Translate this page into:
Exploring the Effectiveness of Ajwain Cream in Treating Taxane-induced Peripheral Neuropathy in Cancer Patients: A Pilot, Randomised and Double-blind Clinical Trial
*Corresponding author: Mehdi Pasalar, Research Center for Traditional Medicine and History of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. pasalar@sums.ac.ir
-
Received: ,
Accepted: ,
How to cite this article: Heydarirad G, Mirzaei H, Gharehgozlou R, Buentzel J, Namazi M, Pasalar M. Exploring the effectiveness of Ajwain cream in treating taxane-induced peripheral neuropathy in cancer patients: A pilot, randomised and double-blind clinical trial. Indian J Palliat Care. 2024;30:71-6. doi: 10.25259/IJPC_32_2023
Abstract
Objectives:
Chemotherapy-induced peripheral neuropathy is a common disorder among cancer patients receiving various chemotherapeutic protocols. The present study aimed to explore the feasibility of ajwain (Trachyspermum ammi [L.] Sprague) cream in treating peripheral neuropathy symptoms triggered by taxane chemotherapeutic agents.
Materials and Methods:
This was a pilot, double-blind, and randomised clinical trial on patients with peripheral neuropathy attributable to chemotherapy with taxane drugs during 2021–2022 in Tehran. Patients received ajwain or placebo cream for four weeks and filled out the chemotherapy-induced peripheral neuropathy assessment tool (CIPNAT) at the start and end finale of the trial. Side effects were also noted.
Results:
Thirty patients suffering from breast, lung, gastro-intestinal, or prostate cancer were allocated to each of the drug and placebo groups. The mean difference in CIPNAT score between the groups was 0.83, demonstrating the statistical ineffectiveness of the drug compared with the placebo (P = 0.372). The safety profile showed promising outcomes at the end of the trial.
Conclusion:
Although the effectiveness of ajwain cream was unacceptable in treating chemotherapy-induced peripheral neuropathy symptoms, multicentre controlled trials with ample sample size are mandatory for an all-inclusive inference.
Keywords
Peripheral neuropathy
Ajwain
Cancer
Chemotherapy
Palliative care
Persian medicine
INTRODUCTION
Peripheral neuropathy is a troubling side effect of chemotherapy, mainly observed with agents such as taxanes, vincristine, platinum analogues, thalidomide, and bortezomib. These chemotherapeutics exert direct and indirect effects on sensory nerves, such as altering the action potential range, reducing transmission speed, or inducing pain.[1-3] Peripheral neuropathy occurs in roughly 59% of patients receiving chemotherapy with taxane and vincristine drugs. Symptoms include pain in the hands and feet, tingling, burning, muscle cramps, drowsiness and temperature sensitivity; such sensory symptoms are more severe than motor symptoms and may interrupt the chemotherapy process.[4] This complication affects the patient’s quality of life, even after treatment completion and drug discontinuation.[5] Common treatments include anticonvulsants (gabapentin and pregabalin), tricyclic antidepressants (amitriptyline), opioids, topical anaesthetics (lidocaine), massage therapy and physiotherapy.[6] However, no definitive treatment is available, rendering it challenging for physicians to manage.[7,8]
Traditional, complementary, and integrative medicine offers the potential to relieve global symptoms and increase the quality of life in breast and gynaecological cancer patients.[9]
In Persian medicine (PM), a disease that is exactly identical to neuropathy has not been defined. Still, some conditions, such as Khádir – numbness of the body, weakness of the limbs, and a tingling sensation in the limbs – share similar symptoms. Furthermore, neuropathy pain seems similar to the pain caused by simple and material coldness in PM, for which local and systemic treatments are available.[10] One local treatment is the Trachyspermum ammi (L.) Sprague plant (traditionally known as Nānkhāh), which PM deems effective for rheumatic pains, nerve pains, headaches, renal stones, and morphine withdrawal syndrome complications.[11] According to PM literature, this plant can effectively treat peripheral nervous system diseases and has analgesic effects.[12] The analgesic effects of this plant have been proven in laboratory studies and animal samples.[13,14] Recent studies have shown that Ajwain seed extract has anti-inflammatory and analgesic activity, mediated through enhancing gamma-aminobutyric acid neurotransmission, suppressing glutamate receptors, and suppressing the nitric oxide pathway. The phenolic compounds of this plant may be responsible for such anti-inflammatory activity.[15] Furthermore, Petramfar et al. showed in a randomised, double-blind clinical trial that ajwain cream significantly reduced the amount of burning, tingling, numbness, and pain compared to a placebo.[16] Therefore, the cream of ajwain seems suitable for reducing neuropathic pain caused by chemotherapy. This study aimed to investigate the feasibility of ajwain cream in treating peripheral neuropathy symptoms caused by taxane chemotherapeutic drugs.
MATERIALS AND METHODS
Study type
We conducted a pilot, double-blind and randomised clinical trial between December 2021 and January 2022 at Shohadaye Tajrish Hospital affiliated with Shahid Beheshti University of Medical Sciences in Tehran, Iran. This study was designed per the ethical criteria of the Helsinki Declaration and was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU. RETECH.REC.1354.1399). Before enrolling subjects, the study was registered in the Iranian Registry of Clinical Trials (IRCT20131218015860N6).
Eligibility criteria
The inclusion criteria were cancer (confirmed by a pathology report), age 18–80 years, current chemotherapy with taxanes or a history of chemotherapy with taxanes in the last two months, having symptoms of peripheral neuropathy (numbness, pain, burning, etc.), a diagnosis of chemotherapy-induced peripheral neuropathy made by the attending physician, and informed consent to participate in the study. The exclusion criteria were wishing to withdraw at any time, developing acute mental and psychiatric symptoms such as depression (according to a psychiatrist), requiring unexpected treatments (e.g., surgery), undergoing radiotherapy or immunotherapy (which was not already part of the patient’s treatment protocol), deficiency or excess of vitamin B6 (according to blood tests), suffering from diabetes, carpal tunnel syndrome, or kidney failure, diagnosis of any new metastases, any history of alcohol abuse, previous history of radiotherapy to the axilla, lumbar or pelvic region, any history of neuropathy due to other diseases that the person had before starting chemotherapy, any history of allergy or side effects following the use of herbal medicines, pregnancy or breastfeeding.
Outcome measures
The primary outcome measure was the effectiveness of topical ajwain on peripheral neuropathy symptoms caused by taxane chemotherapy in cancer patients, and the secondary outcome measure was the side effects of ajwain cream use.
Intervention
Cancer patients who were undergoing chemotherapy with taxanes or had a history of chemotherapy with taxanes in the past two months and complained of peripheral neuropathy symptoms were selected. The study details were explained to each patient, and those who subsequently signed the informed consent form entered the study. The participants administered 5 g of either ajwain or placebo cream twice a day, using a measuring applicator calibrated in grams. The cream was applied in the morning and evening to the distal parts of the upper and lower extremities, encompassing the area from the ankles to the tips of the toes and from the wrists to the fingertips. The duration of the intervention was four weeks.
Assessment
Symptoms were evaluated using the Persian Chemotherapy-Induced Peripheral Neuropathy Assessment Tool (CIPNAT) questionnaire, which is valid and reliable according to previous studies.[17] This questionnaire contains 11 questions on various aspects of peripheral neuropathy, including sensory, motor, and autonomic disturbances. Each question was answered on a scale from 0 (none) to 4 (disabling or life-threatening) before and after the intervention (weeks 0 and 4). The minimum total score is zero, and the maximum is 44. Furthermore, any side effects during the study period were asked about and recorded. Data were recorded in data collection forms.
Randomisation, blinding and allocation concealment
Physicians, patients, and research assistants were blinded to the allocation. A balanced block design with three blocks was used for randomisation. Chains of random numbers from 1 to 5 were generated using the Statistical Package for the Social Sciences (SPSS) software until reaching the desired sample size. Random allocation sequences prepared for individuals were placed in sealed dark envelopes and numbered with a three-digit serial number by a person who had no role in the study. All envelopes had a unique random serial number and were opened immediately after enrolment to allocate participants to the drug or placebo group. In addition, the statistical analyst was unaware of the groups, and the data were analysed based on labels of A or B. After the statistical analysis was completed, the project director opened the codes.
Preparation of Ajwain and placebo creams
Ajwain seeds (T. ammi [L] Sprague) were collected from a medicinal plant garden in Shiraz and were identified and confirmed by Shiraz Pharmaceutical University (Herbarium No.: PM-482). Ajwain seed oil was extracted by the Clevenger apparatus. Using the chromatography-mass spectroscopy technique, thymol, p-cymene, and ɤ-terpinene were identified as the main components of the oil. Similar proportions of Cetomacrogol™, cetostearyl alcohol, Vaseline®, and butylated hydroxytoluene were used in the ajwain and placebo creams. In the formulation of ajwain cream, similar to the Petramfar et al. study, almond oil was used as 5% of the total cream, and the ajwain seed essential oil comprised 10% of the total cream. In the placebo formulation, almond oil again comprised 5% of the total cream, and paraffin oil comprised 10% of the total cream instead of ajwain seed essential oil.[16]
Both creams were filled and sealed in multilayer tubes. Before the clinical trial, the cream samples were evaluated regarding pharmaceutical properties through viscosity, pH and microbial limit tests. Ajwain cream was standardised based on thymol content (2.34 ± 0.02 g per 50 g of cream). To simulate the aroma of the ajwain cream, the inside of the placebo cream tubes was washed with the ajwain distillate before being filled with the placebo cream. The colour of both creams was identical (white).
Sample size
In this pilot study, considering a probability of 5% and a confidence level of 95%, a sample size of 30 people was considered,[18] randomly assigned to the ajwain and placebo groups.
Statistical analysis
Data were summarised using mean and standard deviation. The Chi-square test was used to compare demographic data. The Wilcoxon signed-rank test was used for intragroup comparison among the participants of each group. The correlation of before and after scores in each group was estimated using Spearman’s linear correlation coefficient. The mean change (before and after the study) in the score of each variable was compared between the groups using the exact Kruskal–Wallis Test. The data were evaluated using SPSS version 16 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant.
RESULTS
A total of 49 patients were assessed for eligibility, 30 of whom were randomly divided into two groups of 15 [Figure 1]. Four males and 11 females comprised the drug group, while six males and nine females comprised the placebo group. The mean age was 56.64 ± 5.71 in the drug group and 51.65 ± 7.71 years in the placebo group (P = 0.321). In the drug group, 45.5% had a diploma or lower education, and the rest (54.5%) had tertiary education. These proportions in the placebo group were 71.5% and 28.5%, respectively (P = 0.241). The trial involved patients who were afflicted with different types of cancer: breast (n = 12), lung (n = 6), gastro-intestinal (n = 9) or prostate (n = 3). However, no notable distinction was found among the groups in terms of cancer type.

- CONSORT flowchart of participants. n: Number of participants.
The mean CIPNAT questionnaire score in the drug group before and after the study was 39.09 and 40.82, respectively (P = 0.518), and the score in the placebo group was 24.60 and 25.93, respectively (P = 0.536). Analysis of the mean difference score between the two groups (0.83) demonstrated that the drug was statistically ineffective when compared to the placebo (P = 0.372) [Figure 2].

- Mean of chemotherapy-induced peripheral neuropathy assessment tool scores in both groups.
At the same time, the correlation score of the CIPNAT scores before and after the intervention in the drug (0.238) and placebo (0.631) groups indicated that although the effect of the intervention was not statistically significant, the decrease in scores in the drug group in people who had a higher CIPNAT score (more severe peripheral neuropathy) was more than the decrease in the corresponding placebo group samples. This finding is evident by the distribution of CIPNAT questionnaire scores before and after the trial in the drug and placebo groups [Figure 3].
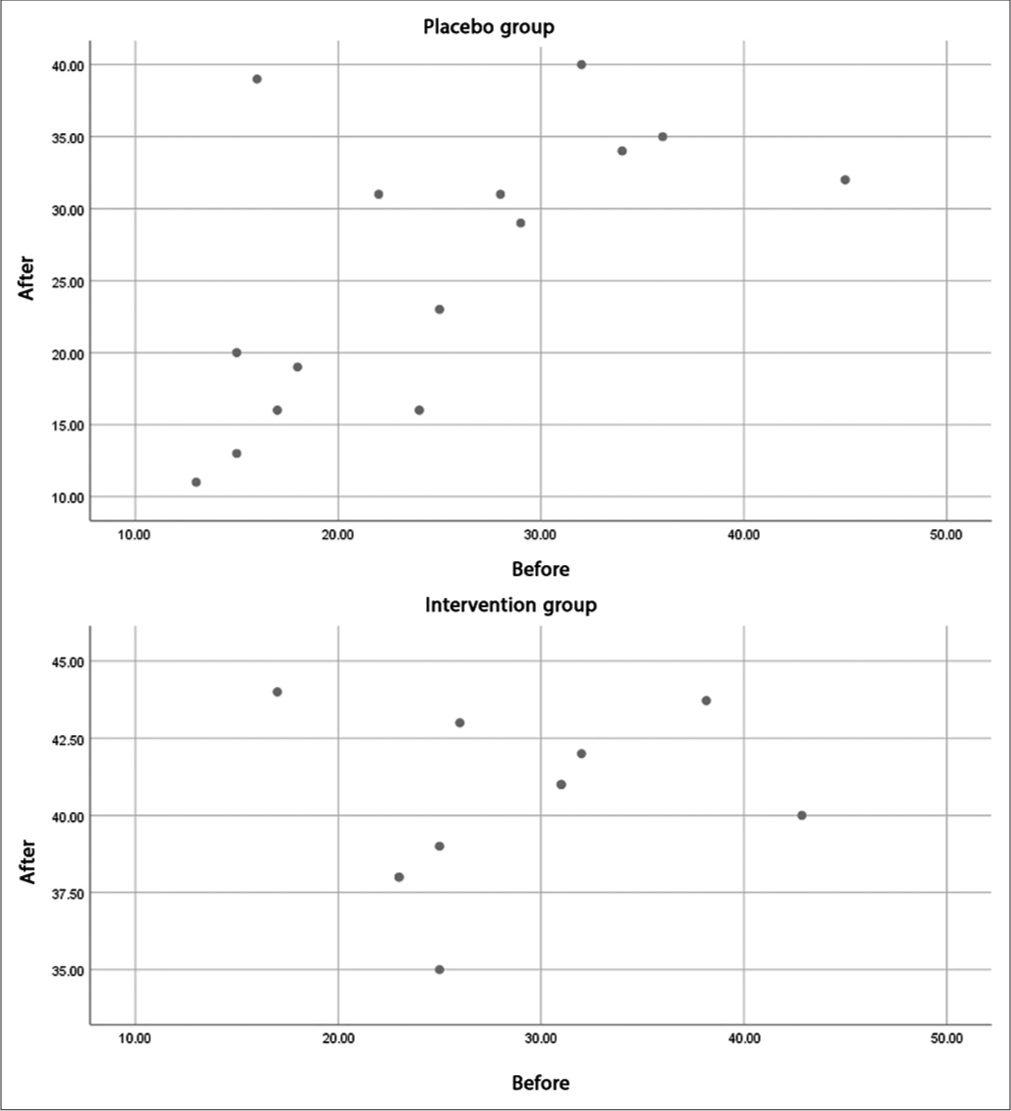
- The distribution of chemotherapy-induced peripheral neuropathy assessment tool scores before and after the trial in both groups.
The side effects recorded at the site of applying the creams included mild erythema with the drug (n = 4) or placebo (n = 1), mild itching with the drug (n = 1), and moderate itching with the placebo (n = 2). Two participants in the placebo group reported mild headaches. As all side effects were mild, none of the patients agreed to discontinue the study.
DISCUSSION
The present study is the first clinical trial to investigate the effectiveness of a plant-derived compound in treating peripheral neuropathy induced by taxane chemotherapeutic agents. Although different drugs and treatment modalities have been used to treat this condition, it is vital to introduce an effective and safe drug for this category of patients. The ajwain cream used in this study had acceptable safety.
There are various open-label studies regarding the use of topical chemical drugs to control peripheral neuropathy, but there are fewer studies on herbal products. In a double-blind and randomised clinical trial on 92 patients, the effect of 10% Ajwain cream on peripheral neuropathy with various causes (diabetic neuropathy, postoperative neuropathy, etc.) was investigated. The results demonstrated that ajwain cream significantly reduced the amount of burning, tingling, numbness, and pain compared with the placebo.[16] The difference in the response observed in our study and Petramfar et al. may be due to the different causes of peripheral neuropathy and dissimilar mechanisms of nerve involvement. Peripheral neuropathy is divided into axonal and demyelinating neuropathy based on the part of the nerve involved. Peripheral neuropathy caused by drug toxicity is often axonal,[1,15,19] which is different from the type of neuropathy of most patients in the mentioned study.[16] Other factors that may explain this discrepancy are the limited number of samples in the present study and different administered tools for measurement (CIPNAT questionnaire versus Mc Gill pain questionnaire).
An open-label trial in which 1% menthol topical ointment was examined in chemotherapy-induced peripheral neuropathy patients also showed a significant reduction in pain. Although it seems that the mechanism of the pain reduction effect of the mint and ajwain plants is the blocking of sodium channels by the active ingredient (menthol and thymol, respectively),[16] the difference in the findings may be related to the degree of skin absorption of the drugs in patients undergoing chemotherapy, which affects the efficacy of topical drugs in treating peripheral neuropathy. The skin of this group of patients may undergo changes due to the use of different courses of chemotherapy and radiotherapy, affecting local drug absorption and, thus, drug efficacy.[20]
The theory of PM, rooted in ancient wisdom and traditional practices, offers a holistic perspective on health and well-being. According to PM sources, an imbalance of the four body humours can lead to disease, such that the dominance of coldness and wetness (phlegm) can cause nervous system diseases like peripheral neuropathy.[21] On the other hand, consuming medicinal plants with the opposite temperament (hot and dry) can effectively balance the humours and cure the disease.[22] From this point of view, ajwain has a hot and dry temperament and can help solve peripheral neuropathy symptoms. Still, the short duration of treatment or the low dosage of the drug may explain the unsuccessful treatment results in the present trial.
Among the other traditional, complementary, and alternative medicine treatments used to control peripheral neuropathy, interventional methods such as herbal therapy, acupuncture, exercise, and massage therapy can be mentioned. Although recent systematic studies have shown relatively good effectiveness for such treatments, limitations such as the unknown active substance, heterogeneity in the treatment protocol and demographic indicators, lack of blinding, and the low number of participants in these studies should be taken into account.[23-26]
One of the limitations of the current study was that it was not feasible to control several factors that affect the incidence of chemotherapy-induced peripheral neuropathy, such as chemotherapy dose intensity, cumulative dose, and treatment duration. Furthermore, the inappropriate use of CIPNAT for measuring pain, as well as the different neuropathy baseline scores of the groups, should be kept in mind. Another constraint is the requirement for patients to possess homogeneity in terms of temperament, and future trial designs may benefit from focusing specifically on patients with painful chemotherapy-induced peripheral neuropathy. However, the randomised design, the control of some concurrent conditions (e.g., diabetes and alcohol abuse), and the use of a local topical agent were strengths of this study. Overall, while the present study highlights the acceptable safety profile of Ajwain cream, it is important to note that the study did not specifically demonstrate a reduction in CIPNAT scores or establish its superiority over the placebo. Therefore, further investigations involving larger multicentre trials with expanded sample sizes are warranted to validate and confirm any potential findings. These future studies should focus on comprehensive evaluations and adhere to evidence-based medicine principles to identify effective treatment options for one of the most common side effects in the field of oncotherapy.
CONCLUSION
Despite the fact that ajwain cream demonstrated inadequate effectiveness in addressing the symptoms of chemotherapy-induced peripheral neuropathy, it is crucial to conduct multicenter controlled trials with a substantial sample size. These trials are necessary in order to obtain a comprehensive and all-encompassing understanding of the subject matter.
Acknowledgements
The authors would like to appreciate all the patients who participated in the study. This manuscript was reviewed in terms of language and grammar by a native English-speaking language editor, Dr. Seyed Ali Hosseini (Native Editor Co., Shiraz, Iran).
Author’s contributions
Ghazaleh Heydarirad, Hamid Reza Mirzaei, Mehdi Pasalar and Reyhaneh Gharehgozlou: Study concept and design. Reyhaneh Gharehgozlou, Hamid Reza Mirzaei, and Ghazaleh Heydarirad: Acquisition of data. Judith Buentzel, Mohammad Reza Namazi, and Mehdi Pasalar: Analysis and interpretation of data. Ghazaleh Heydarirad, Hamid Reza Mirzaei, Mehdi Pasalar: Drafting of the manuscript. Reyhaneh Gharehgozlou, Judith Buentzel, Mohammad Reza Namazi, and Mehdi Pasalar: Critical revision of the manuscript for important intellectual content. Reyhaneh Gharehgozlou: Administrative, technical, and material support. Ghazaleh Heydarirad, Hamid Reza Mirzaei and Mehdi Pasalar: Study supervision.
Ethical approval
The authors declare that the investigations were carried out following the rules of the Declaration of Helsinki and were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1354.1399).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Chemotherapy-induced Neuropathy in Cancer Survivors. J Pain Symptom Manage. 2017;54:204-18.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Chemotherapy-induced Peripheral Neuropathy: Prevention and Treatment Strategies. Eur J Cancer. 2008;44:1507-15.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Costus sp. Preparation as Palliative Care for Chemotherapy-induced Peripheral Neuropathy of Patients: A Randomized Placebo-controlled Pilot Trial. J Altern Complement Med. 2020;26:807-12.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological Management of Chronic Neuropathic Pain: Revised Consensus Statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328-35.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term Chemotherapy-induced Peripheral Neuropathy among Breast Cancer Survivors: Prevalence, Risk Factors, and Fall Risk. Breast Cancer Res Treat. 2016;159:327-33.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of Crocin of Saffron (Crocus sativus L.) against Chemotherapy-induced Peripheral Neuropathy: A Randomized, Double-blind, Placebo-controlled Clinical Trial. J Ethnopharmacol. 2021;281:114511.
- [CrossRef] [PubMed] [Google Scholar]
- Algorithm for Neuropathic Pain Treatment: An Evidence Based Proposal. Pain. 2005;118:289-305.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological Management of Chronic Neuropathic Pain-consensus Statement and Guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of a Patient-tailored Integrative Medicine Program on Gastrointestinal Concerns and Quality of Life in Patients with Breast and Gynecologic Cancer. J Cancer Res Clin Oncol. 2017;143:1243-54.
- [CrossRef] [PubMed] [Google Scholar]
- Moalejat-e Aghili (Persian) Tehran: Institute of Medical History, Islamic Medicine and Complementary Medicine, Iran Medical University; 2008.
- [Google Scholar]
- An Overview on Ajwain (Trachyspermum ammi) Pharmacological Effects; Modern and Traditional. J Nat Remedies. 2014;14:98-105.
- [Google Scholar]
- Toxicological and Anti-rheumatic Potential of Trachyspermum ammi Derived Biogenic Selenium Nanoparticles in Arthritic Balb/c Mice. Int J Nanomedicine. 2020;15:3497-509.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory and Analgesic Effects of Trachyspermum ammi Seed Extract and its GC-MS Analysis. J Pharm Bioallied Sci. 2020;12:886-7.
- [Google Scholar]
- Trachyspermum ammi 10% Topical Cream Versus Placebo on Neuropathic Pain, a Randomized, Double-blind, Placebo-controlled Trial. Neurol Sci. 2016;37:1449-55.
- [CrossRef] [PubMed] [Google Scholar]
- Chemotherapy-induced Peripheral Neuropathy Assessment Tools: A Systematic Review. Oncol Nurs Forum. 2017;44:E111-23.
- [CrossRef] [PubMed] [Google Scholar]
- A Simple Formula for the Calculation of Sample Size in Pilot Studies. J Clin Epidemiol. 2015;68:1375-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of Chemotherapy-induced Peripheral Neuropathy. Front Mol Neurosci. 2017;10:174.
- [CrossRef] [PubMed] [Google Scholar]
- Functional Assessment of a Skin Care System in Patients on Chemotherapy. Skin Pharmacol Physiol. 2007;20:253-9.
- [CrossRef] [PubMed] [Google Scholar]
- Approach of Persian Medicine to Health and Disease. Adv Integr Med. 2022;9:3-8.
- [CrossRef] [Google Scholar]
- A Review on Medical Plants Advised for Neuralgia from the Perspective of “Canon of Medicine”. Adv Integr Med. 2021;8:230-5.
- [CrossRef] [Google Scholar]
- Efficacy and Safety of Acupuncture against Chemotherapy-induced Peripheral Neuropathy: A Systematic Review and Meta-analysis. Evid Based Complement Alternat Med. 2020;2020:8875433.
- [CrossRef] [Google Scholar]
- Can Medical Herbs Stimulate Regeneration or Neuroprotection and Treat Neuropathic Pain in Chemotherapy-induced Peripheral Neuropathy? Evid Based Complement Alternat Med. 2013;2013:423713.
- [CrossRef] [PubMed] [Google Scholar]
- Natural Products Evaluated in Neuropathic Pain Models-a Systematic Review. Basic Clin Pharmacol Toxicol. 2014;114:442-50.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Exercise on Cancer Patients Suffering Chemotherapy-induced Peripheral Neuropathy Undergoing Treatment: A Systematic Review. Crit Rev Oncol Hematol. 2018;121:90-100.
- [CrossRef] [PubMed] [Google Scholar]







