions and educational interventions including psychoeducation, cognitive behavioural therapy and sleep therapy have shown a promising effect in managing the CRF. However, evidence to be generated from more number of randomised controlled trial research studies are in demand to reveal the effectiveness of polarity therapy, bright white light therapy, eurythmy therapy, and restorative therapy. The most common pharmacological agents used for the management of CRF are erythropoietin analogues, methylphenidate, and psychostimulants.[57] Consequently, the enhancement of the quality of life is attained through health adaptation to fatigue. Complementary approaches need to be explored and propitious interventions must be identified for the fatigue. Clinicians and nursing officers should be actively involved in the research projects and systematic reviews to identify the efficacy of various pharmacological and non-pharmacological measures which will pave the way for the generation of evidence-based recommendations for fatigue management. Limitations of this systematic review search were the consideration of two databases only. The screening of the title and abstract was done by one author due to limited resources and time was another limitation.
CONCLUSION
The present systematic review identified four single-item unidimensional scales, six multiple item unidimensional scales, and 13 multidimensional are available for the screening and the evaluation of CRF. Different non- pharmacological and pharmacological approaches are utilised for fatigue management. Among cancer patients, clinical evaluation of fatigue and its management is essential and crucial for boosting their quality of life. This systematic review has many implications for clinical practice and future research and it provides a framework for the guidelines development which facilitate the assessment of fatigue associated with cancer and incorporate various interventions for its management, thereby enhancing cancer patients’ quality of life.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cancer symptom clusters: Clinical and research methodology. J Palliat Med. 2011;14:1149-66.
- [CrossRef] [PubMed] [Google Scholar]
- Symptom clusters and quality of life in breast cancer survivors after cancer treatment in a tertiary hospital in Korea. Eur J Cancer Care (Engl). 2018;27:e12919.
- [CrossRef] [PubMed] [Google Scholar]
- Symptom clusters change over time in women receiving adjuvant chemotherapy for breast cancer. J Pain Symptom Manage. 2017;53:880-6.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of symptom clusters on quality of life outcomes in patients from Japan with advanced nonsmall cell lung cancers. Asia Pac J Oncol Nurs. 2016;3:370-81.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14:101-10.
- [CrossRef] [PubMed] [Google Scholar]
- A literature review of symptom clusters in patients with breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2011;11:533-9.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology Version 2. 2020. 2020 Available from: https://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf [Last accessed on 2020 Jun 15]
- [Google Scholar]
- Progress toward guidelines for the management of fatigue. Oncology (Williston Park). 1998;12:369-77.
- [Google Scholar]
- The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17:367-78.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005;30:433-42.
- [CrossRef] [PubMed] [Google Scholar]
- Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791-801.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16:1689-96.
- [CrossRef] [PubMed] [Google Scholar]
- Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6:448-55.
- [CrossRef] [PubMed] [Google Scholar]
- Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur J Cancer. 2002;38:27-43.
- [CrossRef] [Google Scholar]
- Cancer-related fatigue: Prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11:441-6.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of cancer-related fatigue on the lives of patients: New findings from the fatigue coalition. Oncologist. 2000;5:353-60.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in long-term Hodgkin's disease survivors with chronic fatigue. Eur J Cancer. 2006;42:327-33.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162:777-84.
- [CrossRef] [PubMed] [Google Scholar]
- Joanna Briggs Institute Reviewers' Manual. 2014. Adelaide SA: Joanna Briggs Institute; Available from: http://joannabriggs.org/assets/docs/sumari/ReviewersManual-2014.pdf [Last accessed on 2021 Sep 28]
- [Google Scholar]
- Cancer-related fatigue: Role of oncology nurses in translating national comprehensive cancer network assessment guidelines into practice. Clin J Oncol Nurs. 2008;12:37-47.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the scales used for the measurement of cancer-related fatigue (CRF) Ann Oncol. 2009;20:17-25.
- [CrossRef] [PubMed] [Google Scholar]
- Oncology section EDGE task force on cancer: Measures of cancer-related fatigue-a systematic review. Rehabil Oncol. 2018;36:93-105.
- [CrossRef] [Google Scholar]
- Fatigue Severity Scale Ability Lab. Available from: https://www.sralab.org/rehabilitation-measures/fatigue-severity-scale [Last accessed on 2020 Jun 18]
- [Google Scholar]
- Assessment and management of cancer-related fatigue. J Hosp Palliat Nurs. 2013;15:77-86.
- [CrossRef] [Google Scholar]
- Development and validation of the cancer fatigue scale: A brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5-14.
- [CrossRef] [Google Scholar]
- Fatigue assessment questionnaire: Standardization of a cancer-specific instrument based on the general population. Oncology. 2006;70:351-7.
- [CrossRef] [PubMed] [Google Scholar]
- Psychometric properties of the arabic version of the functional assessment of chronic illnesses therapy-fatigue in arabic cancer patients. J Pain Symptom Manage. 2020;59:130-8.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a fatigue and functional impact scale in anemic cancer patients receiving chemotherapy. Cancer. 2008;113:1480-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-related fatigue: A review of nursing interventions. Br J Community Nurs. 2010;15:214-6, 218-9
- [CrossRef] [PubMed] [Google Scholar]
- Fatigue in cancer patients undergoing chemotherapy: A nursing process approach. Int J Caring Sci. 2019;12:1261.
- [Google Scholar]
- Clinical implementation of exercise guidelines for cancer patients: Adaptation of ACSM's guidelines to the Italian model. J Funct Morphol Kinesiol. 2017;2:4.
- [CrossRef] [Google Scholar]
- Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):52-67.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-60.
- [CrossRef] [PubMed] [Google Scholar]
- Massage therapy for symptom control: Outcome study at a major cancer centre. J Pain Symptom Manag. 2004;28:244-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-related fatigue: Evolving concepts in evaluation and treatment. Cancer. 2003;98:1786-801.
- [CrossRef] [PubMed] [Google Scholar]
- The management of cancer-related fatigue after chemotherapy with acupuncture and acupressure: A randomised controlled trial. Complement Ther Med. 2007;15:228-37.
- [CrossRef] [PubMed] [Google Scholar]
- Acupuncture for postchemotherapy fatigue: A phase II study. J Clin Oncol. 2004;22:1731-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pilot crossover trial of Reiki versus rest for treating cancer-related fatigue. Integr Cancer Ther. 2007;6:25-35.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol. 2005;23:1431-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer Nursing Principles and Practice (7th ed). Sudbury, US: Jones and Barlett Learning; 2010. p. :778-9.
- [Google Scholar]
- Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61:664-75.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-related fatigue treatment: An overview. J Cancer Res Ther. 2017;13:916-29.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of radiotherapy-induced fatigue through a nonpharmacological approach. Integr Cancer Ther. 2005;4:8-13.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: http://www.www.eurythmytherapy.com https://en.wikipedia.org/wiki/Eurythmy [Last accessed on 2020 Dec 19]
- Available from: http://www.verywellhealth.com https://www.anthromedics.org/PRA-0943-EN [Last accessed on 2020 Dec 19]
- The effect of art therapy in women with gynecologic cancer: A systematic review. Evid Based Complement Alternat Med. 2020;2020:8063172.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized clinical trial of adenosine 5'-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2000;92:321-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of bupropion in cancer-related fatigue, a randomized double blind placebo controlled clinical trial. BMC Cancer. 2020;20:158.
- [CrossRef] [PubMed] [Google Scholar]
- Potential role of bupropion sustained release for cancer-related fatigue: A double-blind, placebo-controlled study. Asian Pac J Cancer Prev. 2018;19:1547-51.
- [Google Scholar]
- Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415-20.
- [CrossRef] [PubMed] [Google Scholar]
- Fatigue in palliative care patients--an EAPC approach. Palliat Med. 2008;22:13-32.
- [CrossRef] [PubMed] [Google Scholar]
- Safety, tolerability and symptom outcomes associated with L-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: A phase I/II study. J Pain Symptom Manage. 2006;32:551-9.
- [CrossRef] [PubMed] [Google Scholar]
- Changes of terminal cancer patients' health-related quality of life after high dose Vitamin C administration. J Korean Med Sci. 2007;22:7-11.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of erythropoiesis-stimulating agents on fatigue-and anaemia-related symptoms in cancer patients: Systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111:33-45.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacologic interventions for fatigue in cancer and transplantation: A meta-analysis. Curr Oncol. 2018;25:e152-67.
- [CrossRef] [PubMed] [Google Scholar]
- Control of cancer-related anemia with erythropoietic agents: A review of evidence for improved quality of life and clinical outcomes. Ann Oncol. 2003;14:511-9.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of fatigue in cancer patients. JNCI Monogr. 2004;32:93-7.
- [CrossRef] [PubMed] [Google Scholar]
- Survey of nurses' assessment of cancer-related fatigue. Eur J Cancer Care (Engl). 2000;9:105-13.
- [CrossRef] [PubMed] [Google Scholar]
- Patient-related barriers to fatigue communication: Initial validation of the fatigue management barriers questionnaire. J Pain Symptom Manage. 2002;24:481-93.
- [CrossRef] [Google Scholar]






