Translate this page into:
Conventional Fractionation versus Quad Shot in Advanced Head-and-Neck Cancers: A Randomized Controlled Trial
Address for correspondence: Dr. Akansha Choudhary, Department of Radiotherapy, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India. E-mail: cdrakansha@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
A significant number of patients with head-and-neck cancers have an incurable disease with limited life expectancy. The objective of the present study was to compare two different short courses of hypofractionated palliative radiotherapy regimens to evaluate symptoms, disease response, and acute toxicity.
Materials and Methods:
Previously untreated 50 patients of Stage IV B and IV C head and neck cancers were randomized to receive conventional hypofractionated palliative radiotherapy 30 Gy/10 fractions/2 weeks (control group) or Quad Shot regimen (study group) 14 Gy in 4 fractions given twice a day at least 6 h apart for 2 consecutive days. This regimen was repeated at 4 weekly intervals for a further two courses if there was no tumor progression.
Results:
Symptom relief was similar among the two schedules for pain (60.86 vs. 57.17), dysphagia (60.86 vs. 52.17%), and hoarseness (43.85 vs. 38.09%). Overall response (that is partial response and stable disease) was seen in majority (>70%) of the patients in both the groups. Treatment was very well tolerated with no patient experiencing more than Grade 3 toxicity in the control group and Grade 2 toxicity in the study group.
Conclusions:
Quad Shot regimen is an effective hypofractionated palliative radiotherapy schedule with minimal toxicity, good symptom relief, and response rate as compared to conventionally used regimen (30 Gy/10 fractions/2 weeks).
Keywords
Acute toxicity
hypofractionated palliative radiotherapy
incurable head
neck cancer
Quad Shot regimen
response assessment
INTRODUCTION
Head-and-neck cancer accounts for 14.3% of all cancers in India[1] while globally it is 4.8% of all cancer burden.[1] It contributes to 30% of all cancers in males in India.[2]
Oral cancers are predominant forms of head-and-neck squamous cell cancer in South Asian countries such as Sri Lanka, India, Pakistan, and Bangladesh, whereas in Southeast Asian countries such as China, Malaysia, Indonesia, and Singapore, nasopharyngeal carcinoma is more prevalent. In India, lip, oral cavity, oropharyngeal, and hypopharyngeal cancers constitute >80% of all head-and-neck cancer burden.[1] Histologically head-and-neck cancer is mostly squamous cell carcinoma.
Cigarette smoking and alcohol consumption are the main reasons for head-and-neck squamous cell cancer in the Western population, whereas the use of smokeless tobacco and areca nut is the most common cause in Southeast Asia.[34] The various forms in which smokeless tobacco is used in developing countries include khaini, mava, paan (betel quid), zarda, snuff, and mashiri.[5] The prevalence of HPV in head-and-neck squamous cell cancer is around 50%[6] with the highest prevalence in cancers of the tonsil and base of the tongue,[7] particularly HPV-16 is the most commonly involved type.[8]
A large majority of head-and-neck cancers present in advanced incurable stage[9] because of a very advanced locoregional disease, significant medical comorbidities, poor performance status, distant metastasis, or a combination of these factors. However, they still need some form of treatment to control their locoregional disease[10] which may lie in close proximity to several critical normal tissues such as the spinal cord; salivary glands; mandible; nerves; major blood vessels; and the organs of speech, swallowing, hearing, and respiration.[11]
The management of locoregionally advanced head-and-neck cancers with curative intent is an area of active research and has evolved considerably over time with volumes of research, large randomized control trials, and meta-analysis available for reference. However, there is sparse literature on palliative regimen with several lacunae related to dose and fractionation, identification of patients suitable for palliation, degree or duration of symptom relief, and treatment-related toxicities.[12131415]
With this background, we had undertaken a study to assess the feasibility of a short course of radiation delivered in 2 days for palliation of locally advanced head-and-neck cancers in comparison to the commonly used dose of 30 Gy/2 weeks/10 fractions. The proposed fractionation schedule is likely to decrease the patient visits to the hospital, reduce the travel expenses, and load on the treatment machines, and this, in turn, would cut down the waiting lists for patients. Therefore, it would be very useful, especially in a developing country like India.
MATERIALS AND METHODS
The present prospective randomized controlled study was carried out with 50 previously untreated patients with biopsy-proven squamous cell carcinoma of the head-and-neck region from November 2014 to April 2016. Of a total of 50 patients, 25 patients were selected in each arm. Patients were randomized to receive either 14 Gy/4 fractions/2 days (study group) or 30 Gy/10 fractions/2 weeks (control group) with the help of random number tables. Written informed consent was taken from all the patients.
The inclusion criteria were patients of either sex; age >18 years; histological diagnosis of head-and-neck malignancy; Stage IV B and IV C as per the American Joint Committee on Cancer 2010 classification; Karnofsky Performance Score >60; hemoglobin >10 g%; and blood urea, serum creatinine, serum bilirubin, and serum transaminases within normal limits. The patients with carcinoma of the nasopharynx, salivary gland, nasal cavity, and paranasal sinuses and secondary node with unknown primary site; histology other than squamous cell carcinoma; history of previous oncological treatment; and history of previous malignancy or concomitant second malignancy were excluded from the study.
Technique of radiotherapy
All the patients were subjected to external beam radiation by cobalt-60 teletherapy.
Portals
The treatment volume included the primary tumor site with its extensions and lymphatic drainage up to the first station of lymphatic drainage/maximally involved lymphatic along with 1 cm margin on all sides. Dose was delivered using bilateral opposed lateral fields/anteroposterior arrangements in most cases. In certain situations, oblique/unilateral wedge fields were used. Dose was prescribed at mid-separation level in bilateral/anteroposterior plan. Bolus of 0.5 cm was applied only onto cases where the tumor showed fungation/ulceration, to attain a surface dose of 100% in these cases.
Dose
-
Arm I (study) – 14 Gy/4 fractions over a period of 2 days given twice a day. Each fraction was given at least 6 h apart, for 2 consecutive days. This regimen was repeated at 4 weekly intervals for a further two courses, subject to patient review for tolerance, toxicity, and disease progression
-
Arm II (control) – 30 Gy/10 fractions over a period of 2 weeks given as once a day, 5 days a week.
Evaluation and follow-up
The response to the treatment was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.
The toxicities were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Based on the RECIST criteria for response and the CTCAE criteria for toxicities due to treatment given results were evaluated to assess:
-
Locoregional disease control
-
Toxicity profile
-
Compliance.
This was done:
-
At completion of radiotherapy
-
Postradiotherapy (at monthly intervals up to 4 months).
Follow-up was done using brief history, clinical examination, and relevant blood investigations.
Statistical analysis
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± standard deviation and median. Normality of data was tested by the Kolmogorov–Smirnov test. If the normality was rejected, then nonparametric test was used.
Statistical tests were applied as follows:
-
Quantitative variables were compared using unpaired t-test/Mann–Whitney test (when the data sets were not normally distributed) between the two groups
-
Qualitative variables were compared using Chi-square test/Fisher's exact test.
P < 0.05 was considered statistically significant.
The data were entered in MS Excel spreadsheet, and analysis was done using the Statistical Package for Social Sciences (SPSS) version 21.0. manufactured by IBM, Chicago, Illinois, USA.
RESULTS
The patient characteristics are shown in Table 1. Both the groups were similar with respect to baseline parameters. Of 50 patients enrolled, two patients died, one declined treatment, and another one did not come for follow-up. Hence, the results were analyzed for 46 patients.
| Study group (Quad Shot) | Control group (30 Gy/10#) | |
|---|---|---|
| Age (years) | ||
| Range | 28-78 | 28-76 |
| Median | 55 | 54 |
| Gender, n (%) | ||
| Male | 21 (91.30) | 22 (95.65) |
| Female | 2 (8.69) | 1 (4.34) |
| ECOG PS | ||
| 0 | 6 (26.08) | 8 (34.78) |
| 1 | 17 (73.91) | 15 (65.21) |
| Native place, n (%) | ||
| Rural | 7 (30.43) | 17 (36.95) |
| Urban | 16 (69.56) | 29 (63.04) |
| Site, n (%) | ||
| Oral cavity | 14 (60.86) | 11 (47.82) |
| Buccal mucosa | 6 (26.08) | 7 (30.43) |
| Anterior tongue | 6 (26.08) | 3 (13.07) |
| Floor of mouth | 1 (4.34) | 1 (4.34) |
| Hard palate | 1 (4.34) | 0 |
| Oropharynx | 5 (21.73) | 8 (34.78) |
| Base of tongue | 2 (8.69) | 4 (30.43) |
| Tonsil | 2 (8.69) | 2 (13.07) |
| Soft palate | 1 (4.34) | 2 (4.34) |
| Larynx | 3 (13.07) | 4 (15.79) |
| Hypopharynx | 1 (4.34) | 0 |
| T-stage, n (%) | ||
| T2 | 1 (4.34) | 1 (4.34) |
| T3 | 5 (21.73) | 3 (13.04) |
| T4 | 17 (73.91) | 19 (82.60) |
| N-stage, n (%) | ||
| N0 | 0 (0.0) | 0 (0.0) |
| N1 | 0 (0.0) | 0 (0.0) |
| N2 | 4 (17.39) | 6 (26.08) |
| N3 | 19 (82.60) | 17 (73.91) |
| TNM stage, n (%) | ||
| Stage IV B | 23 (92) | 23 (92) |
| Stage IV C | 2 (8) | 2 (8) |
ECOG PS: Eastern Cooperative Oncology Group performance status, TNM: Tumor, node, metastasis
Response
The response was evaluated according to the RECIST criteria for the primary site as well as nodal disease. At the primary site, 39.13% of patients received a partial response (PR) in the study arm compared to 47.82% in the control arm. About 34.78% of patients had stable disease (SD) in the study arm compared to 30.43% in the control arm at the primary site. Progressive disease (PD) was seen in 26.08% of patients in the study arm compared to 21.73% in the control arm. This was statistically not significant (P = 0.70). At the nodal sites, 43.47% of patients received PR in the study arm compared to 47.82% in the control arm. Almost 39.13% of patients had SD in the study arm compared to 30.43% in the control arm at the nodal site. PD was seen in 17.39% of patients in the study arm compared to 21.73% in the control arm. This was statistically not significant (P = 0.86). Response in two groups was comparable at the primary as well as the nodal site. Response assessment with contrast-enhanced computed tomography is shown in Figures 1–3.
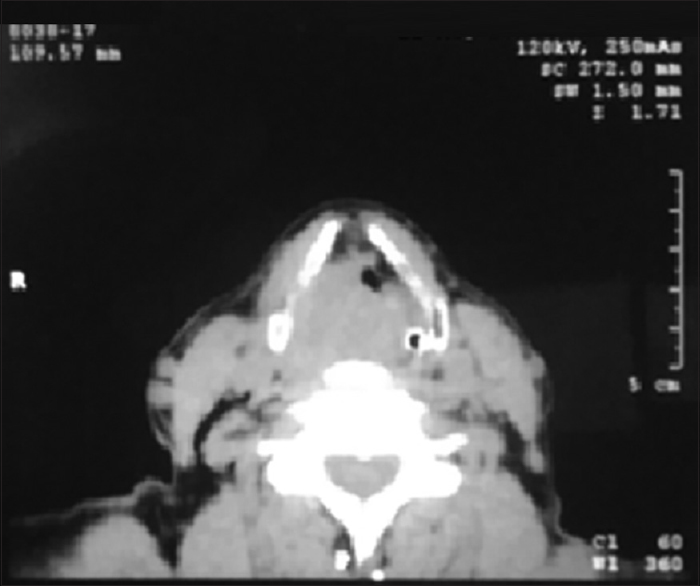
- Baseline contrast-enhanced computed tomography

- Contrast-enhanced computed tomography depicting response at 4 months

- Contrast-enhanced computed tomography depicting response at >4 months
Toxicity
Acute mucosal toxicity, skin reaction, and xerostomia were evaluated according to CTCAE v 4.02. Mucosal toxicity was more severe in the control group with majority of the patients having Grade 3 reactions (56.52%) in comparison to patients in the study arm where no experienced more than Grade 2 acute mucosal toxicity. The incidence of Grade 2 acute mucosal toxicity was similar in both the arms. Grade 1 mucosal toxicity was seen in 34.78% in the study group and 24.73% in the control group. In the study group, there was no acute mucosal toxicity in majority of the patients, i.e., 52.17%, versus only 8.69% of patients in the control group. This observation was statistically significant, acute mucosal toxicity being more in the control group (P ≤ 0.001, Chi-square test). This was managed conservatively with Betadine and steroid gargles and oral fluids. Only 2 (8.69%) patients in the control group required admission for intravenous fluid administration. There was no treatment-related death.
Acute skin toxicity was minimal. In the study group, there was no acute mucosal toxicity in majority of the patients, i.e., 52.17%, versus only 4.34% of patients in the control group. Maximum skin toxicity was of Grade 1 in the study group (47.82%) while 91.3% in the control group experienced this grade skin toxicity. Grade 2 was seen 4.34% of patients in the control arm. None of the Grade 2 reactions required any treatment other than topical application of gentian violet. This observation was statistically significant, acute skin toxicity being more in the control group (P ≤ 0.001, Chi-square test).
Grade 2 xerostomia was observed more in the control arm (47.82% vs. 34.78%). Grade 1 toxicity was found to be almost similar in both the arms (34.38 and 39.13). No xerostomia was seen in 30.43% in the study arm versus 13.04% in the control arm. This required patient counseling and supplementation in the form of lozenges. This observation was statistically significant, acute skin toxicity being more in the control group (P = 0.044, Chi-square test).
Symptom control
Relief in major symptoms that is pain, dysphagia, and hoarseness were evaluated. Patients’ quantification of >50% was taken as symptom relief. Pain and dysphagia were quantified by the patient using a percentage rupee scale.
Pain relief was 52.17% in the study group and 60.86% in the control group. Pain in the patients not relieved by radiotherapy was managed with NSAIDS and weak opioid drugs. Only 4 (17.39%) and 3 (13.04%) patients required oral morphine in the study and control groups, respectively. Pain relief in the two groups was comparable. This observation was statistically not significant (P = 0.656).
Dysphagia relief was 52.17% in the study group and 60.86% in the control group. Dysphagia relief in two groups was comparable. This observation was statistically not significant (P = 0.656). Patients not relieved even after treatment with local anesthetic syrups and steroid gargles required nasogastric tube feeding. Only 2 (8.69%) patients in the control group required admission for intravenous fluid administration.
Hoarseness was present in 91.30% of patients in the study group and control group. Voice was improved in 38% of patients in the study group and 42.85% of patients in the control group. Hoarseness relief in two groups was comparable. This observation was statistically not significant (P = 0.656).
DISCUSSION
An important aspect of any palliative regimen is to offer symptom relief with minimal toxicity. Hypofractionated radiotherapy is increasingly being studied as palliative treatment in advanced head-and-neck cancers, as it confers an effective dose in a short period in a cohort of patients where the prognosis is guarded and late radiation toxicities less relevant.[16] A number of hypofractionated palliative fractionation regimes in head and and neck cancer that have been used are depicted in the Table 2.
| Authors | Years | Number of patients (n) | Dose and fractionation | Dose per fraction | Number of fractions per day | Overall treatment time | Response | Toxicities |
|---|---|---|---|---|---|---|---|---|
| Erkal et al.[25] | 2001 | Total 40 22 10 |
30 Gy/10# 20 Gy/2# |
3 Gy 2 Gy |
OD OD |
2 weeks 1 week (split course 1-week gap) |
1-year response=77% 1-year response=48% |
No patients Sustained severe acute complication |
| Lusinchi et al.[26] | 1990 | 54 | 30 Gy/15# | 2 Gy | OD | 3 weeks | 33% discontinued RT | |
| Wendt et al.[27] | 1987 | 34 | 70.2 Gy/39# | 1.8 Gy | BD | 51 days (3 cycles every 3-4 weeks) Accelerated split course with simultaneous chemotherapy |
Local control rates 87% and 81% | Overall toxicity was tolerable |
| Paris et al.[24] | 1993 (phase 2 study) | 25 | 44 Gy/12# | 3.7 Gy | BD | 9 weeks (3 cycles every 3 weeks) | 84.6% achieved good palliation | The acute toxicity consisted of the expected skin changes, dysphasia, taste blindness, and dryness of the mouth |
| Minatel et al.[28] | 1998 | 58 | 50 Gy/25# | 2.5 Gy | OD | 9 weeks Weeks gap after half the dose | Symptom relief in 81% | Grade 3 mucosal toxicity in 27/58 patients |
| Ghoshal et al.[18] | 2004 | 25 | 30 Gy/10# | 3 Gy | OD | 2 weeks | Significant symptom relief in >91% | 17 patients had Grade 1 and 8 patients had Grade 2 mucositis |
| Mohanti[20] | 2004 | 505 | 20 Gy/5# | 4 Gy | OD | 1 week | 47%-59% symptom relief | Confined to dry desquamation and patchy mucositis |
| Corry et al.[17] | 2005 | 30 | 42 Gy/4# | 3.5 Gy | BD | 2 consecutive days (3 cycles every 4 weeks) | 53% response rate | 3/27 patients had Grade 2 mucositis and 14/27 Patients had Grade 1 dermatitis. No patients |
| Porceddu et al.[29] | 2007 (phase 2 study) | 35 | 30 Gy/5 fractions at 2/week, at least 3 days apart, with an additional boost of 6 Gy for small volume disease (63 cm) in suitable patients |
6 Gy | OD | 1 week | Overall response rate 80% | Grade 3 mucositis and dysphagia were experienced in 26% and 11%, respectively |
| Agarwal et al.[11] | 2008 | 110 | 40 Gy/16# | 2.5 Gy | OD | 3.5 weeks | Symptom relief in 74% | Grade 3 mucositis 69% |
| Ghoshal et al.[19] | 2009 | 15 | 42 Gy/4# | 3.5 Gy | BD | 2 consecutive days (3 cycles every 4 weeks) | 50% objective response 54% Grade 1 and 2 mucositis | 54% Grade 1 and 2 mucositis |
| Paliwal et al.[21] | 2012 | 50 | 20 Gy/5# | 4 Gy | OD | 1 week | Partial response in 92% | Grade 3 mucositis 4% |
| Das et al.[23] | 2013 | 36 | 40 Gy/10# | 4 Gy | OD | 5 weeks (2 fractions per week) | Pain relief in 88% | Grade 3 mucositis and dermatitis was 18% and 3% |
| Chen et al.[30] | 2008 | Total 60 23 13 12 7 5 |
44.4 Gy/3# 70 Gy/35# 30 Gy/10# 37.5 Gy/15# 20 Gy/5# |
3.7 Gy 2 Gy 3 Gy 2.5 Gy 4 Gy |
BD OD OD OD OD |
2 consecutive days (3 cycles every 2-3 weeks) RTOG 85-02 schedule 7 weeks 2 weeks 3 weeks 1 week |
Rate of palliative response 83% Rate of palliative response 77% Rate of palliative response 67% Rate of palliative response 86% Rate of palliative response 60% |
Grade 3+toxicity 9% Grade 3+toxicity 38% Grade 3+toxicity 42% Grade 3+toxicity 29% Grade 3+toxicity 20% |
| Al-Mamgani et al.[10] | 2009 | 158 | 50 Gy/16 # | 3.125 Gy | OD | 22-24 days | Overall response rate of 73% | Acute Grade 3 skin and mucosal toxicities in 45% and 65%, respectively. Severe late toxicity in 4.5% |
| Kancherla et al.[31] | 2011 | 33 | 20 Gy/5# Followed by 2-week gap followed by 20 Gy/5# | 4 Gy | OD | 4 weeks | Response rate in 72% Symptom relief in 79% |
Acute Grade 3 skin and mucosal toxicities in 9% and 6% respectively |
| Nguyen et al.[32] | 2015 | 110 | 24 Gy/8# | 3 Gy | OD | 3 weeks (once a week 0-7-21 regimen) | Overall response>80% | Two (2.1%) patients died unrelated to treatment Three (3.2%) patients required admission during treatments, including one owing to Grade 3 mucositis (1%) |
| Lok et al.[33] | 2012 | 75 | 42 Gy/4# | 3.5 Gy | BD | 2 consecutive days (3 cycles every 4 weeks) RTOG 85-06 study, Quad Shot |
Rate of palliative response 65% | Grade 3 toxicity in 5% |
| Murthy et al.[22] | 2016 | 93 | 32 Gy/8# | 4 Gy | OD | 4 weeks (twice weekly) | Response rate>40% Pain score improved in>76% |
Acute Grade 3 mucositis was seen in one patient (1.2%) while none had Grade 3 skin reactions |
| Straube et al.[34] | 2016 | 27 | SIB IMRT Dose prescription was 50% of PTV receiving the prescribed dose of 40 Gy (D50=40 Gy) in 20 fractions. SIB of 54 Gy in 20 fractions was applied to the GTV+5 mm margin (CTV_SIB) | 2 Gy 2.7 Gy to the SIB site | OD | 4 weeks | 75% of patients showed an early local response | Most patients developed mild-to-moderate acute toxicities; only one patient had Grade 4 mucositis |
| Present study | 2019 | 25 | 42 Gy/4# | 3.5 Gy | BD | 2 consecutive days (3 cycles every 4 weeks) | Overall response 73.91% and 83.60% for primary lesion and nodal site respectively | No Grade 3 toxicity. Acute Grade 2 mucosal toxicities in 13.04% |
| 25 | 30 Gy/10# | 3 Gy | OD | 2 weeks | Overall response 78.26% and 73.91% for primary lesion and nodal site, respectively | Acute Grade 3 mucosal and skin toxicities in 56.52% and 52.17%, respectively |
SIB: Simultaneous integrated boost, IMRT: Intensity modulated radiation therapy, RTOG: Radiation therapy oncology group, CTV: Clinical target volume, GTV: Gross tumour volume, PTV: Planning target volume, OD: Once a day, BD: Twice a day
The Quad Shot regimen[17] described by Corrry et al. designed to giving a biologically equivalent dose below the threshold for producing mucositis. Thirty patients had at least one treatment and sixteen patients completed all three cycles. Sixteen patients (53%) had an objective response and a further seven had SD. No patient experienced Grade 3 or worse toxicity.
Various studies from different parts of India have been carried out. At Chandigarh, two schedules were carried out by Ghoshal et al.[1819] 30 Gy in 10 fractions over 2 weeks and Quad Shot. More than 91% patients significant symptom relief with 30 Gy/10 #. 86 % patients had objective response >50% with the quad shot regimen. Fifty-four percent had Grade 1 and 2 mucositis when they carried out Quad Shot regimen.
In a large prospective study from Delhi by Mohanti et al. who used a schedule of 20 Gy in 5 fractions over 1 week for 505 patients,[20] at 1-month assessment, 37% achieved a PR and were suited for further curative-dose radiotherapy. Good symptom relief (50% or more) was found in 50%–60% of patients. A study from Rajasthan[21] demonstrated symptom relief in >65% of patients with 20 Gy/5#/5 days; almost all patients developed Grade 1 or 2 skin and mucosal toxicities. Agarwal et al.[11] used a schedule of 40 Gy in 16 fractions reported from Mumbai. Patients with PR had a dose escalation up to 50 Gy in 20 fractions. More than 50% symptom relief was seen in 74%. The incidence of Grade 3 mucositis was 69%.
Another study from Mumbai, Murthy et al. published twice-weekly palliative radiotherapy regimen of 32 Gy in 8 fractions Overall response rates were 42% at primary disease and 55% at nodal disease.[22] At conclusion of radiotherapy, 76.3% of the patients reported improvement in pain scores (P = 0.001). At the first followup after 612 weeks significant improvement in pain scores persisted. Acute Grade 3 mucositis was seen in one patient (1.2%) while none had Grade 3 skin reactions. From Vellore, Das et al.[23] using a schedule of 40 Gy/10# have reported significant pain relief (>50%) in about 88% of patients and worsening in 9% of patients at the end of radiotherapy. Incidence of grade III mucositis and dermatitis was 18% and 3% in the same study.
Some landmark studies were carried out globally. In a study by Paris et al.,[24] good palliation was achieved in 84.6% of patients with minimal acute toxicity and no long-term complications. Erkal et al.,[25] in a retrospective study, showed similar response rates and symptom relief. In our study, overall response at 4 months is similar for 73.91% in the study group and 78.26% in the control group as well as nodal site. Mucositis and skin reactions were significantly lower in the Quad Shot arm. Thus, we find that the response and symptom relief in the present study are comparable to previous studies with various time, dose, and fractionation regimens. There are certain limitations of this study. Drawback includes a short follow-up period, small sample size, and inherent biases of a single institutional trial.
CONCLUSIONS
The study shows promising results in terms of locoregional control and symptom relief as well as much lesser toxicities for palliation in patients with advanced head-and-neck cancers with heterogeneous subsites when treated with Quad Shot regimen (14 Gy/4 fractions/2 days). Quad Shot regimen lessens the number of hospital visits, shortens loss of working days, and may make treatment more cost-effective. Quad Shot regimen is more suitable for a developing country like India with limited resources in the form of machines and workforce and a large patient load.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 2013. GLOBOCAN 2012 v1.0 Cancer incidence and Mortality Worldwide IARC Cancer Base No 11. Lyon, France: International Agency for Research on Cancer; Available from: Available at: http://globocan.iarc.fr
- National Cancer Registry Programme India. Available from: www.ncrpindia.org/Annual_Reports. aspx
- Dentition, diet, tobacco, and alcohol in the epidemiology of oral cancer. J Natl Cancer Inst. 1977;59:1611-8.
- [Google Scholar]
- Prevalence of oral cancer and precancerous lesions in ‘pan'/'supari’ chewers. Indian J Public Health. 1978;22:234-45.
- [Google Scholar]
- Tobacco habits in India. In: Tobacco-Related Oral Mucosal Lesions and Conditions in India. New Delhi, India: Jaypee Brothers; 1993. p. :89-99.
- [Google Scholar]
- Age-dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Eur J Cancer B Oral Oncol. 1996;32B:55-62.
- [Google Scholar]
- Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744-54.
- [Google Scholar]
- Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-75.
- [Google Scholar]
- Survival analysis of 5595 head and neck cancers – Results of conventional treatment in a high-risk population. Br J Cancer. 1998;77:1514-8.
- [Google Scholar]
- Hypofractionated radiotherapy denoted as the “Christie scheme”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. 2009;48:562-70.
- [Google Scholar]
- Hypofractionated, palliative radiotherapy for advanced head and neck cancer. Radiother Oncol. 2008;89:51-6.
- [Google Scholar]
- A comparison of radiotherapy or radiochemotherapy with symptomatic treatment alone in patients with advanced head and neck carcinomas. Eur Arch Otorhinolaryngol. 2000;257:164-7.
- [Google Scholar]
- Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancers. Radiother Oncol. 2000;57:119-24.
- [Google Scholar]
- Randomized phase I/II trial of two variants of accelerated fractionated radiotherapy regimens for advanced head and neck cancer: Results of RTOG 88-09. Int J Radiat Oncol Biol Phys. 1995;32:589-97.
- [Google Scholar]
- Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys. 1994;30:795-8.
- [Google Scholar]
- The ‘QUAD SHOT’ – A phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137-42.
- [Google Scholar]
- Palliative radiotherapy in locally advanced head and neck cancer: A prospective trial. Indian J Palliat Care. 2004;10:19-23.
- [Google Scholar]
- Quad shot: A short but effective schedule for palliative radiation for head and neck carcinoma. Indian J Palliat Care. 2009;15:137-40.
- [Google Scholar]
- Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol. 2004;71:275-80.
- [Google Scholar]
- Palliative hypo-fractionated radiotherapy in locally advanced head and neck cancer with fixed neck nodes. Iran J Cancer Prev. 2012;5:178-82.
- [Google Scholar]
- Twice-weekly palliative radiotherapy for locally very advanced head and neck cancers. Indian J Cancer. 2016;53:138-41.
- [Google Scholar]
- Hypofractionated palliative radiotherapy in locally advanced inoperable head and neck cancer: CMC Vellore experience. Indian J Palliat Care. 2013;19:93-8.
- [Google Scholar]
- Phase I-II study of multiple daily fractions for palliation of advanced head and neck malignancies. Int J Radiat Oncol Biol Phys. 1993;25:657-60.
- [Google Scholar]
- Squamous cell carcinomas metastatic to cervical lymph nodes from an unknown head and neck mucosal site treated with radiation therapy with palliative intent. Radiother Oncol. 2001;59:319-21.
- [Google Scholar]
- Radiation therapy for head and neck cancers in the elderly. Int J Radiat Oncol Biol Phys. 1990;18:819-23.
- [Google Scholar]
- Accelerated split-course radiotherapy and simultaneous cis-dichlorodiammine-platinum and 5-fluorouracil chemotherapy with folinic acid enhancement for unresectable carcinoma of the head and neck. Radiother Oncol. 1987;10:277-84.
- [Google Scholar]
- Combined radiotherapy and bleomycin in patients with inoperable head and neck cancer with unfavourable prognostic factors and severe symptoms. Oral Oncol. 1998;34:119-22.
- [Google Scholar]
- Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment–“Hypo trial”. Radiother Oncol. 2007;85:456-62.
- [Google Scholar]
- Palliative radiation therapy for head and neck cancer: Toward an optimal fractionation scheme. Head Neck. 2008;30:1586-91.
- [Google Scholar]
- The role of split-course hypofractionated palliative radiotherapy in head and neck cancer. Clin Oncol (R Coll Radiol). 2011;23:141-8.
- [Google Scholar]
- 0-7-21 hypofractionated palliative radiotherapy: An effective treatment for advanced head and neck cancers. Br J Radiol. 2015;88:20140646.
- [Google Scholar]
- Intensity-modulated radiation therapy in oropharyngeal carcinoma: Effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851-7.
- [Google Scholar]
- Reduced volume SIB-IMRT/IGRT to head and neck cancer in elderly and frail patients: Outcome and toxicity. Radiat Oncol. 2016;11:133.
- [Google Scholar]






