Translate this page into:
Correlation between Symptom Burden and Perceived Distress in Advanced Head and Neck Cancer: A Prospective Observational Study
*Corresponding author: Priti Rashmin Sanghavi, Department of Pain and Palliative Medicine, Gujarat Cancer and Research Institute, Ahmedabad, Gujarat, India. priti.sanghavi@gcriindia.org
-
Received: ,
Accepted: ,
How to cite this article: Ostwal SP, Singh R, Sanghavi PR, Patel H, Anandi Q. Correlation between symptom burden and perceived distress in advanced head and neck cancer: A prospective observational study. Indian J Palliat Care 2021;27:419-25.
Abstract
Objectives:
Head and neck cancer (HNC) account for major cancer burden in the Indian population. Patients often present with a diversity of distressing physical and psychological symptoms, significantly affecting their quality of life. This study aims to determine the correlation between symptom cluster and perceived distress in such patients.
Materials and Methods:
This single center prospective observational study was done on 175 adults advanced HNC patients referred to palliative medicine outpatient clinic. Patients fulfilling eligibility criteria were regularly assessed for their symptoms and distress at baseline and followed up at days 7, 14, and 28.
Results:
Most patients belong to the age group of 40–50 years and having a diagnosis carcinoma of the tongue. The most common symptoms presented were pain, tiredness, loss of appetite, and feeling of well-being. We observed statistically significant correlation between total ESAS score and distress levels in patients at days 0, 7, and 14, respectively, (P = 0.003 vs. 0.0004 vs. 0.002). However, at day 28, no such statistically significant correlation was found (P = 0.085) suggesting attention to other factors during assessment.
Conclusion:
Outpatient palliative care consultations have shown significant improvement in symptom and distress score. Perceived distress in a person can not only be related to physical symptoms. Acute control of symptom may uncover underlying psychosocial and spiritual issues which need to be addressed promptly for better quality of life.
Keywords
Head and neck cancer
Palliative care
Distress
Symptom
INTRODUCTION
Head and neck cancer (HNC) is the most prevalent and third most common cancer found in the Indian population.[1] India accounts for 57% of total HNC cases worldwide with nearly more than one lakh new cases (10.4%) registered every year.[2-4] Compared to worldwide statistics, mouth and tongue cancers in Indian subcontinents has major contribution to overall cancer burden[5,6] Lack of resources such as funding and infrastructure, low awareness, poor patient follow-up compliance, poor record maintenance, and late detection has lead to weak epidemiological data about the actual burden of HNC across country.[7,8] The interplay between established risk factors such as tobacco and alcohol consumption, Human Papilloma Virus infection, betel nut chewing with demographic, behavioral, and environmental factors have been associated with increased surge in numbers.[1,8] Males are significantly more likely to develop HNC than females with an incidence ranging from 2:1 to 4:1.
Standard treatment measures include surgery, radiotherapy, and chemotherapy with more emphasis given to radical surgery whenever possible. Despite advances in diagnostic methodology, treatment protocols, 5 year overall survival rates for Stage III and Stage IV patients remain at 43% and 42%, respectively.[9] Patients with HNC often present with a spectrum of symptoms ranging from physical to spiritual issues.[10-12] Mixed type (nociceptive and neuropathic) pain is seen in more than 2/3rd patients. Changes in body images and involvement of nerves are responsible for severe pain and distress in such patients. This dramatically affects their physical, psychosocial functioning and thus the quality of life.[13] Previous studies showed positive benefits of palliative care interventions in patients suffering from advanced cancers. However, studies correlating symptom burden and distress in HNC are limited in the Indian population. Hence, we decided to conduct an observational trial to determine the correlation between symptom burden and distress in advanced HNC patients at a tertiary cancer center.
MATERIALS AND METHODS
This single-institution, prospective, questionnaire-based study was conducted after Institutional Review Board approval. Consecutive patients who were referred to the palliative medicine department between March 2018 and November 2018 and those meeting inclusion criteria were enrolled in this study. Inclusion criteria for the study were: age more than 18 years; able to understand Hindi or Gujarati language; clinical diagnosis of advanced HNC (Stage III or IV); consenting to participate in the study. Patients with any psychiatric illness or refusing to participate were excluded from the study. A waiver of consent was obtained as patients were regularly assessed for their symptoms as a part of routine outpatient protocol where patients and/or their caregivers complete the Edmonton Symptom Assessment Scale-Revised (ESAS-r) scale. Patients were assisted by nurses in the department when they were unable to understand the study questionnaire.
Sample selection
Of the total 354 advanced HNC patients presenting to pain and palliative care outpatient department between study time-period from March 2018 to August 2018, only 286 (80.79%) agreed to participate in the study. No formal sample size was calculated for our study. Among them patients who had a previous history suggestive of psychiatric disorders (n = 40), or who were unable to give information and/or could not understand/read Gujarati or Hindi, the language spoken by the majority in this part of India (n = 21), unable to follow-up at study time points (n = 50) were excluded from the study, resulting in a total of 175 study participants [Figure 1].
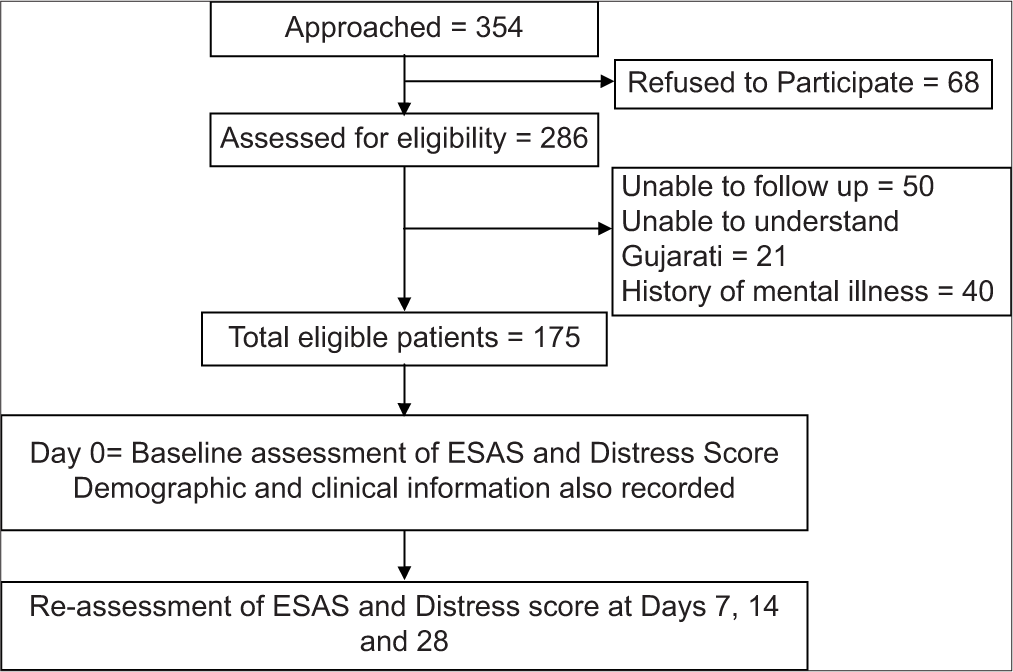
- CONSORT diagram.
Patients were assessed for their symptoms using ESAS-r and for distress using the NCCN distress thermometer (DT)[14] at baseline and at days 7, 14, and 28 of starting palliative care intervention. Changes in symptom burdens were correlated with changes in distress score over different time points. A two-point change in symptom score will be considered as minimally clinically important.
The ESAS-r
The ESAS-r is a ten-item symptom assessment questionnaire where symptom rating has been done from 0 to 10 on a visual analog scale. The scale is easily available in English and Hindi language. This questionnaire involves most common symptoms such as pain, tiredness, nausea, drowsiness, anxiety, shortness of breath, loss of appetite, feeling of well-being, and other problems. It can be completed either by the patient alone or with the assistance of a proxy. A score of 0 indicates the absence of the symptom, and 10 corresponds to the symptom being of the worst possible severity.[15]
Statistical analysis
Descriptive statistics were used to summarize patients’ demographic and clinical characteristics and ESAS-r scores. The overall mean values of each ESAS-r item at baseline/ day 0, day 7, day 14, and day 28 of the treatment course were calculated to assess the significance of demographic or clinical factors on symptom development. To assist with the clinical interpretation of these data, the mean of patient’s severity levels of most common symptoms identified and were calculated across the treatment time. Along with, pearson’s correlation was used to calculate the significance level between symptoms total score and distress according to each follow-up session (day 7, day 14, and day 28). At last, through pictorial representation, the most frequent symptoms among patients in the level of follow-up session were assessed. To identify the influential factors of distress, Pearson correlation was used to calculate the significance level of each complained score and distress, P < 0.05 is considered significant.
RESULTS
Demographic information
All study participants completed ESAS-r and DT at baseline and at follow-up of days 7, 14, and 28. Age was ranged from 25 years to 70 years with most participants belonging to the age group of 40–50 years. Distribution of study participants according to age group were: <30 = 6.3%, 30–40 years = 24.1%, 40–50 years = 32.8%, 50–60 years = 22.88%, and 60–70 years = 13.92%. Whereas 74.1% and 25.9% of patients were male and females, respectively.
Distribution of participants according to cancer site: tongue (35.1%), buccal mucosa (24.1%), nasopharynx and larynx (13.8%), alveolus carcinoma (4.0%), retromolar trigone (4.0%), parotid (4.0%), palate carcinoma (4.0%), and carcinoma of unknown primary (2.8%).
When categorically tested to determine the difference between demographic factors and symptom score across the time period of palliative care treatment: Days 0, 7, 14, and 28 among study participants, there P value was found to be non-significantly correlated with demographic factors in each follow-up session [Table 1].
| Demographic variables | ESAS- | total sore | (Mean±SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Percentage | D-0 | P-value | D-7 | P-value | D-14 | P-value | D-28 | P-value |
| <30 | 6.3 | 40.91±3.88 | 0.76 | 19.63±5.55 | 0.81 | 21.82±5.9 | 0.78 | 9.27±6.77 | 0.91 |
| 30-40 | 24.1 | 42.52±6.24 | 21.54±6.25 | 23.55±4.73 | 10.04±7.09 | ||||
| 40-50 | 32.8 | 40.65±7.36 | 20.38±5.39 | 25.44±4.57 | 11.58±5.37 | ||||
| 50-60 | 22.88 | 43.6±2.36 | 19.39±4.36 | 24.2±3.2 | 10.44±3.1 | ||||
| 60-70 | 13.92 | 39.65±9.7 | 19.42±5.72 | 24.58±4.8 | 10.04±6.62 | ||||
| Gender | |||||||||
| Male | 74.1 | 40.77±6.53 | 0.81 | 20.92±6.29 | 0.91 | 24.12±4.44 | 1.21 | 10.53±6.52 | 0.71 |
| Female | 25.9 | 41.37±8.02 | 19.64±5.9 | 24.73±5.47 | 11.42±6.65 | ||||
| Diagnosis | |||||||||
| Tongue | 35.1 | 41.08±6.45 | 0.83 | 20.23±5.67 | 1.31 | 24.24±4.19 | 1.88 | 10.82±6.61 | 0.65 |
| Alveolus | 4.0 | 38.0±5.51 | 19.14±4.78 | 24.43±3.5 | 6.71±5.82 | ||||
| Buccal mucosa | 24.1 | 41.33±8.03 | 20.97±5.16 | 24.64±4.39 | 12.38±6.12 | ||||
| Retromolar trigone | 13.8 | 39.14±8.07 | 12.00±2.31 | 25.00±5.26 | 11.14±5.14 | ||||
| Nasophyarynx and Larynx | 4.0 | 39.79±4.93 | 20.00±5.39 | 23.04±6.05 | 9.96±6.48 | ||||
| Parotid | 0.6 | 40.86±5.55 | 28.57±13.54 | 29.57±4.5 | 13.43±9.78 | ||||
| Esophagus | 2.3 | ||||||||
| Head | 9.3 | 40.25±6.13 | 20.00±2.16 | 27.5±1.91 | 12.25±7.41 | ||||
| Unknown primary | 2.8 | 41.33±8.03 | 12.25±7.41 | 44.33±10.01 | 21.67±2.52 | ||||
Pattern of Symptom burden during palliative care treatment
Mean total scores for symptom items at baseline (pretreatment), day 7, 14, and 28 were 43.23, 20.93, 24.17, 10.86, respectively.
Mixed model results demonstrated that overall symptom severity for each individual symptom (P < 0.001) and symptom interference (P < 0.001) both progressively improved over the course of treatment. Statistically significant change in mean score has been observed over different study time points. We observed slight increase in mean symptom scores (mainly for pain [1.09 vs. 3.17 vs. 1.91]; tiredness [4.76 vs. 4.21 vs. 1.81] and loss of appetite [2.14 vs. 3.78 vs. 2.91]) and mean distress score (5.2 vs. 4.43 vs. 3.11) at day 14 compared with day 7, which subsequently decreased at day 28 [Figure 2]. A positive correlation between total ESAS-r score and distress was found to be statistically significant at all time points except day 28, suggestive of direct correlation between symptoms and perceived distress.

- Pattern of change in individual symptom across study period: Day 0, Day 7, Day 14, Day 28.
We observed four important symptoms to be directly correlated with overall distress score: pain, tiredness, loss of appetite, and feeling of well-being [Figure 3]. Pearson correlation was used to determine the correlation between the important symptoms and distress. Changes in P values between individual symptom and distress score over various time points (days 0 vs. day 7 vs. day 14 vs. day 28 respectively) were as: feeling of wellbeing versus distress (0.003 vs. 0.0004 vs. 0.0003* vs. 0.076); pain vs distress (0.0002* vs. 0.001 vs. 0.004 vs. 0.081); tiredness vs distress (0.0001* vs. 0.003 vs. 0.004 vs. 0.092); anxiety versus distress (0.0001* vs. 0.001* vs. 0.002 vs. 0.0004*); appetite vs distress (0.003 vs. 0.005 vs. 0.004 vs. 0.096). Changes in R2 values between symptoms and distress score at day 28 were: For feeling of well-being status versus distress (R2 = 0.6952), pain versus distress (R2 = 0.9139), tiredness versus distress (R2 = 0.9923), nausea versus distress (R2 = 0.9099), appetite versus distress (R2 = 0.9351) [Figure 4]. Distress score did not find to be statistically significant (P = 0.085) for common important symptoms, hence considering the possibility of other external factors such as spiritual concerns, psychosocial concerns, etc. contributing for their perceived distress.

- Correlation of common important symptoms with distress across study time points.

- Correlation between distress and symptoms over different study time points. X-axis denotes study time points, while Y-axis denotes symptom score.
DISCUSSION
This was a hospital-based prospective observational study of 175 advanced HNC patients treated over a period of 6 months. HNC constitutes about one-third of cases presenting to hospital. This could be attributed to general trend of HNC in India.
Our study showed males are at more preponderance to develop HNC as compared to females (74.1% vs. 25.9%). This finding is in line with other studies. Most of the patients belonged to 40–50 years of age group. A small proportion of cases belonged to younger age possibly attributed to modifiable risk factors such as tobacco consumption or genetic predisposition. Non-availability of strict government regulations and low awareness could have contributed to cases.
Common symptoms observed in HNC patients were pain either related to oral mucositis or neurovascular bundle invasion or disease extension, inability to take food orally and related fatigue halitosis, malignant wound with occasional myiasis, and changes related to body image and sexuality.[16,17] We have observed psychological distress in both patients and their family members. Various studies have demonstrated the importance of palliative care in advanced HNCs.[18-20]
An interesting observation in our study was that in our patients distress score was found to be directly correlated with symptom score when assessed on days 7 and 14, while on day 28 we observed that in spite of decrease in symptom score patients reported significantly higher levels of distress. The probable explanation for this is that some external factors such as social issues (social stigma, hesitancy to participate in social gatherings, and avoiding social interactions), financial issues (financial exhaustion due to treatment, loss of job), spiritual issues (perceived change in the meaning of life, hopes, despair, etc.) or personal issues (body image and sexuality-related family concerns) might have popped up and contributed for increase in their distress score. We observed that once physical symptoms, which often contribute to sudden and prolonged distress, have been controlled, patients start concentrating and perceiving underlying psychosocial and personal concerns. This might cause sudden change in distress score affecting perceive cognition. This finding is supported by significant levels of anxiety found on day 28. Continuing disease-related treatments such as radiotherapy or chemotherapy and their side effects, both immediate and long term also found to have contributed to symptoms and distress.
Another interesting observation in our study is that patients showed an acute rise in symptom scores mainly of pain, tiredness, and loss of appetite during an assessment on day 14. Possible explanation could be due to sudden change in disease trajectory; compliance-related issues - self stoppage of medication as acute symptoms are well controlled or loss to follow-up; chemotherapy or radiotherapy related side effects.
Our study highlights the importance of palliative care in the control of symptoms and distress in advanced HNC patients, which is similar to the existing available literature.[7,21-23] This study includes patients from Gujarat and nearby areas, hence results from the study can be extrapolated to the region.
Our study also highlights dire need for multi-sitting counseling sessions directed towards psychosocial, spiritual concerns, and body image, timely appropriate interventions, and attention to external factors for symptom control. Meeting requirements of adequate nutrition with emphasis on homemade food forms an integral part of managing advanced cancer patients.
CONCLUSION
Outpatient palliative care services were associated with significant improvement in ESAS- R and distress. A direct correlation was found between distress and symptoms, especially pain, tiredness, loss of appetite, and feeling of well-being on short term period. However, acute control of physical symptoms might have uncovered underlying psychosocial and spiritual issues, increasing perceived distress. A timely, multi-sitting counseling sessions and holistic approach are recommended for better quality of life.
Acknowledgment
We would like to thank Ms. Arunima Datta, researcher at Kolkata for her contribution for statistical analysis.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Head and neck cancer risk factors in India: Protocol for systematic review and meta-analysis. BMJ Open. 2018;8:e020014.
- [CrossRef] [PubMed] [Google Scholar]
- The Global Cancer Observatory May, 2019. 2018-19. India Source: GLOBOCAN 2018 International Agency for Research on Cancer 2019. 468 Available from: https://www.gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf [Last accessed on 2020 Apr 04]
- [Google Scholar]
- 2019. Hindustan Times. Available from: https://www.hindustantimes.com/brandstories/make-sense/india-accounts-for-57-of-head-and-neck-cancer-cases [Last accessed on 2020 Jun 05]
- [Google Scholar]
- Impact of head and neck cancer (HNC) education on HNC knowledge and attitudes toward HNC peer and non-peer education: A school-based pilot study. Yen Med J. 2020;2:47-55.
- [Google Scholar]
- Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133-45.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633-41.
- [CrossRef] [PubMed] [Google Scholar]
- Head and neck cancers in developing countries. Rambam Maimonides Med J. 2014;5:e0009.
- [CrossRef] [PubMed] [Google Scholar]
- Indian clinical practice consensus guidelines for the management of squamous cell carcinoma of head and neck. Indian J Cancer. 2020;57:S1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Survival trends in oral cavity cancer patients treated with surgery and adjuvant radiotherapy in a tertiary Center of Northern India: Where do we stand compared to the developed world? SRM J Res Dent Sci. 2019;10:26-31.
- [CrossRef] [Google Scholar]
- Symptom management during and after treatment with concurrent chemoradiotherapy for oropharyngeal cancer: A review of the literature and areas for future research. World J Clin Oncol. 2016;7:220-6.
- [CrossRef] [PubMed] [Google Scholar]
- Symptom Clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol. 2013;49:360-6.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the University of Texas MD Anderson Cancer Center symptom inventory-head and neck module. Cancer. 2014;120:1975-84.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological distress in patients with head and neck cancer: Review. Br J Oral Maxillofac Surg. 2001;39:67-70.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology: Distress Management. V2. 2019 Referenced with Permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management V.2.2019 In: National Comprehensive Cancer Network, Inc.. 2019.
- [Google Scholar]
- A multicenter study comparing two numerical versions of the Edmonton symptom assessment system in palliative care patients. J Pain Symptom Manage. 2011;41:456-68.
- [CrossRef] [PubMed] [Google Scholar]
- The symptom burden of treatment-naive patients with head and neck cancer. Cancer. 2015;121:766-73.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological factors associated with head and neck cancer treatment and survivorship: Evidence and opportunities for behavioral medicine. J Consult Clin Psychol. 2013;81:299-317.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative and supportive care in head and neck cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130:S198-207.
- [CrossRef] [PubMed] [Google Scholar]
- An enhanced role for palliative care in the multidisciplinary approach to high-risk head and neck cancer. Cancer. 2016;122:340-3.
- [CrossRef] [PubMed] [Google Scholar]
- Role of early palliative care in advanced head-and-neck cancers patients. Indian J Palliat Care. 2019;25:153-5.
- [Google Scholar]
- Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer. 2009;17:573-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of outpatient palliative care (PC) on symptom burden in patients with advanced cancer at a tertiary cancer Center in Jordan. Support Care Cancer. 2017;25:177-83.
- [CrossRef] [PubMed] [Google Scholar]
- Symptom distress: Implementation of palliative care guidelines to improve pain, fatigue, and anxiety in patients with advanced cancer. Clin J Oncol Nurs. 2019;23:149-55.
- [Google Scholar]






