Translate this page into:
Oral Methadone versus Morphine IR for Patients with Cervical Cancer and Neuropathic Pain: A Prospective Randomised Controlled Trial
*Corresponding author: Mikael Segerlantz, Department of Clinical Sciences Lund, Respiratory Medicine, Allergology and Palliative Medicine, Institute for Palliative Care, Faculty of Medicine, Lund University, Lund, Sweden. mikael.segerlantz@med.lu.se
-
Received: ,
Accepted: ,
How to cite this article: Adumala A, Palat G, Vajjala A, Brun E, Segerlantz M. Oral methadone versus morphine IR for patients with cervical cancer and neuropathic pain: A prospective randomised controlled trial. Indian J Palliat Care 2023;29:200-6.
Abstract
Objectives:
In India, cervical cancer is the most common cancer among women and makes up for up to 29% of all registered cancer in females. Cancer-related pain is one of the major distressing symptoms for all cancer patients. Pain is characterised as somatic or neuropathic, and the total pain experience is often mixed. Conventional opioids are the backbone of analgesic treatment but are most often not sufficient in alleviating neuropathic pain, common in cervical cancer. Accumulating evidence of the advantage of methadone compared to conventional opioids, due to agonist action at both μ and q opioid receptors, N-methyl-D-aspartate (NMDA) antagonist activity and the ability to inhibit the reuptake of monoamines has been demonstrated. We hypothesised that, with these properties’, methadone might be a good option for the treatment of neuropathic pain in patients with cervical cancer.
Material and Methods:
Patients with cervical cancer stages ll-lll were enrolled in this randomized controlled trial. A comparison was made between methadone versus immediate release morphine (IR morphine), with increasing doses until pain was controlled. Inclusion-period was from October 3rd to December 31st 2020, and the total patient-study period was 12 weeks. Pain intensity was assessed according to the Numeric Rating Scale (NRS) and Douleur Neuropathique (DN4). The primary objective was to determine whether methadone was clinically superior versus noninferior to morphine as an analgesic for the treatment of cancer related neuropathic pain in women with cervical cancer.
Results:
A total of 85 women were included; five withdrew and six died during the study period, leaving 74 patients completing the study. All participants showed a reduction in mean values of NRS and DN4 from the time of inclusion and to the end of the study period, for IR morphine and methadone 8.4–2.7 and 8.6–1.5, respectively (P < 0.001). The DN4 score mean reduction for Morphine and Methadone were 6.12–1.37 and 6.05–0, respectively (P < 0.001). Side effects were more common in the group of patients receiving IR morphine compared to the patients treated with methadone.
Conclusion:
We found that Methadone had a superior analgesic effect with good overall tolerability compared with morphine as a first-line strong opioid for the management of cancer-related neuropathic pain.
Keywords
Methadone
Cancer
Opioid
Neuropathic pain
Efficacy
INTRODUCTION
Disease-related pain is a distressing condition in patients with cancer.[1,2] Cancer-related pain is caused by pressure from the tumour on adjacent tissues, or by the tumour directly invading the tissue and damaging it. The main types of pain are often described as nociceptive and neuropathic pain. Nociceptive pain arises from nociceptors located throughout our body, which mediate signals to notify us of a potentially harmful stimulus.[3] Neuropathic cancer pain is caused by direct damage to the nervous system per se and is often described with burning, tingling, shooting and electric shock-like pain sensations, while nociceptive pain is a type of pain more often described as aching or throbbing.[4]
The World Health Organisation analgesic ladder for the relief of cancer pain in adults was published in 1996[5] and presented a model of hierarchy in analgesic therapy. In the ladder, paracetamol and non-steroidal anti-inflammatory analgesic drugs (NSAIDs) are represented in the first step followed by mild and strong opioids in the second and third steps, respectively.
Recently, a working group for cancer pain management in low-resource settings (the CAPER working group, the multinational and multidisciplinary Cancer Pain Management in Resource-limited settings) proposed a different strategy to make analgesic therapy more manageable in low-middle-income countries (LMICs). They suggest in their algorithm the introduction of short-acting opioids for opioid naïve patients already when pain assessment is reported to be above three on a numerical rating scale (NRS), NRS of pain intensity, where 0 is freedom from pain and 10 is the worst possible pain.[6]
Treatment of malignant pain often requires a combination of different pain medications and a variety of mechanisms of action.[6] However, conventional opioids are the backbone of the treatment of cancer pain. Nonetheless, cancer pain is still a major issue and not sufficiently alleviated for many patients. Oosterling et al. reported in 2016 the prevalence of neuropathic pain among cancer patients was as high as 40%.[7] In the treatment, where neuropathic cancer pain is suspected to be present, high and rapidly increasing opioid doses are needed. The analgesic treatment is often supplemented with adjuvant drugs, such as antidepressants, anticonvulsants and steroids.[8] Douleur Neuropathique (DN4) questionnaire is a simple and well-validated instrument for diagnosing neuropathic pain and evaluating analgesic treatment.[9,10]
Cervical cancer is the most common cancer in women in India, contributing up to 29% of all cancers in women.[11] It is also one of the leading causes of cancer mortality, accounting for 17% of all cancer deaths among women aged between 30 and 69 years in India. In contrast to developed countries, cervical cancer is a public health problem in LMIC, so much so that India alone accounts for one-quarter of the worldwide burden of cervical cancers.[11]
Many women with cervical cancer develop complex neuropathic pain conditions which are difficult to manage and not well controlled by traditional opioids or other conventional analgesics.[8,11]
Methadone
There is an accumulating body of evidence that methadone has many advantages over traditional opioids for the treatment of neuropathic pain, including agonist action at both μ and δ opioid receptors, 1 N-methylD-aspartate antagonist activity and the ability to inhibit the reuptake of monoamines.[12,13] It is regarded as a long-acting opioid, with an oral bioavailability of 80% (with a range of 41–99%), which is threefold the bioavailability of oral morphine.[14] The side effects of methadone are to a great extent equal to those of morphine: Including nausea, constipation and drowsiness.[15] Pharmacoeconomic issues related to the low cost of generic hydrochloride methadone powder have led to increased use of methadone for the treatment of cancer pain and especially neuropathic pain.[16,17]
Methadone in India
In India, methadone was permitted for pain relief as recently as 2014,[18] but is still only available in a few cancer centres.[19-21] Given the efficacy and low costs, methadone could be a valuable candidate in the treatment of cancer-related pain, in LMIC settings.[18]
Aim
The primary objective was to determine whether methadone was clinically non-inferior to morphine in terms of neuropathic pain relief when administered under controlled self-titrating conditions using an 11-point NRS for pain intensity, NRS, including DN4 in a 12-week-long, randomised study.
Ethics approval
Ethics approval by the local ethical board was obtained before initiation of the study and all participants gave full informed consent to take part in the study. The data were compiled anonymously and the patients could therefore not be identified.
Statistics
Sample size
This study was preceded by a pilot study of 24 patients, that is, 12 patients in each study group (data not shown). Results from the pilot study, and from a previously published randomised controlled trial[22] of a similar design, with similar subjects, we estimated the standard deviation of the pain reduction from baseline to stabilisation (within subjects) to be no more than 2.5. A one-sided two-sample t-test (comparing mean reductions in the two treatment arms) at the alpha = 0.025 level of significance and based on 37 subjects per treatment arm has a 96% power to reject the inferiority of methadone, where inferiority is defined as a difference of two points on the pain scale. We increased the sample size to 50 patients per treatment arm to provide increased confidence and enable us to adjust for site differences and other potential confounders. The primary analysis was to be a head-to-head comparison of methadone versus morphine using a one-sided 95% confidence interval for the difference in pain score reduction.
Descriptive analysis was used to summarise demographic data. Mean (and range) was used as the measure of central tendency, but when obtained data were not symmetrically distributed, with a large variety in the results, the median value was instead presented.
Statistical tests performed included t-tests to compare means between two groups with normally distributed data. Mann– Whitney U-test was used to compare two groups with non-parametric data.
Microsoft Corporation EXCEL 2016 (v16.0), Redmond, Washington, US, was used for descriptive statistical calculation.
For data analysis, SPSS VERSION 25 was used for the calculation of statistical significance.
P-values below 0.05 were considered significant.
MATERIAL AND METHODS
The study, a randomised controlled single-blinded trial comparing methadone with immediate-release morphine (IR morphine), was performed at the outpatient department and in-patient ward at a governmental cancer hospital, a tertiary referral centre, in India.
The design involved examining methadone against an established ‘gold standard’ treatment, in this case IR morphine, in the management of cancer pain with neuropathic features. Our hypothesis was that methadone was at least as good as morphine and designed the trial to test whether it was clinically superior versus non-inferior to morphine.
Inclusion and exclusion
Patients were enrolled consecutively for 3 months, from October 3 to December 31, 2020. Inclusion criteria were patients above the age of 18 and up to 70 years, with cervical cancer stage II or III and with an NRS pain score of >5 including neuropathic pain as established by the DN4 questionnaire of >4 and with a Palliative performance scale[22-24] (PPS) of >50%. Patients with more advanced diseases (stage IV) were not included. Overview of inclusion and exclusion criteria, see Table 1.
| Inclusion criteria | Exclusion criteria |
|---|---|
| All ages between 18 and 70 years | Age <18 y >70 y |
| Cervical cancer stage 2 and 3 | Multiple cancers |
| NRS pain score >5 | Numerical rating scale pain score <5 |
| Neuropathic pain -based on DN4 questionnaire with a score of >4 | Absent neuropathic pain -DN4 questionnaire with a score of <4 |
| PPS >50 | PPS <50 |
| Willing for informed consent | Refused informed consent |
| Normal baseline cognitive function | Patient not mentally competent to report |
| Can be followed up easily | From far places who cannot be followed up |
| No cardiac issues with normal baseline ECG | Patients with cardiac issues, abnormal baseline ECG and documented arrhythmias |
NRS: Numeric rating scale, ECG: Electrocardiogram, DN4: Douleur neuropathique, PPS: Palliative performance scale
End points for the study
The endpoints for the study were to achieve an NRS pain score of <1 and a DN4 score of <1.
Randomisation
A randomisation schedule was prepared by the study biostatistician at the study hospital and was provided to the study pharmacy where the study medication was prepared. The randomisation process was computer-generated and performed in two blocks such that each study site would have an equal number of participants assigned to each treatment. The off-site study pharmacy packaged the medications, assigned participant numbers according to this randomisation list and shipped the study medication to the appropriate pharmacy at each of the study sites.
Methadone and morphine tablets
The pre-packaged tablets were shipped to each of the study locations with labels that included the participant number and no medication name. Once randomised, participants were dispensed the study medication by the study pharmacist according to their participant number.
Outcome measurements
In accordance with the initiative on methods, measurement and pain assessment in clinical trials,[17] outcome measures included assessment in several core domains, the first of which was pain. The pain was measured using the 11-point NRS with anchors at 0 (no pain) and 10 (pain as bad as you can imagine). DN4 questionnaire was used for the assessment of neuropathic pain.
During the first 2 days, NRS and DN4 were assessed every 24 h, followed by one assessment on day 5 and then by weekly scoring.
The PPS was used to assess the performance status, at the time of inclusion, during the study and a final score at the end of the study.
Titration of methadone
Patients in the methadone group were supplied with tablets containing 5 mg of methadone. The dose consisted of 1/2–4 methadone tablets taken twice daily, every 12 h, that is, 2.5– 20 mg/24 h. In view of the uncertain potency ratio between methadone and conventional opioids, the dosing protocol allowed titration to a point where the pain reduction was maximised without side effects. Participants were instructed to start with a dose of half tablet of methadone every 12 h. Within limits of safety and tolerability, participants gradually increased the 12-hourly dose by half tablet every 2nd day such that by the end of 1 week, they were allowed a maximum of 8 tablets/24 h (4 tablets every 12 h). The goal of the titration phase was to reach a target dose so that maximum pain reduction is achieved without causing troublesome side effects. This process was similar to that used when titrating opioid doses at the study hospital.[18] The dose titration phase took place over 4 weeks and treatment continued for 8 more weeks, allowing 2 weeks for the pain treatment to stabilise and 2 weeks to maintain a steady state, totalling 84 days (12 weeks) of treatment. Monitoring of dose and the analgesic effect was done on participants on 11 occasions (during the 12 weeks) either by phone call or when the patient came to the site for a follow-up. Assessment of pain scores and side effects was repeatedly performed at each of these 11 occasions.
For breakthrough pain, NSAIDs were recommended, such as ketorolac 10 mg orally. If the requirement of NSAIDS was more than three doses per day, then the daily dose of methadone was increased by 5 mg/day in two divided doses.
Titration of IR morphine
Morphine was administered in the form of 10 mg tablets, 1/2– 6 tablets every 4 h, that is, 30–360 mg/24 h. For breakthrough pain, 2–3 doses of IR morphine were allowed per day. If the requirement was more than three breakthrough doses, the daily dose was increased by 30 mg/day in divided doses. Monitoring of dose and analgesic effect was done in the same way as with methadone and during follow-up, pain scores were evaluated at 11 occasions during the 12 weeks.
Side effects
Side effects of opioids (constipation, nausea, vomiting, dry mouth and fatigue) were noted when expressed by the patients during their visits and were treated accordingly.
Concomitant analgesic medications
Patients were on weak opioids (Tramadol) or NSAIDs (Diclofenac, Aceclofenac) before the start of the study. All drugs were stopped at the start of the study. However, appropriate coanalgesics were prescribed, whenever required, for additional management of neuropathic pain and thus noted in the study. The available coanalgesic for neuropathic pain was the anticonvulsant drug, sodium valproate. The dose of coanalgesics was escalated as per requirement. Medication types and dosages were recorded. The use of other opioids was not permitted during the 12 weeks trial.
Tumour-specific treatment
Synchronously, patients continued prescribed oncological treatment: radiotherapy and/or chemotherapy if deemed appropriate by the treating oncologist.
RESULTS
Patients
One hundred patients were initially screened, ten were not eligible due to exclusion criteria and five patients denied participation leaving 85 patients included, whereof 35 patients had a stage II disease and remaining 50 stage III. Five participants withdrew at some point during the study, due to side effects (n = 2) and travel problems (n= 3) in view of COVID lockdown and six patients died during the study period, leaving a total of 74 patients for analysis, see Figure 1. Overview of demographic information, see Table 2. Treatment related side-effects reported in the methadone or IR morphine group were nine and 24 patients respectively, whereof the most common was constipation, 3 and 14 respectively and nausea, vomiting as 3 and 4, dry mouth 1 and 4 respectively, giddiness 2 and 2 respectively.

- Flowchart of the study.
| Patient demographics | ||
|---|---|---|
| Parameters | Morphine group | Methadone group |
| Age | ||
| 30–45 years | 17 | 18 |
| 46–60 years | 15 | 11 |
| 61–70 years | 5 | 8 |
| Education | ||
| Literate | 26 | 25 |
| High school | 10 | 10 |
| Graduate | 1 | 2 |
| Weight | ||
| 40–50 kg | 18 | 20 |
| 51–60 kg | 12 | 10 |
| 61–70 kg | 7 | 7 |
Baseline pain score
Baseline NRS in the group of patients receiving methadone (n = 37) and morphine (n = 37) was 8.6 and 8.4, respectively, see Table 3. The neuropathic pain score, DN4, for the morphine group and the methadone group was 6.0 and 6.1 respectively at start of our study, see Table 4.
| Hours | Weeks | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Methadone | 8.6 | 7.1 | 5.6 | 4.5 | 3.8 | 3.4 | 3.1 | 2.7 | 2.5 | 2.2 | 2.1 | 1.9 | 1.7 | 1.6 | 1.5 |
| Morphine | 8.4 | 7.4 | 6.8 | 6.2 | 5.6 | 5.3 | 4.6 | 4.6 | 4.4 | 4.2 | 3.9 | 3.5 | 3.3 | 2.9 | 2.7 |
At start of study (0 h), during the first 2 days (24 and 48 h) and then weekly until end of study (12 week), NRS: Numeric rating scale
| Hours | Weeks | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Methadone | 6.05 | 6.05 | 5.63 | 4.53 | 3.29 | 2.32 | 1.76 | 0.82 | 0.61 | 0.24 | 0.21 | 0.11 | 0.08 | 0 | 0 |
| Morphine | 6.12 | 6.12 | 5.12 | 4.73 | 4.60 | 4.55 | 4.5 | 3.68 | 3.29 | 3.12 | 3.07 | 2.63 | 2.12 | 1.81 | 1.37 |
At start of study (0 h), during the first 2 days (24 and 48 h) and then weekly until end of study (12 week), DN4: Douleur neuropathique
PPS at baseline and at the end of the study
At baseline, all included patients had a mean PPS score of ≥50, for the methadone group and the morphine group. The PPS score increased in both groups, after 12 weeks, to 80 and 70, respectively.
Pain score, time to analgesic effect, final opioid doses, coanalgesics and side-effects
Methadone-treated group
In patients treated with methadone, the mean NRS score decreased from 8.6 to 3.8 already after 2 weeks and at the end of the study; after 12 weeks, the NRS score was 1.5 [Table 3]. The neuropathic pain score, DN4, decreased from 6.1 to 3.3 after 2 weeks and after 12 weeks, at the end of the study, no neuropathic pain component could be detected, see Table 4. Only one patient was prescribed coanalgesics for additional control of neuropathic pain, sodium valproate (n = 1), 400 mg and one patient was prescribed NSAIDs (n = 1) during the study period. The final stable dose of methadone was at a median of 20 (12.5–37.5) mg. Side effects registered were constipation (n = 3), nausea (n = 3), vomiting (n = 3), dry mouth (n = 1) and giddiness (n = 2).
IR Morphine-treated group
In the IR morphine group, the NRS score decreased from 8.4 to 3.9 but with a timespan of almost 8 weeks [Table 3]. At the end of the study (12 weeks), the NRS score was 2.7. The neuropathic pain score, DN4, decreased from 6.1 to 4.5 after 2 weeks and at the end of the study, after 12 weeks, to 1.4 [Table 4]. More than half of the patients were prescribed coanalgesics for control of neuropathic pain, sodium valproate (n = 22), with a mean dose of 400 mg distributed as follows; 200 mg (n = 5), 400 mg (n = 11) and 600 mg (n = 6), and NSAIDS (n = 3), during the study period. The final stable dose of IR morphine was 180 (80–200) mg.
Side effects registered were constipation (n = 14), nausea (n = 4), vomiting (n = 4), dry mouth (n = 4) and giddiness (n = 2).
Pain comparison between the methadone- and morphine-treated groups
Patients treated with methadone experienced a significantly greater reduction in NRS pain score, from baseline to end of the study, compared to patients receiving IR morphine (P < 0.001) [Figures 2 and 3].

- Pain (numeric rating scale).
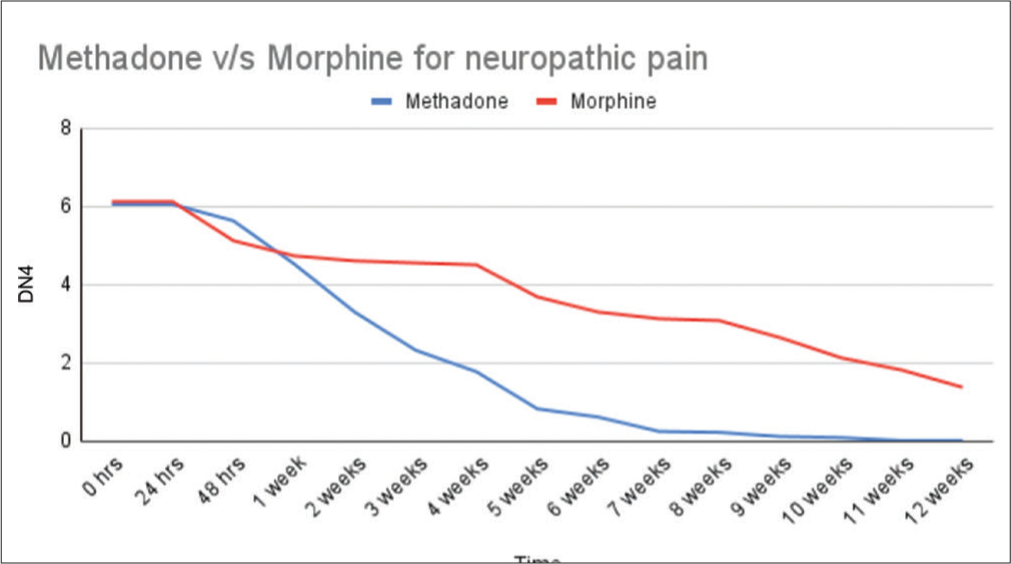
- Neuropathic pain (Douleur neuropathique).
DISCUSSION
In the current randomised prospective study of patients with advanced cervical cancer, prompter pain relief was found in patients treated with methadone, as a 60% mean pain relief was noted already after 2 weeks, compared to morphine, where the same level of pain relief was reached first after 8 weeks of treatment. At the end of the study, the methadone-treated patients reported 90 % neuropathic pain relief compared to the morphine group reporting 70 % neuropathic pain relief.
Pain relief is central in the management of patients suffering from cancer. Opioids are the main analgesics and the most important and significant means to achieve pain control. However, the usage of opioids for the treatment of pain is most unevenly spread globally, within 2010 an estimation that four HI countries make up 68% of all legal usage of morphine while all LMIC regions accounted for only 7% of the global use, thus representing a high burden of suffering.[25-27] Hence, it is estimated that 83% of the world’s population is left without effective pain treatment.[28] The costs of morphine are low, and drugs can be produced locally but obstacles are found in lagging legislation, unmotivated fear of addiction and side-effects from opioids in patients and families and lack of education within the medical service sector.[18] It is thus important to facilitate the process of making opioids available for a large number of cancer patients without access to efficient and cost-effective pain treatment.[18,19,29] Furthermore, when conventional opioids provide unsatisfactory pain alleviation a neuropathic element must be suspected, and alternative drugs applied. Methadone has pharmacological and pharmacodynamic advantages over morphine with actions against neuropathic pain elements and a high grade of bioavailability following oral intake. In a study on opioid treatment of cancer-related pain from a palliative unit in a developing country in South America, methadone was in line with our study and found to be a viable option for first-line analgesic treatment, with a lower oral morphine equivalent dose for patients receiving methadone.[30,31] Moreover, the addition of low-dose methadone to morphine has been recommended when other adjuvant analgesics are not sufficient, in the treatment of cancer-related pain.[30] In the present study, we wanted to compare methadone to IR morphine in severe pain with neuropathic characteristics. Both drugs were associated with a reduction in pain during the 12-week observation time, however with a significantly more prompt and earlier onset of analgesia for the methadone group compared to morphine. Side effects were manageable, obstipation was more common in patients treated with IR morphine and this is probably due to the relatively high doses of morphine needed in the IR morphine group. The introduction of methadone in low doses as an analgesic has been hampered partly by the anticipation of problems in monitoring effects and side effects because of the long T½, fear of side effects such as respiratory depression and sedation and a social stigma connected with methadone. These fears seem exaggerated in view of the growing experience of introducing low-dose methadone in the treatment of malignant pain.[19] Barriers to surpassing include a lack of knowledge and mistrust within the medical profession and among the public.
The study aimed to provide support showing a safe introduction of methadone in a low-resource setting and to overcome mistrust of this useful drug. These results may add to the growing body of evidence that methadone is safe and feasible in the treatment of malignant pain with neuropathic features, in a low-resource setting in a LMIC.
Limitations
Limitations to this randomised study are among others that grading of side effects was not consistently performed, mainly due to the busy setting of a governmental cancer hospital. Concomitant treatment, oncological, is not recorded but is expected to be similar in the two treatment arms. The use of IR morphine consistently throughout the study was done to be certain of opioid-equivalent doses, but it can be argued that in the real-world slow-release preparations would be preferred when an analgesic effect is obtained with IR morphine.
It can also be argued that PPS differed between the groups and that coanalgesics were not equal. COVID lockdown also created travel problems for follow-up.
CONCLUSION
Based on our study results, methadone produced a superior and more prompt analgesic effect for neuropathic pain relief with good overall tolerability compared with IR morphine as a first-line strong opioid for the treatment of pain in patients with cervical cancer.
Acknowledgment
We wish to thank Mehdi Nawaz Jung Institute of Oncology and regional cancer centre and the Department of Pain and Palliative Care for providing resources and records for this study and above all the patients participating in the study, making this work possible in the interest of future patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Psychosocial issues in cancer pain. Curr Pain Headache Rep. 2011;15:263-70.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer pain and psychosocial factors: A critical review of the literature. J Pain Symptom Manag. 2002;24:526-41.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing cancer pain management in resource-limited settings. Support Care Cancer. 2019;27:2113-24.
- [CrossRef] [PubMed] [Google Scholar]
- Nociceptors: The sensors of the pain pathway. J Clin Invest. 2010;120:3760-72.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer Pain Relief with a Guide to Opioid Availability: Report from a WHO Expert Committee. 1996. (2nd ed). Geneva: World Health Organization. WHO Library Cataloguing in Publication Data; :14-15. Available from: https://apps.who.int/iris/bitstream/handle/10665/37896/9241544821.pdf?sequence=1&isAllowed=y"9241544821.pdfwho.int [Last accessed on 2022 May 05]
- [Google Scholar]
- Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9-19.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic pain components in patients with cancer: Prevalence, treatment, and interference with daily activities. Pain Pract. 2016;16:413-21.
- [CrossRef] [PubMed] [Google Scholar]
- Multicenter, cross-sectional observational study of the impact of neuropathic pain on quality of life in cancer patients. Support Care Cancer. 2017;25:3759-67.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29-36.
- [CrossRef] [PubMed] [Google Scholar]
- Translation to portuguese and validation of the douleur neuropathique 4 questionnaire. J Pain. 2010;11:484-90.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of cervical cancer and role of screening in India. Indian J Med Paediatr Oncol. 2016;37:278-85.
- [CrossRef] [PubMed] [Google Scholar]
- Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell Mol Life Sci. 2019;76:1889-99.
- [CrossRef] [PubMed] [Google Scholar]
- Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000;4:5-15.
- [CrossRef] [PubMed] [Google Scholar]
- Methadone for relief of cancer pain: A review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73-83.
- [CrossRef] [PubMed] [Google Scholar]
- Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain. 2003;4:231-56.
- [CrossRef] [PubMed] [Google Scholar]
- Methadone for cancer pain. Cochrane Database Syst Rev. 2017;2017:CD003971.
- [CrossRef] [PubMed] [Google Scholar]
- Methadone as a first-line opioid in cancer pain management: A systematic review. J Pain Symptom Manage. 2018;55:998-1003.
- [CrossRef] [PubMed] [Google Scholar]
- Practical guide for using methadone in pain and palliative care practice. Indian J Palliat Care. 2018;24:S21-9.
- [CrossRef] [PubMed] [Google Scholar]
- The introduction and experiences of methadone for treatment of cancer pain at a low-resource Governmental cancer center in India. Indian J Palliat Care. 2021;27:382-404.
- [CrossRef] [PubMed] [Google Scholar]
- The introduction and experiences of methadone for treatment of cancer pain at a low-resource governmental cancer center in India. Indian J Palliat Care. 2021;27:382-404.
- [CrossRef] [PubMed] [Google Scholar]
- The use of Methadone in adult patients with cancer-pain at a governmental cancer centre in India. Indian J Palliat Care. 2021;27:139-45.
- [CrossRef] [Google Scholar]
- Methadone vs. morphine SR for treatment of neuropathic pain: A randomized controlled trial and the challenges in recruitment. Can J Pain. 2019;3:180-9.
- [CrossRef] [PubMed] [Google Scholar]
- A reliability and validity study of the palliative performance scale. BMC Palliat Care. 2008;7:10.
- [CrossRef] [PubMed] [Google Scholar]
- Using the palliative performance scale to estimate survival for patients at the end of life: A systematic review of the literature. J Palliat Med. 2018;21:1651-61.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5-11.
- [CrossRef] [PubMed] [Google Scholar]
- Availability and utilization of opioids for pain management: Global issues. Ochsner J. 2014;14:208-15.
- [Google Scholar]
- Global Access to Pain Relief Evidence for Action. 2020. European Society for Medical Oncology. Available from: https://www.esmo.org/content/download/14123/252826/file/global-access-to-pain-relief-evidence-for-action.pdfglobal-access-to-pain-relief-evidence-for-action.pdf.esmo.org [Last accessed on 2023 May 21]
- [Google Scholar]
- A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25:6-18.
- [CrossRef] [PubMed] [Google Scholar]
- Model List of Essential Medicines. 2019. Geneva: World Health Organization; Available from: https://www.apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1 [Last accessed on 2019 Dec 12]
- [Google Scholar]
- Methadone as first-line opioid treatment for cancer pain in a developing country palliative care unit. Support Care Cancer. 2016;24:3551-6.
- [CrossRef] [PubMed] [Google Scholar]
- Improved pain control in terminally ill cancer patients by introducing low-dose oral methadone in addition to ongoing opioid treatment. J Palliat Med. 2018;21:177-81.
- [CrossRef] [PubMed] [Google Scholar]






