Translate this page into:
Fluoroscopy-guided Neurolytic Splanchnic Nerve Block for Intractable Pain from Upper Abdominal Malignancies in Patients with Distorted Celiac Axis Anatomy: An Effective Alternative to Celiac Plexus Neurolysis - A Retrospective Study
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The pain from upper gastrointestinal malignancy leads to considerable morbidity. The celiac plexus and splanchnic nerve neurolysis are good therapeutic options. Although splanchnic nerve neurolysis less frequently performed, but it has an edge over celiac plexus as it can be performed in patients with altered celiac plexus anatomy by enlarged lymph nodes.
Methods:
The fluoroscopy-guided splanchnic nerve neurolysis was done in about 21 patients with intractable upper abdominal pain with pain intensity of ≥7 in numerical rating scale (NRS) from upper gastrointestinal cancers with distorted celiac plexus anatomy from enlarged celiac lymph nodes as seen by computed tomography scan after positive diagnostic splanchnic nerve neurolysis. The demographic features, pain intensity, daily opioid dose, functional status and quality of life was measured at baseline and 1 week, 1 and 3 months after the procedure.
Results:
There was a significant improvement in pain intensity, opioid requirement, functional status, and physical components quality of life after the neurolysis (P < 0.05) and this improvement had continued till 3 months. There were also more than 50% reduction in pain intensity and significant decrease in opioid requirement in all the patients after neurolysis.
Conclusion:
The fluoroscopy-guided splanchnic nerve neurolysis results significant pain relief, decrease in opioid intake, improvement in functional status, and quality of life for up to 3 months in upper abdominal pain from gastrointestinal cancers in patients with distorted celiac lymph node anatomy not amenable to celiac plexus neurolysis.
Keywords
Celiac plexus
fluoroscopy
splanchnic nerve neurolysis
upper gastrointestinal cancer pain
INTRODUCTION
Celiac plexus and splanchnic nerve block had been described by Kappis and Popper, respectively.[12] The use of neurolytic agents in these two blocks was first described by Jones and its role in the treatment of pain from upper gastrointestinal malignancies was first described by Bridenbaugh et al.[34]
There was a significant improvement in the technique of celiac plexus block with the advent of new imaging techniques. Nowadays, the celiac plexus block can be given by endoscopic ultrasound, computerized tomography, ultrasound, and fluoroscopy-guided technique.[56789101112]
The pain relief from celiac plexus neurolysis is good to excellent for pain arising out from upper gastrointestinal malignancies.[12] Most of the studies and reviews had reported good to excellent pain relief after a celiac plexus block, and it decreases the opioid burden and improves the quality of life.[131415161718]
However, in advanced malignancies, the celiac plexus anatomy can be distorted by the underlying malignancy or the enlarged celiac lymph nodes, making the access the celiac ganglia is difficult, or there is inadequate spread of the neurolytic agent.[1920] In these circumstances, the splanchnic nerve neurolysis can be useful as its anatomy is not affected by the disease process or enlarged lymph nodes. The literature for splanchnic nerve neurolysis for relief of upper abdominal malignancies is limited. There were many studies which showed that the mechanical neurolysis of splanchnic nerves is effective in pain control in patients with pain arising out of pancreatic cancer or chronic pancreatitis. The mechanical splanchnicectomy can be performed through multiple approaches, which are transhiatal bilateral splanchnicectomy, unilateral thoracoscopic splanchnicectomy, and bilateral thoracoscopic splanchnicectomy.[2122232425] Moreover, there were a few major complications associated with these procedures.[26] Thus, we tried to find a safer alternative to the celiac plexus for pain relief in upper gastrointestinal malignancies. Till date, there were few studies on computed tomography (CT)-guided splanchnic nerve block.[272829] In addition recently in last few years, three studies on fluoroscopy-guided splanchnic neurolysis have showed promising results as compared to celiac neurolysis for upper abdominal pain from gastrointestinal cancer.[30313233] Furthermore, recently, the radiofrequency thermocoagulation of splanchnic nerves has showed promising results for pain upper gastrointestinal structures.[34]
We have retrospectively analyzed the data from about 21 patients who had underwent the splanchnic nerve neurolysis after a positive diagnostic splanchnic nerve block. We tried to find the safety and efficacy of splanchnic nerve block. The primary objective of our study was 50% reduction of the pain intensity after the neurolysis, and the secondary objective is 50% reduction in opioid dose, functional status, and quality of life.
METHODS
The retrospective study of the 21 patients who had undergone the fluoroscopy-guided splanchnic nerve alcohol neurolysis for intractable upper abdominal pain (numerical rating scale [NRS]>7) from upper gastrointestinal cancers, who had not responded to oral opioid therapy as per the WHO analgesic ladder and who had a positive diagnostic splanchnic block, over a period of 1 year from October 2014 to September 2015 after a positive diagnostic splanchnic nerve neurolysis. These patients were not amenable for celiac plexus neurolysis due to distortion of anatomy by the disease or enlarged celiac lymph nodes as shown in the computerized tomography or magnetic resonance scan. The patients were diagnosed with gastrointestinal cancers where chemotherapy was continued.
They were considered for splanchnic plexus neurolysis if they fulfill the following:
-
Diagnosed with upper gastrointestinal cancer
-
Severe pain with pain intensity > NRS
-
Not responding to oral opioid or having intolerable side effects from opioid therapy
-
Not amenable to celiac plexus neurolysis due to distorted celiac plexus anatomy due to disease or enlarged lymph nodes
-
Positive diagnostic splanchnic nerve block, i.e., more than 50% relief in pain intensity for more than 2 hour.
Two patients had refused neurolysis because of coagulopathy.
Diagnostic splanchnic nerve block
The patients were kept fasting for 6 h before the procedure and were prehydrated with 500 ml of normal saline. The oral immediate release morphine was stopped 4 h and the delayed release one was stopped 12 h before the procedure. The other analgesics, for example, paracetamol were stopped 6 h before the injection, and the other nonsteroidal anti-inflammatory drugs were stopped either 12–24 h preoperatively. In the operation theater table, the patient was placed in prone position with a pillow under the hip and chest to reverse thoracolumbar lordosis and to increase the distance between iliac crests and rib cage. The monitors, electrocardiography, noninvasive blood pressure, and pulse oximetry were connected as per the American Society of Anesthesiologists standard and 20-gauge intravenous cannulae were inserted, and lactated ringer solution was started. The T12 vertebrae were visualized with fluoroscopy, and the c-arm was rotated to the ipsilateral side by 20–30° until the T12 transverse processes is flushed with the anterolateral border of the vertebrae. The skin and subcutaneous tissue was infiltrated with 1% lignocaine. A 22-gauge Chiba needle (Cook Medical Inc., Bloomington, IN, USA) was directed toward the anterolateral border of T12 vertebrae under fluoroscopy guidance, and the final position was confirmed by the spread of contrast along the anterolateral border of the vertebra with no posterior leaking of contrast [Figures 7-9]. The diagnostic splanchnic nerve block was given using 8 ml of 0.25% bupivacaine through the Chiba needle on both sides after a negative aspiration of blood or fluid (contrast). The diagnostic block was considered positive if there was 50% decrease in pain intensity (measured in NRS) for at least 2 h after the injection. The patients were observed for any immediate hemodynamic complication and for the delayed effects such as diarrhea and were placed on overnight intravenous fluid. The regular analgesics were started once the pain started after the procedure as per WHO guidelines.

- The pain intensity in numerical rating scale at various times in different diagnostic categories and different body mass index
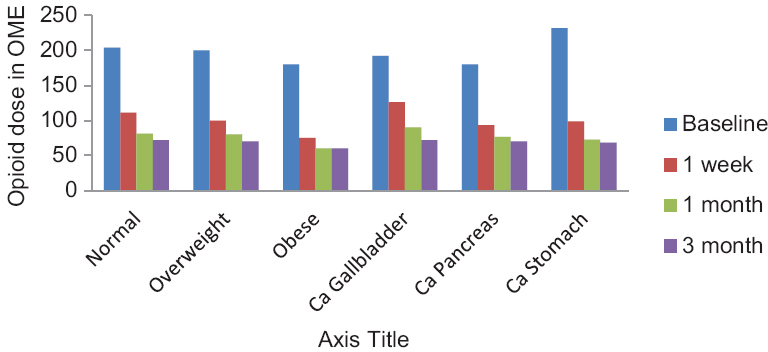
- Opioid dose at various times in different types of cancer and different body mass index
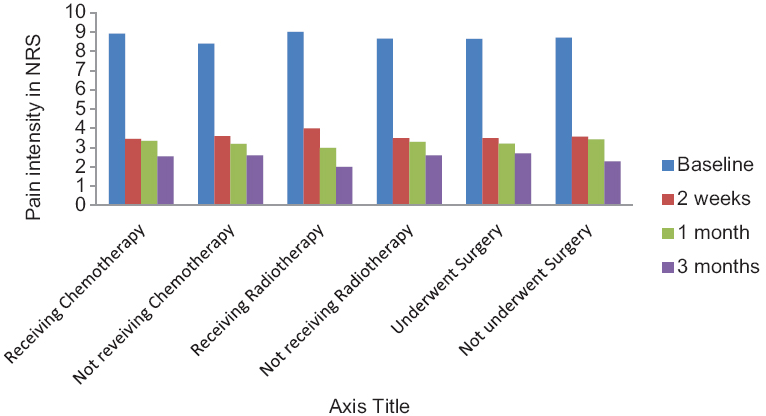
- Pain intensity in numerical rating scale at different types in patients with or without chemotherapy, with or without radiotherapy, and with or without surgery
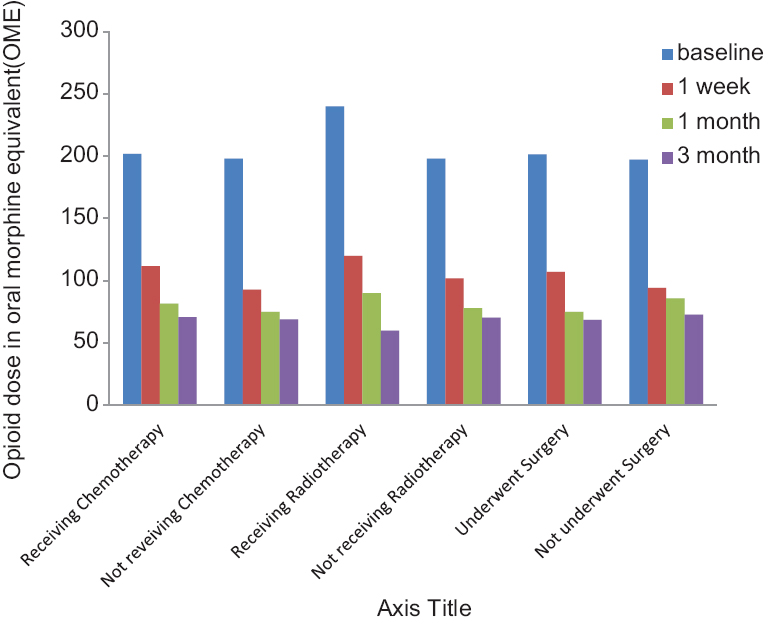
- Opioid dose in patients with or without chemotherapy, radiotherapy or surgery
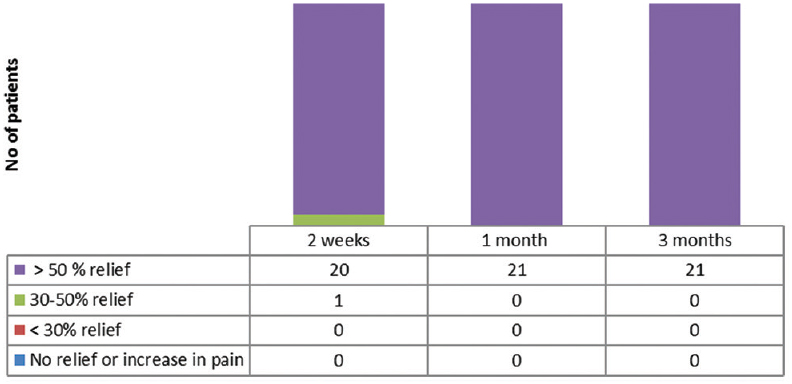
- The number of patients with varying degree of relief in pain intensity after the neurolysis
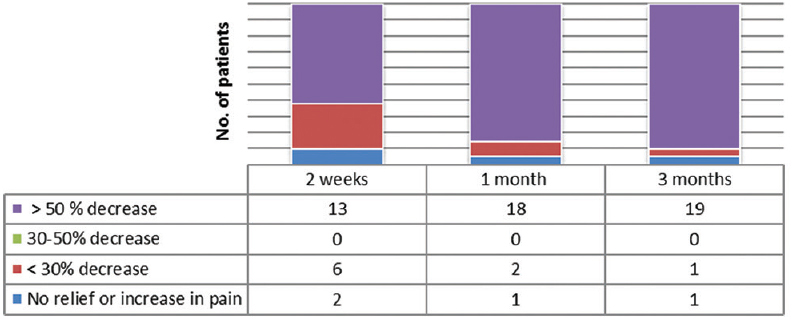
- The number of patients with varying degree of decrease in opioid dose measured in oral morphine equivalent after the neurolysis
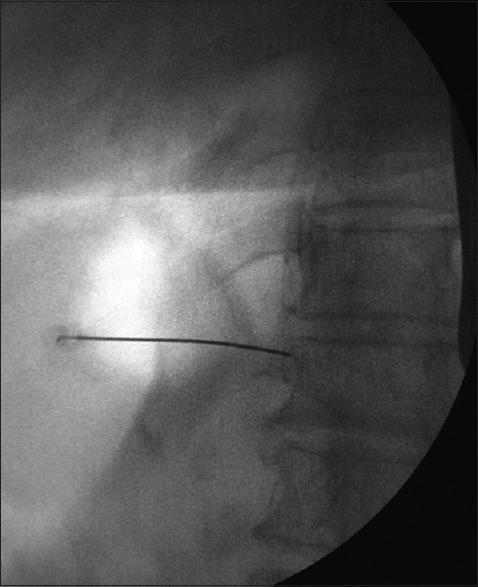
- The anteroposterior view of the needle position in the splanchnic nerve block
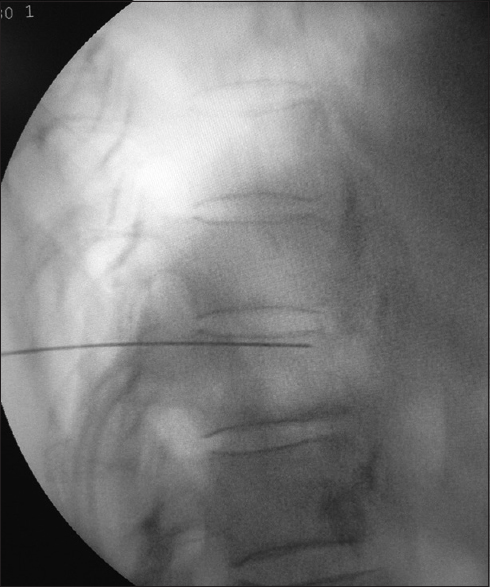
- The lateral view of the needle position in the splanchnic nerve block

- The anteroposterior view of the contrast spread in the splanchnic nerve block
Therapeutic splanchnic nerve block
The therapeutic splanchnic plexus block was given in a similar way as the diagnostic splanchnic nerve block, and the neurolysis was done using 6 ml of 50% alcohol in 0.25% bupivacaine on both sides after negative aspiration of blood or cerebrospinal fluid and confirmation of site by the use of contrast under fluoroscopy [Figures 7-9]. There was initially increase in pain in two patients, which resolved in a few minutes. The patients were observed for any immediate hemodynamic complication and delayed effects such as diarrhea and were placed on overnight intravenous fluid.
Additional interventions
No additional intervention like additional neurolysis was done during the study period, except the titration of opioid and other analgesics.
Postprocedure follow-up
The patients were followed up at 1 week, 1 and 3 months after the procedure. The baseline pain intensity (NRS), opioid dose (in oral morphine equivalent [OME]), functional activity in Karnofsky scale, and quality of life by short form (SF)-36 questionnaire were measured by a resident who was not involved in the procedure. The other analgesics such as nonsteroidal anti-inflammatory drugs were kept constant for all patients. All patients were given oral paracetamol 4 g/day throughout the study period and oral etoricoxib 60–90 mg for 5 days. Furthermore, any complication or adverse effects were noted.
Statistical analysis
The statistical analysis was done using SPSS (version 16, SPSS Inc., Chicago, Illinois, USA) for Windows, and the variables were expressed as mean and standard deviation. The paired t-test was used to assess the effectiveness of the splanchnic nerve block by measuring the changes in variable (pain intensity, opioid dose, functional status, and quality of life) with time. The correlation among the variables was measured by Pearson correlation. Level of significance was set at <0.05.
Evaluations
The pain intensity was measured with NRS. The daily opioid dose was converted to OME and was calculated at baseline and at 2 weeks, 1 and 3 months after the procedure. The functional status assessment of the patient was done using the Karnofsky score, which was scored 0–100, with higher number indicates better function. The quality of life was measured by the SF-36 questionnaire, which assesses the various dimensions in the quality of life, including physical functioning, role physical, bodily pain, general health, social functioning, role emotional, vitality, social functioning, and mental health.
Outcome measures
The primary outcome was measured by 50% decrease in the pain intensity (NRS) from baseline to subsequent visits after the procedure. The secondary outcome measures were 50% improvement in functional status (measured by Karnofsky status), quality of life as measured by SF-36 questionnaire, decrease in opioid consumption (measured in OME, oral morphine equivalent), and incidence of adverse effects.
RESULTS
Patients
About 25 patients underwent diagnostic splanchnic nerve block, and out of them, only 23 had a successful outcome. Out the 23 successful splanchnic nerve blocks, one patient refused for the therapeutic splanchnic nerve block, and out of the 22 patients who had underwent the therapeutic splanchnic nerve block, one patient was lost to follow-up immediately after the procedure and was excluded from the analysis.
Demographics
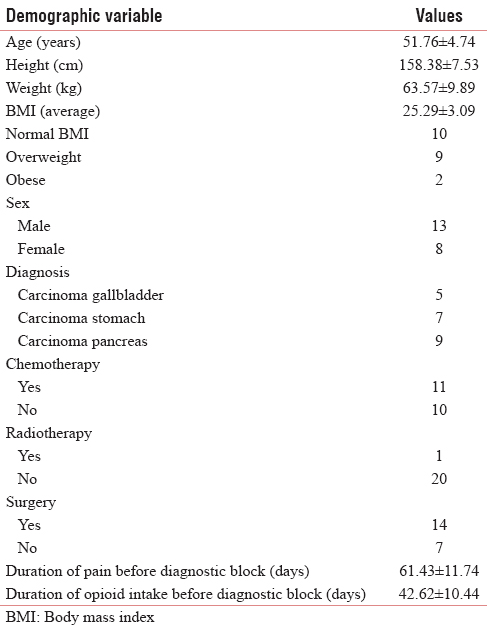
The mean age of the patients was 51.76 ± 4.74 years. The patients were 13 males and 8 females. There was no significant difference in the pre- or post-procedure pain intensity, opioid dose, functional status, or quality of life among the patients with different age or sex (P > 0.05).
Diagnosis
The patients included in the study were diagnosed with carcinoma of gallbladder (five patients), stomach (seven patients), and pancreas (nine patients), respectively. There was no significant difference in the pre- or post-procedure pain intensity, opioid dose, functional status, or quality of life among the patients with different diseases [Figures 1 and 2] (P > 0.05).
Preprocedure chemotherapy, radiotherapy, and surgery
The data on preprocedure chemotherapy, radiotherapy, and surgery are given on Table 1. There was no impact of the preprocedure chemotherapy, radiotherapy, and surgery on the results of the block [Figures 3 and 4] (P > 0.05).
Body mass index
The average body mass index (BMI) of the patients was 25.29 ± 3.09. There were nine overweight and two obese patients. However, there was no significant difference in the pre- or post-procedure pain intensity, opioid dose, functional status, or quality of life among them (P > 0.05).
Preprocedure pain and opioid intake duration
The mean duration of pain and opioid intake before the diagnostic block was 61.43 ± 11.74 days and 42.62 ± 10.44 days, respectively. However, there was no significant difference in the pre- or post-procedure pain intensity, opioid dose, functional status, or quality of life among them (P > 0.05).
Pain intensity
There was a significant reduction in pain intensity after the neurolysis, from a baseline value of 8.67 ± 0.483–3.52 ± 0.68, and it was maintained at that level for 3 months (P < 0.05) [Table 2]. There was 50% reduction in pain intensity in twenty patients 2 weeks after the neurolysis, whereas later, after 1 month, all the patients had got more than 50% reduction in pain intensity [Figure 5].

Opioid dose
There was a significant reduction of opioid dose from a baseline value of 200 ± 78.88 mg to 102.86 ± 40.88 mg after 2 weeks and it reduced further at 1 and 3 months (P < 0.05) [Table 2]. There was 50% reduction in opioid dose in 13, 18, and 19 patients at 1 weeks, 1 month, and 3 months, respectively [Figure 6].
Functional status and quality of life
There was also nonsignificant improvement in functional status as measured by Karnofsky score from a baseline value of 51.33 ± 7.43–61.33 ± 7.43, and it remained at that level throughout the study [Table 2]. There was also significant improvement in the physical components of quality of life as measured by SF-36 after the neurolysis (P < 0.05), whereas emotional components showed only minimal improvement [Table 3].

There was no correlation among the demographic variables and preprocedure pain and opioid intake duration with relief in pain intensity and decrease in opioid dose after the neurolysis.
Multiple regression analysis also showed that neither the demographic variables nor the preoperative pain and opioid dose or duration were related to postprocedure pain intensity, opioid dose, functional status, and quality of life.
Complications
There were few minor complications following diagnostic and therapeutic splanchnic blocks. Of them one patient developed neuritis after the splanchnic neurolysis, which resolved after a few days. Furthermore, Transient hypotension and diarrhea occurred in 5 (23.8%) and 5 (23.8%) patients after diagnostic block and 4 (19%) and 3 (14.2%) patients after neurolysis, but it had resolved within 24–48 h. There were no major complications after the diagnostic or neurolytic block.
DISCUSSION
In our study, splanchnic neurolysis resulted in significant and sustained relief in pain intensity and decrease in opioid intake in patients with upper abdominal pain from upper gastrointestinal malignancies with distorted celiac anatomy with severe pain not responding to opioid as per the WHO analgesic ladder. Furthermore, there was also improvement in functional status and quality of life after the neurolysis.
Efficacy of splanchnic nerve neurolysis
There are only few comparative studies on the splanchnic neurolysis with celiac neurolysis.[30313233] The recent studies had showed that patients with splanchnic nerve neurolysis lead to better pain relief, less opioid consumption, fewer complications, and a better quality of life as compared to celiac plexus neurolysis.[3032] In our study, also we had found that more than 50% relief in pain intensity was obtained in all patients and more than 50% decrease in opioid dose was observed in >75% patients at 1 month. Furthermore, there was improvement in both functional status and quality of life in all patients. Our study also confirms that splanchnic neurolysis is a good option for patients with upper abdominal pain from upper gastrointestinal malignancy.
Distorted celiac anatomy
Furthermore, in advanced malignancies, the celiac plexus anatomy can be distorted by the underlying malignancy or the enlarged celiac lymph nodes, making the celiac plexus block difficult to access the celiac ganglia is difficult, or there is inadequate spread of the neurolytic agent.[1920] In our study, the celiac anatomy was distorted in all patients making the celiac plexus block difficult, and thus we had opted fr the splanchnic neurolysis which had resulted in significant pain relief, decrease in opioid intake, and improvement in functional status and quality of life. Hence, it confirms that in these circumstances, the splanchnic nerve neurolysis can be an effective alternative to celiac plexus neurolysis as there is no change in the anatomy of the anatomy splanchnic nerve with the disease progress.
Mechanical splanchnicectomy
There were many studies on the mechanical splanchnicectomy. There are three techniques for mechanical splanchnicectomy, which are transhiatal bilateral splanchnicectomy, unilateral thoracoscopic splanchnicectomy, and bilateral thoracoscopic splanchnicectomy.[2122232425] The procedures had resulted in significant and prolonged pain relief and improvement in quality of life.[25] However, these procedures had been associated with complications such as pneumothorax, chylothorax, splenic injury, and intercostal neuralgia.[25] In our study, we had encountered only mild adverse effects such as hypotension and diarrhea, which had resolved spontaneously; while one patient had developed neuritis, which resolved in a few days and was well controlled with oral medications. Hence, fluoroscopy-guided splanchnic nerve block can be effective and safer alternative to mechanical splanchnicectomy for patients with pain from upper gastrointestinal cancers.
Thoracic splanchnic nerve radiofrequency thermocoagulation
The radiofrequency thermocoagulation had also resulted in good pain relief, decrease in opioid consumption, and improvement in quality of life in patients with pain from pancreatic cancers.[34] However, radiofrequency thermocoagulation needs a specialized equipment, whereas fluoroscopy-guided neurolysis only requires fluoroscope which is readily available in most of the centers. Thus, fluoroscopy-guided neurolysis can be good alternative to those centers which do not have the radiofrequency equipment or cannot afford to procure it.
Onset of maximum relief
The time for maximal relief from the celiac neurolysis is variable; some studies are showed immediate relief while other showed variable onset in relief.[3536] In our study, we had the obtained data only after 1 week from the procedure, and at that time, all the patients had got substantial significant relief in pain intensity which is similar to the study for splanchnic nerve block by Shwita AH and et al.[30]
Effect of body mass index
One previous study on celiac plexus block had found that with the increasing BMI, the success rate of celiac plexus block decreases, whereas in our study, we did not found any change in the success of the block with the increasing BMI.[37] The previous authors have postulated that the needle placement may not be accurate with increasing BMI, and in their technique, the verification of the needle placement was either not done or only by plain films and the verification by fluoroscopy or by CT scan in only 7.7% patients.[37] However, in our study, the verification was done by the fluoroscopy in all patients, which may the reason of higher success rate as the landmarks may change with the increasing BMI and with fluoroscopy, the exact target for block can be visualized in real time, and also the needle path and final position of the needle can be confirmed by the use of contrast, which is not possible with the blind technique or plain films in the study quoted.
Effect of chemotherapy, radiotherapy, or prior surgery
In our study, we did not find any influence of chemotherapy, radiotherapy, or prior surgery on the results of the procedure, which was similar to previous study by Koyyalagunta et al.[31]
Effect on survival
The splanchnic neurolysis also improves the survival as found out by Lillemoe et al., but in our study, we did not have the data beyond 3 months; Hence, its impact on survival cannot be analysed.[33]
Effect of diagnosis
There was no role of the cancer type on the performance of the neurolysis, which was similar to previous studies.[3031]
Effect of duration of pain and opioid intake
There was no effect of the duration of pain and opioid dose or duration of opioid intake on the performance of block. There was also no correlation between duration of pain and opioid intake with the preprocedure pain intensity or opioid dose.
Complications
There are few complications of celiac and splanchnic neurolysis which are described in literature, for instance hypotension and diarrhea, gastric or bowel perforation, vascular injury, hematoma, and chemical peritonitis.[26] The chances of gastric or bowel perforation, vascular injury, hematoma, and chemical peritonitis are rare in splanchnic nerve neurolysis as there is no vessels nearby the splanchnic nerve and the nerve is extraperitoneal, so neither bowel perforation nor vascular injury occurs with splanchnic nerve neurolysis. There was orthostatic hypotension in 23% and 19% of patients after the diagnostic and neurolytic block, which is similar to the previous study by Amera H and et al., where one previous study by Papadopoulos et al. found a very high incidence of 53% after splanchnic nerve block.[3034] In our study, transient diarrhea occurred in 23.8% and 14% patients after diagnostic and neurolytic block, which is less than the previous study, which reported a incidence of 30%.[30] However, there is still a rare possibility of paraplegia with splanchnic neurolysis, as described for celiac neurolysis, which fortunately did not occurred in our study.[34] In contrast, there is no serious complications reported in splanchnic neurolysis, as compared to celiac plexus block such as paraplegia, and no such complications had occurred in our study except one patient with moderate neuritis which resolved spontaneously within a few days.
Limitations of study include:
-
Small sample size
-
Nonrandomized study
-
Limited follow-up
-
Nonsham-controlled study
-
Nonblind study
-
Retrospective study.
CONCLUSION
This study confirms that splanchnic nerve neurolysis results in relief in pain intensity, decrease in opioid intake, and also improvement in functional status and quality of life in patients with pain from upper abdominal malignancy with distorted celiac anatomy. However, to conclude the findings, a large randomized sham-controlled trial with adequate sample size and prolonged follow-up is needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Experiences with local anesthesia in abdominal surgery. Verh Dtsch Ges Circ. 1914;43:87.
- [Google Scholar]
- Acute pancreatitis; an evaluation of the classification, symptomatology, diagnosis and therapy. Am J Dig Dis. 1948;15:1-4.
- [Google Scholar]
- A technic for injection of the splanchnic nerves with alcohol. Anesth Analg. 1957;36:75-7.
- [Google Scholar]
- Management of upper abdominal cancer pain: Treatment with celiac plexus block with alcohol. JAMA. 1964;190:877-80.
- [Google Scholar]
- Anterior approach to celiac plexus block during interventional biliary procedures. Radiology. 1988;167:562-4.
- [Google Scholar]
- Celiac plexus neurolysis with the modified transaortic approach. Radiology. 1990;175:274-6.
- [Google Scholar]
- Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656-62.
- [Google Scholar]
- A prospective randomized comparison of endoscopic ultrasound-and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-5.
- [Google Scholar]
- Bedside ultrasound-guided celiac plexus neurolysis with bilateral paramedian needle entry technique can be an effective pain control technique in advanced upper abdominal cancer pain. J Palliat Med. 2008;11:1195-9.
- [Google Scholar]
- CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging. 2006;31:710-8.
- [Google Scholar]
- CT-guided celiac plexus neurolysis: A review of anatomy, indications, technique, and tips for successful treatment. Radiographics. 2011;31:1599-621.
- [Google Scholar]
- Neurolytic celiac plexus block for treatment of cancer pain: A meta-analysis. Anesth Analg. 1995;80:290-5.
- [Google Scholar]
- Techniques for neurolytic neural blockade. In: Cousins MJ, Bridenbaugh PO, eds. Neural Blockade (3rd ed). Philadelphia: JB Lippincott; 1998. p. :1007-61.
- [Google Scholar]
- Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 1998;85:199-201.
- [Google Scholar]
- Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64:597-602.
- [Google Scholar]
- Celiac plexus block versus analgesics in pancreatic cancer pain. Pain. 1993;52:187-92.
- [Google Scholar]
- Single-needle celiac plexus block: Is needle tip position critical in patients with no regional anatomic distortions? Anesthesiology. 1997;87:1301-8.
- [Google Scholar]
- Celiac plexus block: Injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology. 2001;94:561-5.
- [Google Scholar]
- A comparison of two invasive techniques in the management of intractable pain due to inoperable pancreatic cancer: Neurolytic celiac plexus block and videothoracoscopic splanchnicectomy. Eur J Surg Oncol. 2005;31:768-73.
- [Google Scholar]
- Transhiatal bilateral splanchnicotomy for pain control in pancreatic cancer: Basic anatomy, surgical technique, and immediate results in fifty-one cases. Surgery. 1992;111:640-6.
- [Google Scholar]
- Thoracoscopic splanchnicectomy for control of intractable pain due to advanced pancreatic cancer. Surg Endosc. 2001;15:129-31.
- [Google Scholar]
- Bilateral thoracoscopic splanchnicectomy: Effects on pancreatic pain and function. Ann Surg. 1999;230:785-90.
- [Google Scholar]
- Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86:264-6.
- [Google Scholar]
- Neurolytic block of the celiac plexus and splanchnic nerves with computed tomography. The experience in 150 cases and an optimization of the technic. Radiol Med. 1999;98:183-8.
- [Google Scholar]
- Computerized tomography-guided neurolytic block of the splanchnic nerve. Radiol Med. 1997;93:739-42.
- [Google Scholar]
- Comparative study of the effects of the retrocrural celiac plexus block versus splanchnic nerve block, C-arm guided, for upper gastrointestinal tract tumors on pain relief and the quality of life at a six-month follow up. Korean J Pain. 2015;28:22-31.
- [Google Scholar]
- The effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra-abdominal cancer pain. Pain Physician. 2016;19:281-92.
- [Google Scholar]
- Efficacy of coeliac plexus and splanchnic nerve blockades in body and tail located pancreatic cancer pain. Eur J Pain. 2004;8:539-45.
- [Google Scholar]
- Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217:447-55.
- [Google Scholar]
- Bilateral thoracic splanchnic nerve radiofrequency thermocoagulation for the management of end-stage pancreatic abdominal cancer pain. Pain Physician. 2013;16:125-33.
- [Google Scholar]
- Celiac plexus blockade in a 7-year-old child with neuroblastoma. J Pain Symptom Manage. 1995;10:321-4.
- [Google Scholar]
- The role of celiac plexus block in intractable upper abdominal pain. In: Racz GB, ed. Techniques of Neurolysis. Boston: Kluwer Academic; 1989. p. :161.
- [Google Scholar]
- Neurolytic celiac plexus block for pancreatic cancer pain. Anesth Analg. 1987;66:869-73.
- [Google Scholar]






