Translate this page into:
Cognitive Training Intervention for Heart Failure Patients: A Randomised Controlled Trial
*Corresponding author: Monika Dutta, Department of Nursing Education, Post Graduate Institute of Medical Education and Research, Chandigarh, India. monika.dutta75@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumari S, Dutta M, Otaal PS, Das K. Cognitive Training Intervention for Heart Failure Patients: A Randomised Controlled Trial. Indian J Palliat Care. 2025;31:167-76. doi: 10.25259/IJPC_83_2024
Abstract
Objectives
The aim of this study was to evaluate the impact of ‘Cognitive Training’ on cognitive functions, self-care, medication adherence, QOL, functional capacity and satisfaction level among HF patients.
Materials and Methods
In the current randomised controlled trial, 60 HF patients were enrolled from the cardiology outpatient department by total enumeration sampling technique and randomised into the 30 control and 30 experimental group participants using a random number table. A socio-demographic cum clinical profile sheet, Montreal cognitive assessment, self-care of HF index, Hill-Bone medication adherence scale, Minnesota living with HF questionnaire (MLHFQ), 6-min walk test and satisfaction questionnaire were used for collecting data and pre-test was done at the time of enrolment. The experimental group participants received cognitive training through face-to-face counselling sessions provided by the researcher along with routine care. Control group participants received routine care. Post-test was done at the end of the 12th week to assess the impact of Cognitive training on cognitive functions and other variables. Descriptive and inferential statistics were used to analyse the data.
Results
Significant improvement was observed in cognitive functions which include memory (P < 0.001), executive functions (P < 0.001) and attention and concentration (P < 0.001) among the experimental group participants in the 12th week. Furthermore, significant improvement was observed in self-care maintenance (P < 0.001), self-care management (0.04), self-care confidence (P < 0.001), medication adherence (P < 0.001), QOL (P < 0.001), functional capacity (0.002) as well as satisfaction with care provided.
Conclusion
Cognitive training was found to be effective in terms of improvement in cognitive functions and it should be part of the routine intervention in healthcare settings for better patient outcomes.
Keywords
Cognitive function
Cognitive impairment
Cognitive training
Heart failure
INTRODUCTION
Heart failure (HF) is a significant global public health issue. Since HF affects about 26 million people globally, it has been considered a global pandemic.[1] According to the global data, India is the country in Southern Asia spending the most on HF, estimating the amount as ~ $1186 million (1.1% of total global HF spending).[2]
Patients with HF experience a variety of physical and psychological symptoms that limit patients’ daily physical and social activities and lead to poor quality of life (QOL).[3] Mild cognitive impairment (CI) has been reported in 50–80% of people with HF.[4] Working memory, learning memory, delay recall, attention, processing speed and executive function are some of the key cognitive areas that are negatively impacted by HF.[5] Patients might struggle with self-care tasks such as performing activities of daily living, reaching short- and long-term health objectives and adhering to a treatment plan.[6]
HF population is increasing, but there are no standard guidelines to address their CI leading to unfavourable outcomes. Cognitive training is one such initiative involving interventions where structured activities are planned and performed by patients to enhance their cognitive abilities. Nurse-led cognitive training has shown promising results for improving cognitive functions and reducing the burden on healthcare resources.[7] In the current Indian scenario, there is a paucity of research studies on the impact of cognitive training interventions among HF patients. Hence, nurses as part of the healthcare workforce can play a significant role in cognitive risk assessment and provide training, thus preventing cognition decline among HF patients, which contributes to improving self-care and QOL.
MATERIALS AND METHODS
A randomised controlled trial to assess the effectiveness of cognitive training was carried out in the cardiology outpatient department of the advanced cardiac centre, Postgraduate Institute of Medical Education and Research, Chandigarh, by enrolling 60 participants using a total enumeration sampling technique.
Inclusion criteria
Chronic HF New York Heart Association (NYHA) class II and III, left ventricular ejection fraction ≤40%, 18–60 years of age and Montreal cognitive assessment (MoCA) score <26.
Exclusion criteria
Unstable angina, recent Myocardial Infarction (MI), end-stage organ failure, End-Stage Renal Disease (ESRD) patient on maintenance haemodialysis and mental health disorders. Enrolled patients were randomised into 30 experimental and 30 control group participants using a random number table. The experimental group participants received cognitive training through face-to-face counselling sessions provided by the researcher along with routine care. Cognitive training sessions were started on patient enrolment itself and then individual face-to-face sessions were conducted on the 2nd, 4th, 8th and 12th week. Control group participants received routine care. Post-test was done at the end of the 12th week to assess the impact of cognitive training on cognitive functions and other variables. Primary outcome measures were cognitive functions (memory, executive functions and attention/concentration), whereas self-care in HF, medication adherence, health-related QOL (HQOL), functional capacity and satisfaction level were taken as secondary outcomes.
Tools were selected as well as prepared based on an extensive review of relevant literature and validated by experts in the fields of Nursing and Cardiology. A tryout was conducted to check the feasibility of the study. Sociodemographic cum clinical profile sheet, MoCA, self-care of HF index (SCHFI), Hill-Bone medication adherence scale (HB-MAS), Minnesota living with HF questionnaire (MLHFQ), 6-min walk test (6MWT) and satisfaction questionnaire are the tools used in the study. Content validity of the sociodemographic cum clinical profile sheet and satisfaction questionnaire was ensured by seeking experts’ opinions.
MoCA
It is a 30-item valid and reliable instrument used to measure cognition and has a coefficient alpha for the reliability of 0.885, whereas, for subscales, it ranged between 0.878 and 0.893. It tests a person’s cognition in the following areas: memory, executive functions and attention/concentration. The total score for MoCA ranges from 0 to 30. CI has been categorised according to the MoCA score: A score between 27 and 30 means ‘no to light CI’; a score between 18 and 26 means ‘mild CI’, a score between 10 and 17 means ‘moderate CI’ and a score lower than 10 means ‘severe CI’.[8]
SCHFI v.6.2
It is a standardised instrument used to evaluate the effectiveness of HF patients’ self-care. It has three self-care domains-maintenance, management and confidence. The root mean square error of approximation, a measure of test-retest reliability is 0.05, 0.07 and 0.02 for self-care maintenance, self-care management and self-care confidence, respectively.[9]
HB-MAS
It is an effective tool for assessing medication adherence in individuals with a variety of conditions including HF. Cronbach’s alpha for the tool is.80.[10]
MLHFQ
This 21-item patient-focused tool is recommended for assessing HF patient’s patients’ HQOL. The total score ranges from 0 to 105 and is based on a 6-point Likert scale (0–5) for each item. A higher score indicates a more significant impairment in HQOL. The MLHFQ has two domains: the physical domain (8 items, score range from 0 to 40) and the emotional domain (5 items, score range from 0 to 25). Cronbach’s alpha for the tool is ≥0.80.[11]
6MWT
A submaximal exercise evaluation involves measuring the distance walked over 6 min. Pearson product moment correlation coefficient is r = 0.984 (P < 0.000).[12]
Satisfaction questionnaire
It is a self-structured questionnaire used to assess the level of satisfaction of the experimental group with cognitive training and the control group with routine care. It is a Likert-type scale where responses are recorded from 1 to 5. The total items are 6 and the total score is 30.
Cognitive training intervention
Cognitive training was provided to the experimental group participants along with routine care and it comprised:
Information in their understandable language regarding the disease process and its management.
An educational booklet containing the same information was given.
Information cum demonstration on cognitive and physical exercises to enhance cognitive abilities was provided.
Cognitive exercises workbook (beading, number cancellation, spot the difference, mazes, colouring, trail making, etc.) was also given to practice cognitive exercises at home and checked at the time of follow-up.
Counselling sessions on a healthy heart diet and soft copies of content were also shared with patients.
Breathing exercises and meditation audio for promoting relaxation.
Telephonic reminders twice a week to reinforce the patient’s adherence to cognitive training interventions. Patient queries were handled during the study period from 5 pm to 8 pm every day.
Data collection
Data collection was done using various tools after obtaining written informed consent from participants. A structured interview schedule was used to gather information from both groups. Experimental group participants received cognitive training provided by the researcher under the supervision of a clinical psychologist. Each cognitive training session comprised 40–50 min. Cognitive training sessions were started on patient enrolment itself and then follow-up was done on the 2nd, 4th, 8th and at the beginning of the 12th week. Control group participants continued with routine hospital care services. Reinforcement for adherence to cognitive training and follow-up was done through telephonic reminders for experimental group participants. At the end of the 12th week of enrolment, reassessment was done for both groups.
Ethical approval for the study was obtained from the Institute Ethics Committee with the number INT/IEC/2022/SPL-204 and the trial was registered in Clinical Trials Registry- India (CTRI) (Clinical trial registration no. CTRI/2022/06/043422).
Statistical analysis
Descriptive and inferential statistical tests were applied to analyse the data using the Statistical Package for the Social Science program version 23. The measurable data were checked for their normality using the Shapiro–Wilk test. The chi-square test or Fisher’s exact test was applied for categorical variables. Independent sample t-test was applied for between-group comparisons of normally distributed data, whereas paired t-test were used to compare each group before and after intervention. P < 0.05 is taken as the level of significance. Tables and figures were used to present the analysed data.
RESULTS
A total of 60 eligible HF patients were enrolled in the study. At baseline, both the groups were comparable in sociodemographic [Table 1] and clinical profile [Table 2]. The mean age was nearly 50 years in both groups. More than half of the participants in both groups were male. More than 90% of participants were married. More than half of the participants were vegetarians in both groups. Twenty per cent of the control and 43.3% of the experimental participants had a history of previous substance use. Family history of cardiac illness was positive in 20% of the control and 36.7% of the experimental participants [Table 1]. Nearly, 90% of participants in both groups had a history of hospitalisation. The reason for hospitalisation was chest pain in half of the participants followed by shortness of breath. Acute coronary syndrome (ACS) and dilated cardiomyopathy (DCMP) were the two main etiological factors responsible for HF in the present study. Diabetes mellitus (DM) was the most common comorbidity present in participants of both groups. Almost all the study patients received diuretics, Angiotensin - converting enzyme inhibitors / Angiotensin II receptor blockers / Angiotensin receptor-neprilysin inhibitors and beta-blockers. Most of them were on antiplatelet and antihyperlipidemics. Devices such as ICD and CRT were implanted in 6.6% of control and 16.7% of experimental group participants [Table 2].
| Variables | Control group (n1=30) f (%) | Experimental group (n2=30) f (%) | χ2(df ) P-value |
|---|---|---|---|
| Age (years) | |||
| ≤40 | 1 (3.3) | 4 (13.3) | 0.87 (1) 0.35# |
| 41–60 | 29 (96.7) | 26 (86.7) | |
| Mean±SD 51.90±6.06 | Mean±SD 50.20±6.96 | ||
| Gender | |||
| Male | 18 (60) | 22 (73.3) | 1.20 (1) 0.27 |
| Female | 12 (40) | 8 (26.7) | |
| Marital status | |||
| Married | 29 (96.7) | 28 (93.3) | 1.19 (2) 1.00f |
| Unmarried | 0 | 1 (3.3) | |
| Widow | 1 (3.3) | 1 (3.3) | |
| Education | |||
| Primary | 10 (33.3) | 5 (16.7) | 2.26 (2) 0.32 |
| Secondary | 18 (60.0) | 22 (73.3) | |
| Sr. Secondary graduate or above | 2 (6.7) | 3 (10.0) | |
| Occupation | |||
| Skilled worker | 5 (16.7) | 8 (26.7) | 1.52 (2) 0.46 |
| Unskilled worker | 13 (43.3) | 14 (46.7) | |
| Unemployed | 12 (40.0) | 8 (26.7) | |
| Dietary habits | |||
| Vegetarian | 21 (70.0) | 17 (56.7) | 4.42 (2) 0.11 |
| Non-vegetarian | 9 (30.0) | 13 (43.3) | |
| Drug or substance use | |||
| Previous use | |||
| No | 24 (80.0) | 17 (56.7) | 3.77 (1) 0.09 |
| Yes | 6 (20.0) | 13 (43.3) | |
| Current use | |||
| No | 26 (86.7) | 27 (90.0) | 0.16 (1) 0.68 |
| Yes (Alcohol, cigarette/bidi) | 4 (13.3) | 3 (10.0) | |
| Family history of cardiac disorders | |||
| No | 24 (80.0) | 19 (63.3) | 2.05 (1) 0.15 |
| Yes | 6 (20.0) | 11 (36.7) | |
| Relation (n1=6, n2=11) | |||
| First-degree relative | 5 (83.3) | 9 (81.8) | |
| Second-degree relative | 1 (16.6) | 2 (18.2) | |
| Relation with primary caregiver | |||
| Spouse | 22 (73.3) | 23 (76.7) | 1.26 (2) 0.76f |
| Parents | 0 | 1 (3.3) | |
| Children | 8 (26.7) | 6 (20.0) |
f: Fisher’s exact test, #yates’ continuity correction, SD: Standard deviation, df: degrees of freedom
| Clinical parameters | Control group (n1=30) f (%) | Experimental group (n2=30) f (%) | χ2(df) P-value |
|---|---|---|---|
| Age at diagnosis (years) | |||
| ≤40 | 6 (20.0) | 7 (23.3) | 0.65 (2) 0.71 |
| 41–50 | 12 (40.0) | 15 (50.0) | |
| 51–60 | 12 (40.0) | 8 (26.7) | |
| Heart failure classification (NYHA) | |||
| NYHA II | 25 (83.3) | 27 (90.0) | 0.14 (1) 0.70# |
| NYHA III | 5 (16.7) | 3 (10.0) | |
| LVEF (%) | |||
| ≤20 | 3 (10.0) | 5 (6.7) | 0.89 (2) 0.63 |
| 21–30 | 14 (46.7) | 11 (36.7) | |
| 31–40 | 13 (43.3) | 14 (46.7) | |
| Duration of heart failure follow-up | |||
| ≤1 Year | 13 (43.3) | 11 (36.7) | 0.36 (2) 0.83 |
| 2–5 years | 9 (30.0) | 11 (36.7 | |
| >5 years | 8 (26.7) | 8 (26.7) | |
| Number of hospitalisation post diagnosis | |||
| 0 | 3 (10.0) | 2 (6.7) | 0.88 (2) 0.64 |
| 1–2 | 24 (80.0) | 23 (76.6) | |
| 3–4 | 3 (10.0) | 5 (16.6) | |
| Reason for hospitalisation (n1=25, n2=28) | |||
| Chest pain | 16 (64.0) | 15 (53.5) | 4.28 (4) 0.38f |
| Shortness of breath | 9 (36.0) | 10 (35.7) | |
| Tachycardia | 0 | 1 (3.57) | |
| Dizziness/syncope | 0 | 2 (7.14) | |
| Baseline aetiology of heart failure | |||
| ACS | 12 (40.0) | 12 (40.0) | 6.83 (7) 0.43f |
| Rheumatic heart disease/valvular heart disease | 1 (3.3) | 1 (3.3) | |
| DCMP | 10 (33.3) | 8 (26.7) | |
| Arrhythmias/conduction abnormalities | 0 | 1 (3.3) | |
| HTN | 1 (3.3) | 1 (3.3) | |
| ACS+HTN | 0 | 4 (13.3) | |
| Cardiomyopathy+HTN | 5 (16.7) | 2 (6.7) | |
| ACS+Cardiomyopathy | 1 (3.3) | 1 (3.3) | |
| Co-morbidities/associated conditions (n1=8, n2=11) | |||
| Diabetes mellitus | 7 (87.5) | 11 (100.0) | 0.02 (1) 0.87 |
| Hypothyroidism | 1 (12.5) | 0 | |
| Medications | |||
| Diuretics | 27 (90.0) | 28 (93.3) | 0.21 (1) 0.64 |
| Beta-blockers | 29 (96.7) | 28 (93.3) | 0.35 (1) 0.55 |
| ACE inhibitors/ARB/ARNI | 27 (90.0) | 26 (86.6) | 0.31 (1) 0.71 |
| PAI | 21 (70.0) | 24 (80.0) | 0.80 (1) 0.37 |
| Antihyperlipidaemic | 22 (73.3) | 25 (83.3) | 0.88 (1) 0.34 |
| Antianginals | 5 (16.7) | 5 (16.7) | - |
| Other drugs (hypoglycaemic agents, multivitamins, proton pump inhibitors, etc.) | 26 (86.7) | 28 (93.3) | 0.74 (1) 0.38 |
| Interventions performed | |||
| CART | 13 (56.5) | 13 (48.1) | 2.55 (2) 0.63 |
| PCI | 7 (30.4) | 10 (37.0) | |
| Device implantation (Permanent pacemaker, AICD ) | 3 (13.0) | 4 (14.8) | |
f: Fisher’s exact test, # yates continuity correction. LVEF: Left ventricular ejection fraction, ACS: Acute coronary syndrome, PAI: Platelet aggregation inhibitors, PCI: Percutaneous coronary intervention, HTN: Hypertension, DCMP: Dilated cardiomyopathy, NYHA: New York Heart Association, df: degrees of freedom, ACE: Angiotensin - converting enzyme ARB: Angiotensin II receptor blockers ARNI: Angiotensin receptor-neprilysin inhibitors, CART: Coronary Artery Revascularization Therapies, AICD: Automatic Implantable Cardioverter-Defibrillator
After the intervention, all parameters of cognitive assessment (memory, executive functions and attention/concentration) showed statistically significant improvement (P < 0.001) among participants of the experimental group which was reflected through significant improvement (P < 0.001) in total MoCA score [Table 3]. After the intervention, significant improvement (P < 0.001) was observed in all self-care domains among participants of the experimental group as compared to the control group [Table 4]. Medication adherence improved significantly (P < 0.001) among participants of experimental group after intervention as mean score increased from 30.28 to 34.83 [Table 5]. Post-intervention all the participants of the experimental group had high medication adherence [Figure 1]. The within-group comparison revealed a significantly high reduction (P < 0.001) in the mean score of physical dimension, emotional dimension and total QOL among experimental group participants after intervention (decreased mean score means a significant improvement in overall QOL and its dimensions) [Table 6]. After the intervention, the mean distance covered in 6MWT significantly increased (P = 0.002) among experimental group participants [Figure 2]. All participants of the experimental group were satisfied with dietary modification and physical exercises. The majority of experimental group participants were satisfied with the medication regimen (96%), cognitive training (89.6%) and stress management and relaxation therapy [Table 7].
| Cognitive assessment parameters of MoCA | Pre-intervention mean±SD n=60 | Post-intervention mean±SD n=58 | Paired t-test (P-value) |
|---|---|---|---|
| Memory score (0–17) | |||
| Control group | 11.69±1.83 | 11.83±1.81 | 0.64 (0.52) |
| Experimental group | 11.66±2.14 | 12.59±2.29 | 4.55 (<0.001) |
| t -test (P-value) | 0.13 (0.89) | 1.39 (0.17) | |
| Executive functions score (0–7) | |||
| Control group | 2.45±1.82 | 2.24±1.80 | 1.79 (0.08) |
| Experimental group | 2.55±1.70 | 3.52±1.76 | 5.11 (<0.001) |
| t-test (P-value) | 0.15 (0.88) | 2.72 (<0.001) | |
| Attention/concentration score (0–6) | |||
| Control group | 3.55±1.50 | 3.69±1.44 | 0.84 (0.40) |
| Experimental group | 3.93±1.51 | 4.55±1.43 | 3.41 (<0.001) |
| t-test (P-value) | 1.05 (0.30) | 2.29 (0.02) | |
| Total MoCA score (0–30) | |||
| Control group | 18.62±3.84 | 18.69±4.20 | 0.25 (0.80) |
| Experimental group | 19.03±3.82 | 21.48±4.51 | 7.15 (<0.001) |
| t -test (P-value) | 0.51 (0.61) | 2.39 (0.02) |
MoCA: Montreal cognitive assessment, SD: Standard deviation
| Self-care domain | Groups | Pre-intervention (Baseline) n=60 mean±SD | Post-intervention (12thweek) n=58 mean±SD | Paired t-test (P-value) |
|---|---|---|---|---|
| Self-care maintenance | Control group | 35.22±10.92 | 36.63±9.22 | 0.73 (28) 0.47 |
| Experimental group | 35.55±10.29 | 74.25±10.61 | 15.34 (<0.001) | |
| t-test (P-value) | 0.12 (0.90) | 14.40 (<0.001) | ||
| Self-care management | Control group | 51.43±14.63 | 43.33±10.89 | 0.34 (0.74) |
| Experimental group | 46.67±14.02 | 70.00±10.00 | - | |
| t-test (P-value) | 0.59 (0.56) | 3.73 (<0.001) | ||
| Self-care confidence | Control group | 37.25±15.32 | 31.80±11.72 | 2.05 (0.05) |
| Experimental group | 38.36±10.96 | 69.60±9.47 | 13.24 (<0.001) | |
| t-test (P-value) | 0.32 (0.74) | 13.50 (<0.001) |
SCFHI: Self-care for heart failure index, SD: Standard deviation
| Groups | Medication adherence score mean±SD | Paired t-test (P-value) | |
|---|---|---|---|
| Pre-intervention (Baseline) n=60 | Post-intervention (12th week) n=58 | ||
| Control group | 29.69±3.25 | 31.07±2.99 | 3.24 (0.003) |
| Experimental group | 30.28±2.65 | 34.83±0.92 | 8.06 (<0.001) |
| t-test (P-value) | 0.36 (0.71) | 6.44 (<0.001) | |
HB-MAS: Hill-Bone medication adherence scale, SD: Standard deviation
| Dimensions of quality of life | Groups | Pre-intervention (Baseline) n=60 Mean±SD | Post-intervention (12th week) n=58 Mean±SD | Paired t-test (df)P-value |
|---|---|---|---|---|
| Physical dimension score (0–40) | Control group | 28.33±6.98 | 26.59±6.94 | 1.65 (0.11) |
| Experimental group | 28.40±6.94 | 16.38±5.69 | 11.59 (<0.001) | |
| t-test (P-value) | 0.04 (0.97) | 6.12 (<0.001) | ||
| Emotional dimension score (0–25) | Control group | 16.37±5.43 | 14.48±4.90 | 2.19 (0.04) |
| Experimental group | 14.73±4.41 | 10.38±3.47 | 6.34 (<0.001) | |
| t-test (P-value) | 1.28 (0.20) | 3.67 (<0.001) | ||
| Total quality of life score (0–105) | Control group | 72.13±15.35 | 67.55±16.94 | 2.08 (0.05) |
| Experimental group | 69.40±12.58 | 47.45±12.67 | 11.88 (<0.001) | |
| t-test (P-value) | 0.75 (0.45) | 5.11 (<0.001) |
MLHFQ: Minnesota living with heart failure questionnaire, SD: Standard deviation, df: degrees of freedom
| Satisfaction parameters | Control group (n1=29) f (%) | Experimental group (n2=29) f (%) | ||||
|---|---|---|---|---|---|---|
| Satisfied | Neutral | Dissatisfied | Satisfied | Neutral | Dissatisfied | |
| Dietary modification | 14 (48.3) | 0 | 15 (51.7) | 29 (100.0) | 0 | 0 |
| Medication regimen and its importance | 17 (58.6) | 0 | 12 (41.4) | 28 (96.6) | 1 (3.4) | 0 |
| Management of disease condition and follow-up | 11 (37.9) | 2 (6.9) | 16 (55.1) | 22 (75.9) | 3 (10.3) | 4 (13.8) |
| Physical exercises | 4 (13.8) | 2 (6.9) | 23 (79.3) | 29 (100.0) | 0 | 0 |
| *Cognitive training | - | - | 26 (89.6) | 3 (10.3) | 0 | |
| *Stress management and relaxation therapy | - | - | 28 (96.6) | 1 (3.4) | 0 | |
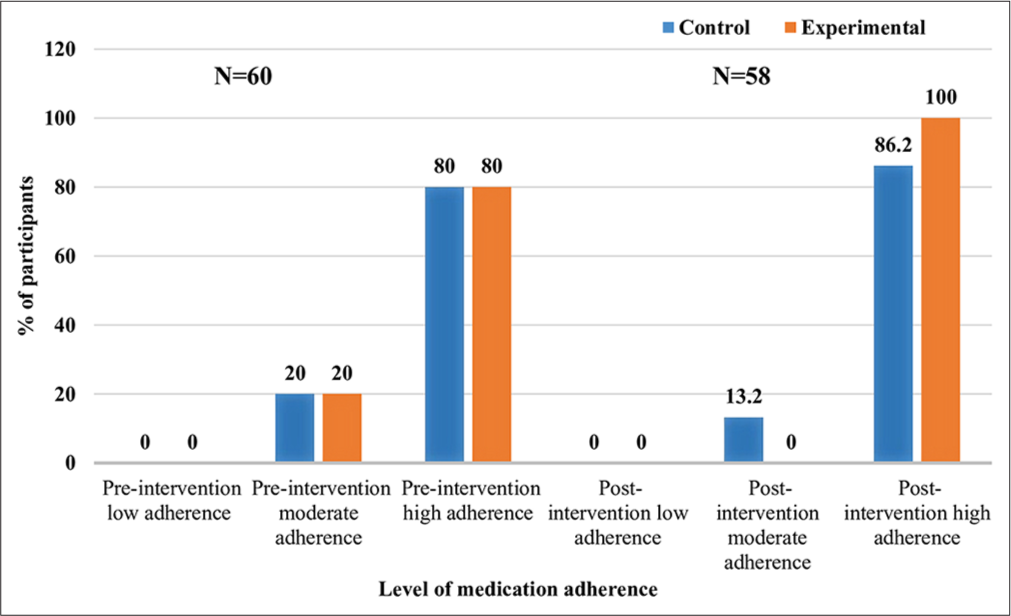
- Level of medication adherence among participants.
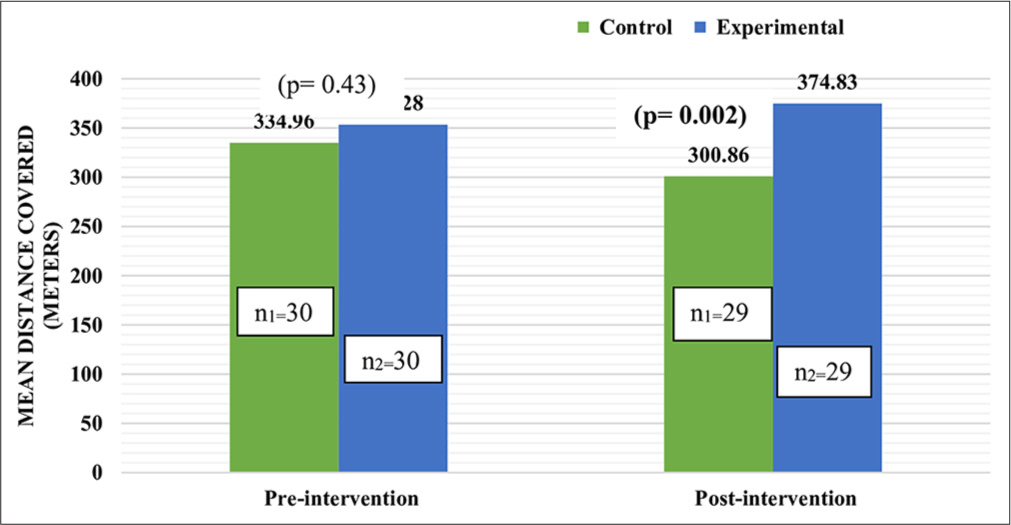
- Comparison of mean distance covered by participants as per 6-min walk test.
DISCUSSION
In the present study, the mean age of the participants in both the groups was same, that is 50 years. More than half of the participants were male. These findings are congruent with the study conducted by Seth et al. (2015)[13] where the mean age of
HF patients was 53 years and 63% of participants were male. Some of the study participants had non-vegetarian dietary habits, a history of substance use, a family history of cardiac illness and associated comorbidities (diabetes, hypertension [HTN], coronary artery disease, etc.). These risk factors are strongly linked with heart disease and increase the likelihood of HF. A case-control study revealed similar findings that coronary heart disease, HTN, DM, obesity and smoking are responsible for 52% of HF cases in the population.[14]
Eighty-three per cent of the control and 90 % of experimental group participants were categorised as NYHA class II HF and few were categorised as NYHA III. Since haemodynamic stability and better functional ability are required to deliver different aspects of cognitive training, NYHA class IV patients were excluded from the study. Cognitive decline is not prominent among HF NYHA class I.
ACS and DCMP were the most common etiological factors of HF among current study participants. It highlights the global burden of ischemic heart disease (IHD) which could lead to cardiac remodelling and eventual progression to HF. These findings are in line with the International Congestive Heart Failure Study (INTER-CHF)[15] data which shows CAD is the most common aetiology (48%) of HF in Asia and according to Trivandrum Registry[16] IHDs accounted for 71% of HF. Chest pain was the major reason for previous hospitalisation in almost half of the study participants as myocardial infarction is one of the adverse cardiac events associated with HF. These findings were in line with previous study findings where the most common precipitating factor for hospitalisation in the new-onset HF group was ACS (39.2%) presented with chest pain.[17]
India is considered the diabetes capital of the world and this has been also reflected in current study findings where DM was the most prevalent co-morbid condition. History of HTN is another comorbidity reported in our study and the majority of the subjects were receiving antihypertensive therapy. Both diabetes and HTN are independent risk factors for HF development. These findings are parallel with the findings of a study conducted by Shukkoor et al. (2021)[18] that reported type 2 DM as the most prevalent comorbidity (62.8%) in HF patients with reduced ejection fraction followed by HTN (53.3%).
In our study, almost all the patients received diuretics (91.67%), ACEI/ARB/ARNI (88.33%) and beta-blockers (95%). Most of them were on antiplatelets (75%) and antihyperlipidemics (78.33%). A similar trend for drug therapy in HF has been seen in a study conducted by Vellone et al. (2020)[19] where medications for HF patients comprised ACEI/ARB/ARNI, beta-blocker, MRA, digoxin and diuretics (83.6%), (86.9%), (48%), (10%) and (65%), respectively.
Patients enrolled in our study had MoCA score <26 which reflected initiation of decline in cognition related to HF. A possible reason for cognitive decline in HF patients can be decreased cerebral blood perfusion due to low cardiac output in HF. These findings reflect the need for cognitive training programs to improve the intellectual functions of HF patients for better clinical outcomes. These findings were consistent with the findings of a systematic review which found that patients with HF are at increased risk for cognitive decline over the long-term compared with patients without HF.[20] Another study conducted by Harkness et al. (2011)[21] revealed that CI was detected in >70% of HF patients. Early intervention (Cognitive training) at this point can be beneficial in preventing further decline in cognition which, in turn, will contribute toward better self-care, treatment adherence and QOL.
In the present study, experimental group participants received cognitive training for 12 weeks along with routine health care services whereas the control group received only routine care. Based on the present study findings, it can be said that cognitive training is an effective strategy for enhancing cognitive functions among HF patients as statistically significant improvement was noticed in all three dimensions of cognition (memory, executive functions and attention/concentration) as well as in total MoCA score among experimental group participants. The effectiveness of cognitive training has also been reported by Tang et al. (2019)[22] where MOCA score improved significantly (P = 0.013) in the cognitive training group compared to their counterpart. Similarly, Gary et al. (2019)[23] reported that participants in the combined aerobic exercise and cognitive training program had significant improvement (P = 0.02) in verbal memory at 3 months and a trend for sustained improvement at 6-months compared to exercise alone or the attention control usual care group. A randomised controlled trial by Pressler et al. (2015)[24] also showed significant improvement (P = 0.02) in working memory as a result of computerised cognitive training over the 8-week intervention. Working memory as measured by CogState one-back accuracy scores for patients in the brain fitness group improved over time as compared to the control group. CI may impact the ability to perform self-care activities of daily living in HF as self-care is a mentally demanding activity that requires decision-making, disease knowledge and skills in self-management tasks. A systematic review conducted by Lovell et al. (2019)[25] found that cognitively impaired HF patients tend to display lower levels of self-care as evidenced by low scores on the self-care 64 of the HF Index. After the intervention, a significant improvement was noticed in different self-care domains among experimental group participants. Guidance and counselling related to self-care management in HF might have contributed toward the enhancement of the confidence of participants in carrying out self-care activities. These findings are in line with a previous study by Davis et al. (2012)[26] where they found that mean scores of all three SCHFI sub-scales showed greater improvement in self-care for the intervention group when compared with the control group.
In general, study participants had high medication adherence levels. Possible reasons for this finding can be life-threatening symptoms as well as prior sensitisation of the participants by the physician about the importance of medication compliance to avoid adverse events. However, a significant proportion of participants had a moderate level of medication adherence which highlights the need for continuous reinforcement for better medication compliance. There was significant improvement in medication adherence among experimental group participants after the intervention. The effect of guidance on the importance of medication adherence was reflected in terms of 100% therapeutic compliance in experimental group participants as compared to their counterparts in which it remained at the pre-intervention level (80%). Daily dairy records of medications consumed may have further improved compliance with treatment regimens. Findings of a systematic review showed a statistically significant improvement in medication adherence in the intervention group which supports the present study findings.[27]
It was seen that 93.3% of control and 96.7% of experimental group participants had poor QOL at baseline. This may be due to physical and emotional symptoms produced by HF which limit patients’ activities of daily living as well as social interaction ultimately affecting the QOL. The study conducted by Aggelopoulou et al. (2017)[28] supported these findings and highlighted poor QOL among HF patients (the mean score of MLHFQ was 65.4 ± 20.6). After the intervention experimental group, participants experienced and communicated better QOL and this enhancement in different parameters of QOL can be related to significant improvement in MoCA score, that is cognitive functions and improvement in different domains of self-care (as discussed earlier) made the present study participants in experimental group experience better QOL which has been revealed by study findings. A randomised control trial by Gary et al. (2022) [29] revealed that combined exercise and a computerised cognitive training program resulted in significant improvement (P < 0.039) in QOL among HF patients.
Patients’ satisfaction level reflects the efficacy of health care services of that health institute. The majority of the experimental group participants (89.6%) were satisfied with the cognitive training comprised of an educational guidebook, meditation audio, diet video, and cognitive and physical exercises. Health education, counselling, demonstration and reinforcement are provided for each component as per the content and needs of the patient. On the other hand, just more than half of the control group participants were dissatisfied with the care provided. The findings of the present study were supported by a study carried out by Pressler et al. (2011)[30] that HF patients had a high level of satisfaction with nurse-enhanced memory intervention and they were interested in improving their cognitive health.
Limitation
Prolonged follow-up and limited period of data collection made it difficult to enrol more subjects. Hence, a larger sample size was not possible. A longitudinal study of a similar type can be planned on a larger sample size.
Later on effects of cognitive training could not be assessed due to the completion of research project.
CONCLUSION
Cognitive training is significantly effective in improving cognitive functions (memory, executive functions and attention/concentration), self-care adequacy, medication adherence, QOL and functional capacity among HF patients.
Acknowledgements
We acknowledge the participation of all study subjects.
Ethical approval
The research/study was approved by the Institutional Ethics Committee at the Post Graduate Institute of Medical Education and Research, Chandigarh, number INT/IEC/2022/SPL-204, dated 21st March 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Heart Failure: Preventing Disease and Death Worldwide. ESC Heart Fail. 2014;1:4-25.
- [CrossRef] [PubMed] [Google Scholar]
- Financial Burden of Heart Failure in A Developing Country: Cost Analysis from Manipal Heart Failure Registry, India. J Public Health. 2021;29:585-94.
- [CrossRef] [Google Scholar]
- Quality of Life in Patients with Heart Failure: Ask the Patients. Heart Lung. 2009;38:100-8.
- [CrossRef] [PubMed] [Google Scholar]
- Key Elements of Interventions for Heart Failure Patients with Mild Cognitive Impairment or Dementia: A Systematic Review. Eur J Cardiovasc Nurs. 2020;19:8-19.
- [CrossRef] [PubMed] [Google Scholar]
- Detecting and Managing Cognitive Impairment to Improve Engagement in Heart Failure Self-care. Curr Heart Fail Rep. 2017;14:13-22.
- [CrossRef] [PubMed] [Google Scholar]
- Healthcare Resource Use among Heart Failure Patients in A Randomized Pilot Study of A Cognitive Training Intervention. Heart Lung. 2013;42:332-8.
- [CrossRef] [PubMed] [Google Scholar]
- EPA-1593-Psychometric and Clinometric Properties of the Montreal Cognitive Assessment (MoCA) in a Greek Sample. Eur Psychiatry. 2014;29:1.
- [CrossRef] [Google Scholar]
- Psychometric Testing of the Self-care of Heart Failure Index Version 6.2. Res Nurs Health. 2013;36:500-11.
- [CrossRef] [PubMed] [Google Scholar]
- About the Hill-Bone Scales. Available from: https://nursing.jhu.edu/faculty_research/research/projects/hill-bone/about-hill-bone-scales.html [Last accessed on 2022 Mar 03]
- [Google Scholar]
- Psychometric Properties of the Minnesota Living with Heart Failure Questionnaire (MLHF-Q) Clin Rehabil. 2001;15:489-500.
- [CrossRef] [PubMed] [Google Scholar]
- Six-minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest. 2020;157:603-11.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of Acute Decompensated Heart Failure in India : The AFAR study (Acute Failure Registry Study) J Pract Cardiovasc Sci. 2015;1:35.
- [CrossRef] [Google Scholar]
- Risk Factors for Heart Failure: A Population-based Case-control Study. Am J Med. 2009;122:1023-8.
- [CrossRef] [PubMed] [Google Scholar]
- Heart Failure in Africa, Asia, the Middle East and South America: The INTER-CHF study. Int J Cardiol. 2016;204:133-41.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Presentation, Management, In-hospital and 90-day Outcomes of Heart Failure Patients in Trivandrum, Kerala, India: The Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17:794-800.
- [CrossRef] [PubMed] [Google Scholar]
- Precipitating Factors for Hospitalization with Heart Failure: Prevalence and Clinical Impact Observations from the Gulf CARE (Gulf Acute Heart Failure Registry) Med Princ Pract. 2020;29:270-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Characteristics and Outcomes of Patients Admitted with Acute Heart Failure: Insights from a Single-center heart Failure Registry in South India. Egypt Heart J. 2021;73:38.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive Impairment in Patients with Heart Failure: An International Study. ESC Heart Fail. 2020;7:46-53.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive Change in Heart Failure: A Systematic Review. Circ Cardiovasc Qual Outcomes. 2013;6:451-60.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for Cognitive Deficits Using the Montreal Cognitive Assessment Tool in Outpatients =65 Years of age with Heart Failure. Am J Cardiol. 2011;107:1203-7.
- [CrossRef] [PubMed] [Google Scholar]
- The Effects of 7-week Cognitive Training in Patients with Vascular Cognitive Impairment, no Dementia (The Cog-VACCINE Study): A Randomized Controlled Trial. Alzheimers Dement. 2019;15:605-14.
- [CrossRef] [PubMed] [Google Scholar]
- Exercise and Cognitive Training as a Strategy to Improve Neurocognitive Outcomes in Heart Failure: A Pilot Study. Am J Geriatr Psychiatry. 2019;27:809-19.
- [CrossRef] [PubMed] [Google Scholar]
- Nurse-enhanced Computerized Cognitive Training Increases Serum Brain-derived Neurotropic Factor Levels and Improves Working Memory in Heart Failure. J Card Fail. 2015;21:630-41.
- [CrossRef] [PubMed] [Google Scholar]
- Self-management of Heart Failure in Dementia and Cognitive Impairment: A Systematic Review. BMC Cardiovasc Disord. 2019;19:99.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted Intervention Improves Knowledge But Not Self-care or Readmissions in Heart Failure Patients with Mild Cognitive Impairment. Eur J Heart Fail. 2012;14:1041-9.
- [CrossRef] [PubMed] [Google Scholar]
- Medication Adherence Interventions for Older Adults With Heart Failure: A Systematic Review. J Gerontol Nurs. 2017;43:37-45.
- [CrossRef] [PubMed] [Google Scholar]
- The Level of Anxiety, Depression and Quality of Life among Patients with Heart Failure in Greece. Appl Nurs Res. 2017;34:52-6.
- [CrossRef] [PubMed] [Google Scholar]
- Exercise and Cognitive Training Intervention Improves Self-Care, Quality of Life and Functional Capacity in Persons With Heart Failure. J Appl Gerontol. 2022;41:486-95.
- [CrossRef] [PubMed] [Google Scholar]
- Nurse-Enhanced Memory Intervention in Heart Failure: the MEMOIR study. J Card Fail. 2011;17:832-43.
- [CrossRef] [PubMed] [Google Scholar]






