Translate this page into:
Comparison Between Efficacy of Oral Melatonin and Oral L-theanine in Improving Sleep in Cancer Patients Suffering From Insomnia: A Randomised Double-blinded Placebo-controlled Study
*Corresponding author: Athira AS, Department of Anaesthesiology, Karnataka Institute of Medical Sciences, Hubli, Karnataka, India. athira.asha93@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kurdi MS, Athira AS, Ladhad DA, Mitragotri MV, Baiju A. Comparison Between Efficacy of Oral Melatonin and Oral L-theanine in Improving Sleep in Cancer Patients Suffering from Insomnia: A Randomised Double-blinded Placebo-controlled Study. Indian J Palliat Care. 2024;30:176-81. doi: 10.25259/IJPC_89_2023
Abstract
Objectives:
The primary objective was to compare the hypnotic efficacy of oral melatonin, oral L-theanine, and placebo in improving sleep in cancer patients with insomnia by the Athens Insomnia Scale (AIS). The secondary objective was to know the prevalence of insomnia in patients with cancer.
Materials and Methods:
A prospective, double-blinded, placebo-controlled study was conducted after obtaining Institutional Ethics Committee approval. One hundred and twenty patients were randomly assigned to either Group A (melatonin), Group B (L-theanine), or Group C (placebo). They were instructed to take the tablets for 14 consecutive days, two h (hours) before bedtime, and evaluated with AIS on the 1st day, 7th day, and 14th day.
Results:
There were seven dropouts: Two in Group A, two in Group B, and three in Group C. Significant differences in favour of melatonin treatment were found. There were statistically significant improvements in insomnia in cancer patients on melatonin intake compared to L-theanine. L-theanine had statistically significant improvements in insomnia in comparison to placebo. The mean ± standard deviation AIS on day one in Group A was 14.82 ± 1.29; on day seven was 10.92 ± 1.12; and on day 14 was 5.00 ± 0.70. Similarly, in Group B, the mean ± standard deviation AIS was 15.39 ± 1.03, 13.05 ± 1.06, and 9.55 ± 1.01, and in Group C, the mean AIS was 14.92 ± 1.40, 14.54 ± 1.35 and 13.05 ± 1.61 on the 1st, 7th and 10th day, respectively. There was an improvement in sleep from 1 to 7 days, 7 days to 14 days, and 1 day to 14 days in all the groups (P < 0.005).
Conclusion:
The hypnotic efficacy of oral melatonin 3 mg was better than oral L-theanine 200 mg in cancer patients having insomnia. Furthermore, L-theanine had a better hypnotic efficacy when compared to a placebo.
Keywords
Athens insomnia scale
Insomnia
L-theanine
Melatonin
INTRODUCTION
Sleep is vital to all human functioning. The circadian rhythm, the 24-hour internal clock in our brain, regulates cycles of alertness and sleepiness by responding to slight variations in our surroundings.[1] According to the Diagnostic Statistical Manual (DSM)-V criteria, insomnia is defined as dissatisfaction with sleep quantity and quality associated with one or more of the following symptoms: Difficulty in initiating sleep, difficulty in maintaining sleep or frequent awakenings or difficulty in returning to sleep after awakenings.[2] Insomnia can exacerbate cancer-associated medical conditions such as pain, psychiatric comorbidities, fatigue, and the use of sedative drugs.[3] Insomnia is a frequently overlooked problem in cancer practice, and patients may fail to report it, assuming it to be a normal and temporary reaction to a cancer diagnosis or treatment. The sequelae of insomnia include daytime fatigue, reduced alertness, irritability, and impaired concentration, and all these symptoms have a major negative impact on the quality of life.[2]
Melatonin is a hormone secreted by the pineal gland. Melatonin exhibits both hypnotic and chronobiotic properties for inducing sleep and treating sleep disorders. Melatonin is different from benzodiazepines and their derivatives in that it exerts a sleep-promoting effect by amplifying day/night differences in alertness and sleep quality. It also displays a modest sleep-inducing effect, quite mild as compared to that seen with benzodiazepines.[4]
L-theanine (g-glutamyl ethyl amide) is a unique non-protein amino acid found in green tea (Camellia sinensis), a widely consumed beverage associated with human health. As the structure of L-theanine resembles that of L-glutamic acid, its mechanism of action may be potentially mediated through glutamate receptors, a possibility supported by its partial co-agonistic effect on the N-methyl-D-aspartate receptor. L-theanine has been reported to modulate alpha activity as well as provide beneficial effects on the mental state, including sleep quality. While the anti-stress effects of L-theanine have been observed following once and twice-daily administration, its attention-improving effects have been observed in response to treatment of 100 mg/day on four consecutive days and also following 200mg/day.[5]
With this background, we conducted a prospective, double-blinded, randomised controlled study. Our primary objective was to compare the hypnotic efficacy of oral melatonin, oral L-theanine and placebo in improving sleep quality in cancer patients having insomnia by means of the Athens Insomnia Scale (AIS). Our secondary objectives were to estimate the prevalence of insomnia in cancer patients quantitatively.
MATERIALS AND METHODS
This study was performed on cancer patients satisfying DSM IV criteria of insomnia visiting the pain and palliative care clinic at Karnataka Institute of Medical Sciences, Hubballi. Approval for the study was obtained from the institutional ethics committee (ECR/489/Inst/KA/2013/RR16 date 4/10/2021). The protocol of the study was registered prospectively with the Clinical Trials Registry-India (CTRI/2022/02/051604). The study was conducted over the period from February 2022 to February 2023. The study followed all the principles of the Declaration of Helsinki. The sample size was calculated from a previous study, where it was found that insomnia improved in 46.5% of the participants who were administered melatonin as compared to 11.3% in the placebo group after 14 days of medication.[6] Hence, assuming the effect size to be 35% and a similar amount of difference between the performance of melatonin and L-theanine and placebo, the minimal sample size required was noted to be 40 in each group after adjusting for Fleiss continuity correction at 80% power and 95% confidence interval. One hundred and twenty patients of either gender, aged between 18 and 55 years, were included in the study. Patients with neuropsychiatric illness, deaf and dumb patients, patients with a previous history or currently taking sleep medications, opioids or anti-anxiety medications (fluvoxamine) or on oral contraceptive pills, on immunosuppressants including steroids and other adjuvants that affect sleep, pregnant and lactating females were excluded from the study.
All the cancer patients on arrival at the pain and palliative clinic were randomly assigned to Group A, Group B, and Group C by fish bowl technique by the pharmacist. Age, gender, height, weight, body mass index (BMI), comorbidities, type of cancer, stage of cancer, and history of surgical interventions were noted. Patients were given either a capsule of melatonin 3 mg (‘Sleep Easy’ from Healthkart pharmaceuticals) or a capsule of L-theanine 200 mg (‘Healthy Hey’ from Healthkart pharmaceuticals-) or a placebo capsule as per randomisation by the pharmacist. The capsules in all three groups were wrapped in similar-looking opaque red-colored envelopes. All three capsules were yellowish-white coloured, were similar looking, and had the same consistency. The identity of the drug was revealed by the palliative care pharmacist to the evaluator at the end of the study. The patients were instructed to take the tablets every day for 14 consecutive days, two h before routine bedtime. They were evaluated with AIS. The AIS assesses the severity of insomnia using diagnostic criteria set forth by the International Classification of Diseases-10. The eight items of the scale evaluate sleep onset, night and early-morning waking, sleep time, sleep quality, frequency and duration of complaints, distress caused by the experience of insomnia, and interference with daily functioning.
Data were entered in Microsoft Excel and were analysed using the Statistical Package for the Social Sciences version 22.0 (ZoomInfo: United States of America). The data analysis of this study included univariate and bivariate analysis as per the study objectives. As a first step, normality testing for the data was done using the Shapiro– Wilk test. The univariate analysis was done to describe the socio-demographic information, weight, height, presence of any comorbid condition, and severity of insomnia as assessed by AIS for all the patients as well as groups as per the drug administered. Quantitative variables such as age, BMI, weight, and height were presented as mean along with standard deviation (SD), that is, (mean±SD) or median with interquartile range (median [interquartile range]) depending on the type of data distribution. Categorical variables such as gender, presence of comorbidity, anxiety, and insomnia severity as derived from the AIS were presented as numbers and percentages. The comparison was done within the group for all the independent variables (weight, height, and comorbid conditions) and dependent variables (insomnia and its severity) amongst the three drug groups at the baseline and subsequent follow-ups. We did a comparison between groups of all the variables mentioned above, that is, the baseline AIS, 1st day AIS, and 7th day AIS of Group A versus Group B, Group B versus Group C, and the Group A versus Group C using one-way analysis of variance (ANOVA) followed by post hoc pairwise Tukey’s test. Intragroup analysis of the patients was done using repeated measures ANOVA followed by post hoc pairwise Bonferroni comparison. P < 0.05 was considered significant to reject the null hypothesis.
RESULTS
A total of 400 patients were recruited for the study, out of which 120 patients in the age group between 18 and 55 years with cancer and suffering from insomnia were randomly allocated into three groups. About 28% of the total cancer patients recruited were suffering from insomnia. About 45% of the patients included had comorbidities, and the most common comorbidity was hypertension. (25%) Insomnia was noted to be more severe in cervical cancer patients. Out of 19 patients evaluated with cervical cancer, nine patients (47.3%) had severe insomnia. There were a total of seven dropouts, including two in Group A, two in Group B, and three in Group C [Figure 1]. The mean age in Group A was 44.11 ± 8.07 years; in Group B was 45.03 ± 7.92 years; and in Group C was 43.27 ± 8.40 years. Group A had 47.37% males and 52.63% female cases, Group B had 42.11% males and 57.89% females and Group C had 54.05% males and 45.95% female cases. The age and gender in all three groups were comparable.
On day 1 of the trial, patients in all three groups had similar AIS. The AIS subsequently improved more in Group A, followed by Group B, with the least improvement noted in Group C in the 1st and 2nd week [Table 1]. The AIS of Group A improved on day seven and day 14 which was statistically significant in comparison to Group B and Group C (P = 0.0001) [Figure 2].
Intragroup comparison of mean AIS was done using repeated measures ANOVA, and it was found to be statistically significant in all three groups. Post hoc pairwise comparison (Bonferroni) in all three groups between day 1 and day 7, day 7 and 14, and day 1 and day 14 was also noted to be significant [Table 2 and Figure 3].
DISCUSSION
Melatonin and L-theanine are naturally occurring compounds known for their hypnotic property. The current study is the first study in which the hypnotic efficacy of melatonin has been compared with that of L-theanine. The demographic profile of patients in the current study was comparable to that in other studies comparing the hypnotic efficacy of melatonin with placebo in cancer patients.[6] No statistical differences were observed with respect to age, weight, and BMI.
There was a significant improvement in the AIS in patients receiving melatonin in comparison with patients receiving L-theanine and placebo on the 7th day and 14th day. These results were similar to the study by Kurdi and Muthukalai on the effect of oral melatonin in improving sleep in cancer patients with insomnia, where the authors noted that regular daily intake of oral melatonin 3 mg 2 h before bedtime along with non-pharmacological measures improved sleep.[6]
Intragroup comparison in the melatonin group showed that there was considerable and significant improvement after the 7th and 14th days compared to the 1st day. Shahrasbi et al. used a higher dose of 6 mg melatonin at night in breast cancer patients and concluded that during the first 30 days, total score, sleep quality, sleep duration, sleep delay, and daily performance improved considerably with melatonin.[7] Our study findings were also similar to the results of the systematic review by Jafari-Koulaee and Bagheri-Nesami and the study conducted by Kunz and Mahlberg on the effect of melatonin on sleep quality and insomnia.[8,9]
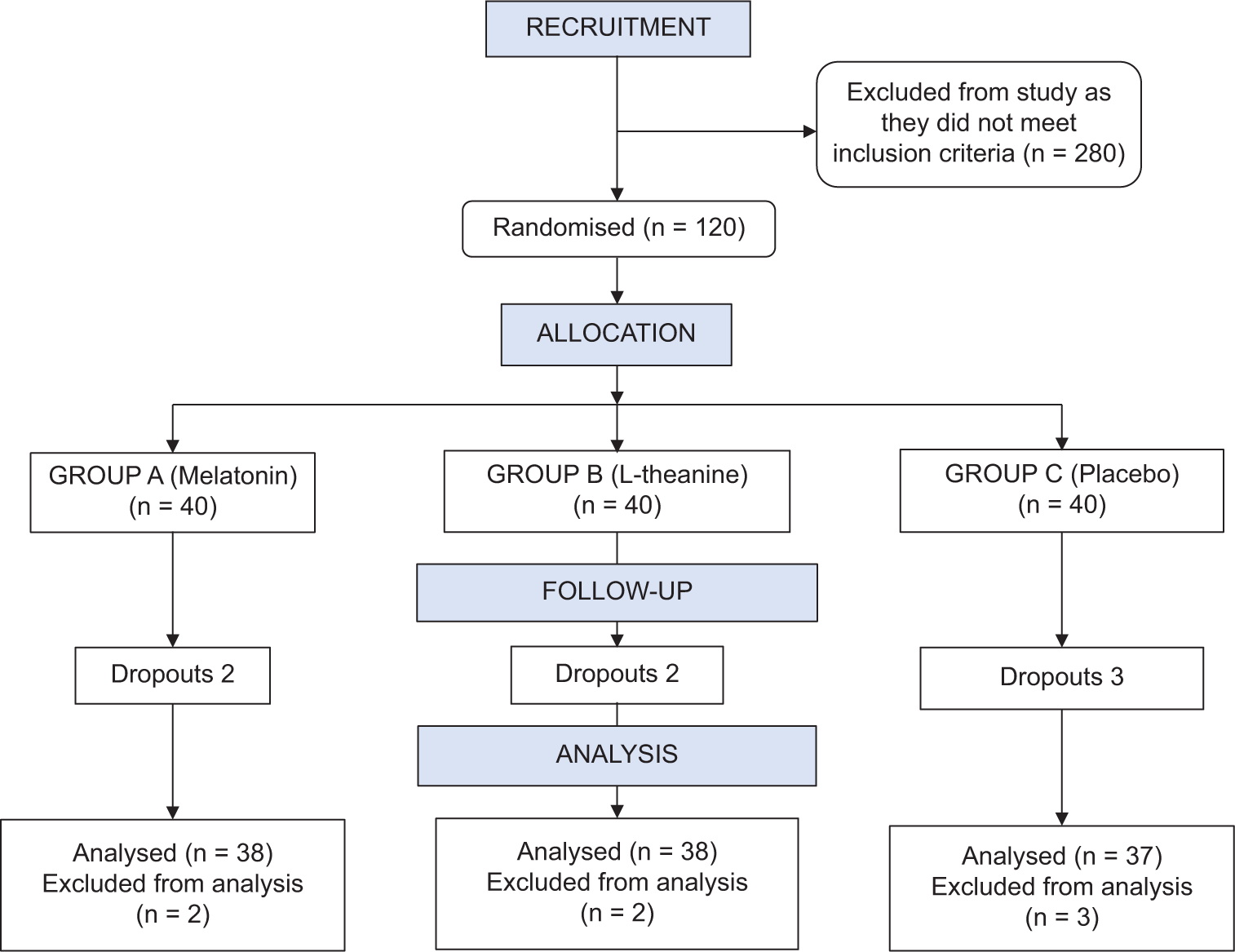
- Consolidated standards of reporting trials (CONSORT) diagram. n=Number.
| Time points | Groups | Mean | SD | P-value | Post hoc pairwise comparison (Tukey’s test) | |
|---|---|---|---|---|---|---|
| 1st day | Group A | 14.82 | 1.29 | 0.1030 | Group A vs. Group B | P=0.112 |
| Group B | 15.39 | 1.03 | Group B vs. Group C | P=0.932 | ||
| Group C | 14.92 | 1.40 | Group A vs. Group C | P=0.229 | ||
| 7th day | Group A | 10.92 | 1.12 | 0.0001* | Group A vs. Group B | P<0.005 |
| Group B | 13.05 | 1.06 | Group B vs. Group C | P<0.005 | ||
| Group C | 14.54 | 1.35 | Group A vs. Group C | P<0.005 | ||
| 14th day | Group A | 5.00 | 0.70 | 0.0001* | Group A vs. Group B | P<0.005 |
| Group B | 9.55 | 1.01 | Group B vs. Group C | P<0.005 | ||
| Group C | 13.05 | 1.61 | Group A vs. Group C | P<0.005 | ||
| Range of days | Groups | Mean difference | SE | Post hoc pairwise comparison (Bonferroni) (P-value) |
|---|---|---|---|---|
| 1st day to 7th day | Group A | 3.89 | 0.92 | 0.0001* |
| Group B | 2.34 | 0.78 | ||
| Group C | 0.38 | 0.55 | ||
| 1st day to 14th day | Group A | 9.82 | 1.16 | 0.0001* |
| Group B | 5.84 | 1.00 | ||
| Group C | 1.86 | 1.21 | ||
| 7th day to 14th day | Group A | 5.92 | 0.88 | 0.0001* |
| Group B | 3.50 | 0.89 | ||
| Group C | 1.49 | 1.02 |
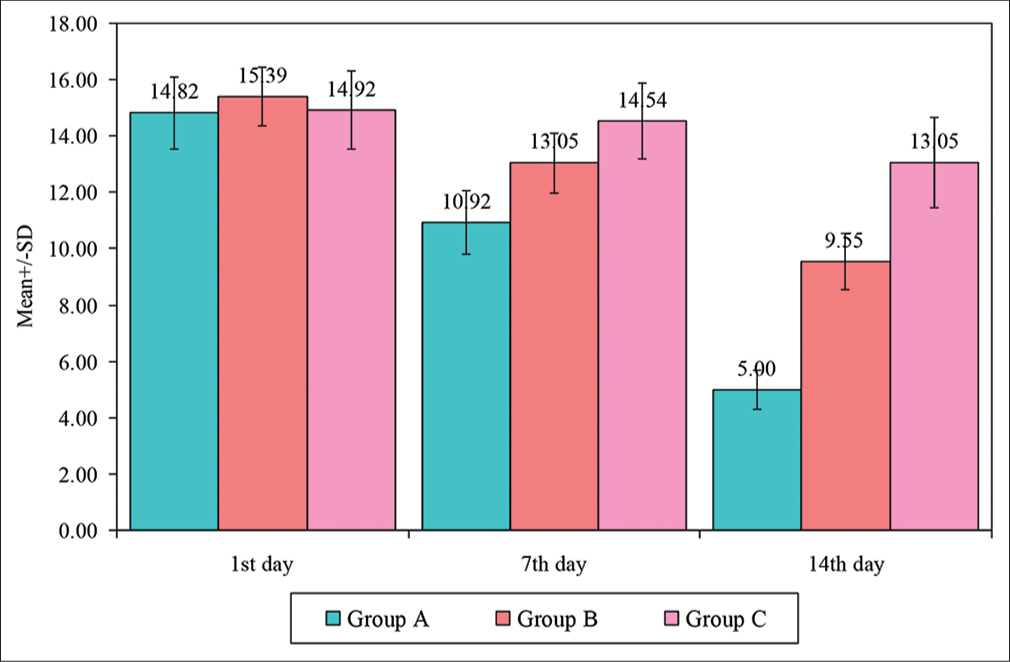
- Intergroup comparison of AIS of the three groups. AIS: Athen’s Insomnia Scale, SD: Standard deviation, Group A: Melatonin, Group B: L-theanine, Group C: Placebo.
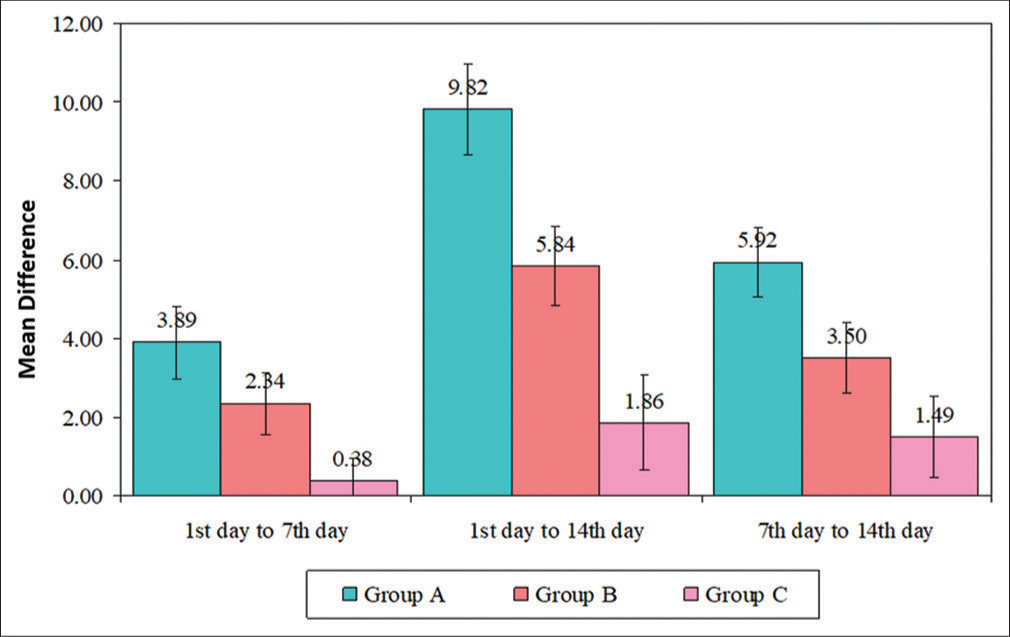
- Intragroup comparison of three groups between different follow-up times. AIS: Athen’s Insomnia Scale, SD: Standard deviation, Group A: Melatonin, Group B: L-theanine, Group C: Placebo.
The AIS in those receiving L-theanine was significantly better than in patients receiving placebo on the 7th day and 14th day in our study suggesting that L-theanine was effective in reducing insomnia in cancer patients when compared to placebo. Intragroup comparison in the L-theanine group also showed significant improvement. Our results correlate with the study done by Sarris et al., which concluded that L-theanine improved sleep satisfaction as well as resolved the symptoms of sleep disturbance in generalised anxiety disorders.[10] However, they used L-theanine at a higher dose of 450 mg/day for eight weeks.
Although the placebo group had the least improvement in comparison to melatonin and L-theanine, the intragroup comparison of patients receiving placebo showed significant improvement. This could only be explained as due to the ongoing treatment of the cancer patients or the nutrient effects of placebo causing improvement in AIS.
A study was conducted by Hidese et al. on the effect of L-theanine administration on stress-related symptoms and cognitive functions in healthy adults.[5] Nevertheless, the Pittsburgh sleep quality index (PSQI) scale was used in this study to assess insomnia, and the authors concluded that oral L-theanine reduces sleep latency.[5]
There are many other studies that have used the daily sleep diary and PSQI rated by the bed partner or roommate.[11,12] Most of our study population had a poor educational background, and hence, the maintenance of a sleep diary and the evaluation of PSQI would have been improbable and time-consuming. Actigraphy and polysomnography are other objective monitors that can be used to record the sleep pattern.[12] Since getting these objective monitors was not feasible for us, we could not use them. We preferred using AIS which is a well-known validated tool used to screen and diagnose insomnia and to assess the severity of insomnia. AIS has high sensitivity (93%) and specificity (85%).[13]
28% of the cancer patients interviewed had insomnia, whereas other studies state that it ranges between 24% and 95%.[5] 45% of patients had comorbidities, and the most common comorbidity was hypertension (25%). Fowler et al., in their study on comorbid prevalence in cancer patients, noted that although chronic obstructive pulmonary disease was most common amongst oral cancer and lung cancer patients, hypertension was a common comorbidity in all other cancer patients.[14] Insomnia was more severe in cervical cancer patients in our study compared to other cancer patients. Out of 19 patients evaluated with cervical cancer, nine patients (47.3%) had severe insomnia. In other studies, it was found that breast and lung cancer patients had the highest degree of insomnia, followed by cervical cancer.[4] This contrasting result of our study could be due to the exclusion of patients on opioids and other sedative analgesics, where we noted that most of the breast and lung cancer patients were on opioids. Melatonin is a hormone secreted by the pineal gland that regulates the sleep-wake cycle and circadian rhythm.[6] It is easily available in the market in the form of syrups, capsules, and tablets. We used a 3 mg tablet of melatonin, within the safe dose range, two h before routine bedtime as in other studies and as the peak plasma concentrations are reached in 60–150 min.[15] L-theanine was used in the dose of 200 mg in the present study as it is the most routinely used dose and L-theanine in the dose range of 200–450 mg has been found to have a relaxation effect on the brain and reduces anxiety.[7,16] Both drugs were used for a period of 2 weeks, as in other studies. Two weeks allows for synchronisation of drug levels with the circadian cycle, and hence, we noted considerable improvement in sleep when these drugs were taken for the entire period of 2 weeks.[16,17]
The current study is associated with a few limitations. First, patients in advanced stages of oesophageal and oral cancer who were on a liquid diet and had dysphagia were excluded for obvious reasons leading to bias. This could have been circumvented in future studies using spray-dried pectin microspheres of melatonin and L-theanine for targeted nasal delivery. The second limitation is the inclusion of patients with all stages of cancer in the study. In advanced stages of cancer, insomnia can be due to pain, while in the early stages of cancer, insomnia may be due to depression and anxiety. We could not standardise all these factors in our study because of the different types of cancer, varying socioeconomic status of the study population, and poor educational background of the patients. Acceptance and compliance with adjuvant oncological treatment modalities such as radiotherapy and chemotherapy can also be added as parameters in future studies. Patients who are compliant with the treatment modalities will show better AIS scores even in the advanced stages of cancer. Third, as both melatonin and L-theanine are naturally occurring compounds, the intake of nutrients in the study cases could have confounded the results. Melatonin is present in fish, meat, and dairy products, and L-theanine is present in green tea. Fourth, we did evaluate whether patients were at home or institutionalised which could have affected the change in environs leading to change in sleep quality. We recommend future studies to evaluate the role of these dietary habits and sources on sleep quality, like home or admitted, and also the use of more sensitive methods of assessing insomnia by objective monitors such as actigraphy, polysomnography, and electroencephalography.
CONCLUSION
The results of the current study lead to the conclusion that the hypnotic efficacy of oral melatonin 3 mg is better than oral L-theanine 200 mg in improving sleep in cancer patients suffering from insomnia, and oral L-theanine 200 mg is better than placebo. The prevalence of insomnia in cancer patients is 28%, and the severity of insomnia is highest in cervical cancer patients.
Ethical approval
The research/study was approved by the Institutional with Ethics Committee at Karnataka Institute of Medical Sciences, number ECR/489/Inst/KA/2013/RR16, dated 4/10/2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The DSM-5: Classification and Criteria Changes. World Psychiatry. 2013;12:92-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep Disturbance in Adults with Cancer: A Systematic Review of Evidence for Best Practices in Assessment and Management for Clinical Practice. Ann Oncol. 2014;25:791-800.
- [CrossRef] [PubMed] [Google Scholar]
- A Comparative Study between Oral Melatonin and Oral Midazolam on Preoperative Anxiety, Cognitive, and Psychomotor Functions. J Anesthesiol Clin Pharmacol. 2015;31:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-related Insomnia. Am J Hosp Palliat Med. 2014;31:777-85.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients. 2019;11:2632-69.
- [CrossRef] [PubMed] [Google Scholar]
- The Efficacy of Oral Melatonin in Improving Sleep in Cancer Patients with Insomnia: A Randomized Double-blind Placebo-controlled Study. Indian J Palliat Care. 2016;22:295-300.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin Effect on Sleep Disorders in Breast Cancer Patients Receiving Adjuvant Chemotherapy. J Clin Oncol. 2022;40:e12512.
- [CrossRef] [Google Scholar]
- The Effect of Melatonin on Sleep Quality and Insomnia in Patients with Cancer: A Systematic Review Study. Sleep Med. 2021;82:96-103.
- [CrossRef] [PubMed] [Google Scholar]
- A Two-part, Double-blind, Placebo-controlled Trial of Exogenous Melatonin in REM Sleep Behaviour Disorder. J Sleep Res. 2010;19:591-6.
- [CrossRef] [PubMed] [Google Scholar]
- L-theanine in the Adjunctive Treatment of Generalized Anxiety Disorder: A Double-blind, Randomised, Placebo-controlled Trial. J Psychiatr Res. 2019;110:31-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prolonged-release Melatonin for Insomnia-an Open-label Long-term Study of Efficacy, Safety, and Withdrawal. Ther Clin Risk Manag. 2011;7:301-2.
- [CrossRef] [PubMed] [Google Scholar]
- The Effect of Melatonin on Sleep and Quality of Life in Patients with Advanced Breast Cancer. Support Care Cancer. 2015;24:1097-105.
- [CrossRef] [PubMed] [Google Scholar]
- The Diagnostic Validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55:263-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity Prevalence among Cancer Patients: A Population-based Cohort Study of Four Cancers. BMC Cancer. 2020;20:2.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin in the Treatment of Insomnia in Children and Adolescents. Psychiatr Bull. 2004;28:222-4.
- [CrossRef] [Google Scholar]
- Efficacy of Prolonged Release Melatonin in Insomnia Patients Aged 55-80 Years: Quality of Sleep and Next-day Alertness Outcomes. Curr Med Res Opin. 2007;23:2597-605.
- [CrossRef] [PubMed] [Google Scholar]
- Membrane Melatonin Receptors Activated Cell Signaling in Physiology and Disease. Int J Mol Sci. 2021;23:471-5.
- [CrossRef] [PubMed] [Google Scholar]






