Translate this page into:
Reversal of Opioid-Induced Toxicity
Address for correspondence: Dr. Shrenik P Ostwal, Department of Palliative Medicine, Tata Memorial Hospital, Parel, Mumbai - 400 012, India. E-mail: drshrenikostwal@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Opioids are commonly used for pain control in palliative care setting. Accumulation of active metabolites of opioids can cause a well-recognized toxidrome including respiratory depression (RD), decreased conscious level, pinpoint pupils, and drop in blood pressure. Opioid toxicity is often associated with amount ingested and its speed of absorption in the body. This can have life-threatening effects on various body systems. Naloxone is an opioid antagonist that competitively binds to opioid receptors and reverses all their effects. The indication for use is RD because of known or suspected opioid overdose. This article presents a case report of 61-year-old female, a case of advanced pancreatic cancer, progressed on disease-modifying treatments and referred to palliative care for best supportive care. She developed features of morphine toxicity that was promptly identified and managed with use of naloxone and other supportive measures.
Keywords
Morphine
naloxone
opioid antagonist
opioid-induced toxicity
INTRODUCTION
Pain is one of the most common and most feared symptoms in palliative care practice. About 52%–77% patients suffer from pain despite World Health Organization recommendations. A systematic review by van den Beuken-van Everdingen MH et al. reported the prevalence of pain in 62%–86% of patients suffering from advanced cancer.[1] Of these, 70%–80% patients have moderate to severe pain.[2]
Opioid remains the mainstay of cancer pain management. Strong opioids are commonly used to control pain for patients with advanced, life-limiting disease (palliative care) and to manage chronic, severe pain.[3] Long-term use of opioids can lead to accumulation of its toxic metabolites leading to various adverse events, known as opioid toxicity. This condition is often missed and misdiagnosed in clinical practice and hence seldom managed appropriately. Classic signs of opioid toxicity include pinpoint pupils, hallucinations, drowsiness, vomiting, respiratory depression (RD), confusion, and myoclonic jerks.[4] Although serious RD is an unlike adverse event of opioid use, it should be promptly recognized and treated with opioid antagonist due to its life-threatening nature.[567] Naloxone is a potent opioid antagonist and is commonly used in reversal of opioid-induced RD, either in overdose or in those patients who have suffered exaggerated response to conventional doses.[58]
CASE REPORT
A 61-year-old female, known diabetic, hypertensive, asthmatic with past history of resolved stroke and currently on medications for comorbidities and was diagnosed with locally advanced pancreatic cancer (body of pancreas) with metastases to liver and retroperitoneal lymph nodes. She had received palliative chemotherapy as a disease-modifying treatment. She was referred to Palliative Medicine Department for best supportive care in view of her disease progression and declining health.
She presented with severe pain in the lower back and left buttock region, radiating to the left lower limb. Pain score was 7–8 out of 10. The pain descriptors suggested mixed nociceptive and neuropathic type pain. It affected her activities of daily living and disturbed her sleep significantly. It resulted in significant distress to both the patient and her caregivers. On examination, she was found to have mass over her left buttock area associated with tenderness. No local changes were found. She was referred to radiotherapy for palliative radiation to left buttock mass region. All her laboratory parameters were within normal range. Analgesia was optimized on a titrated dose of oral morphine 30 mg sustained release tablet twice a day. In addition, adjunct analgesics that included pregabalin and etoricoxib were commenced alongside preemptive side effect-limiting medications. Her pain was well controlled with palliative radiotherapy and opioid analgesics.
After 8 days of regular medications, she presented to outpatient department with a 2-day history of involuntary movements over the right arm with ataxia. On examination, she was conscious and oriented to time, place, and person. Pupils were constricted and sluggishly reactive to light. Right upper arm showed involuntary movements likely to be focal seizures, with decreased palmar grasp of the right hand. All routine laboratory tests were sent and an MRI brain was ordered. She received injection phenytoin sodium as a loading dose for her suspected focal seizures and tablet morphine was stopped immediately. Over a period of 1 h, she became drowsy, minimally responding to painful stimuli. She also developed hypotension with a blood pressure of 94/64 mmHg and hypoventilation with a respiratory rate of 8/min. Pulse rate was 100 beats/min, and saturation on room air was 98%. On examination, her pupils were pinpoint and nonreactive to light. Deep tendon reflexes were also sluggish. She was diagnosed of having opioid-induced neurotoxicity and was immediately given injection naloxone intravenously, starting with bolus of 40 mcg and assessed for response. Bolus was repeated every 2–3 min. The patient became conscious and oriented with five bolus doses (total dose – 200 mcg). Her pupils became normal and were equally reactive to light. Respiratory rate normalized to 18 breaths/min. On chest examination, she was found to have wheeze over the right infrascapular area. She was observed for any signs of recurrence of opioid toxicity for the next 2 h. Meanwhile, she received supportive intravenous fluids for her low blood pressure. Her laboratory investigations revealed acute kidney injury (mainly related to dehydration) with serum creatinine of 2.1 mg/dl. Other parameters were within normal range. Her MRI brain was normal. She was admitted for observation and management of acute kidney injury. After 5 days of treatment, she did not had any signs of recurrence, and her creatinine dropped down to 1.1 mg/dl. She was discharged with good pain control with fentanyl transdermal patch 12.5 mcg/hr, paracetamol, and pregabalin.
Possible contributors for opioid toxicity in her are acute renal injury, old age, and frail condition.
DISCUSSION
Opioids cause a well-recognized toxidrome including RD, decreased conscious level, pinpoint pupils, and drop in blood pressure. Opioid toxicity is often associated with amount ingested and its speed of absorption in body.[9] Opioid toxicity can occur as a result of (1) doses are increased too rapidly; (2) in renal impairment; (3) morphine nonresponsive pain; and (4) pain relieved with other interventional methods (e.g., radiotherapy and nerve blocks).[4] Warning signs include drowsiness, confusion, pinpoint pupils, myoclonic jerks, hallucinations (auditory and visual), vomiting, and delirium. Table 1 depicts various signs and symptoms seen in opioid or toxicity.[10111213]
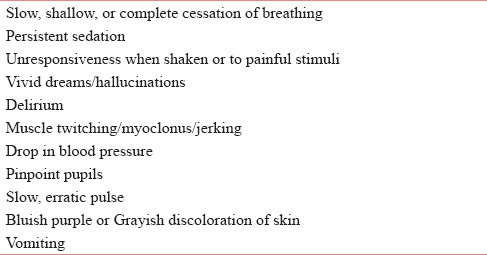
RD is one of the most feared consequences of opioid use. RD due to opioid use is postulated to be caused by chemical and behavioral actions on μ and ƙ receptors.[14] The American Society of Anesthesiologists defined RD as a RR <10/min in their guidelines for the management of RD with spinal opioids.[15] RD should be promptly diagnosed and treated to prevent death of the patient.
Strategies for the management of opioid toxicity involve:[101116171819]
-
Treat from ABCDE perspective:[20]
-
Airway: Position the patient - Head tilt/jaw thrust/ chin-lift +/- use airway adjuncts if there are signs of airway compromise
-
Breathing: oxygen if saturation <95%
-
Circulation: Intravenous access
-
Disability: Review ABCs/check pupils and blood glucose levels/GCS
-
Exposure.
-
-
Address possible other causes for the condition, for example, dehydration
-
Stop opioids and remove any patches (e.g., fentanyl or buprenorphine)
-
Reassess analgesic requirement of patient: use opioid-sparing techniques – for example, nonopioid analgesics
-
Consider naloxone[21] administration if there is evidence of reduced GCS and RD.
-
RD can be defined as:
-
Respiratory rate < 12/min and patient having cyanosis or in coma
-
Respiratory rate <8/min.
-
-
Naloxone is an opioid antagonist acting competitively at the mu-, kappa-, and sigma-opioid receptors. Therefore, it reverses all pharmacologic effects of opioids including neurologic depression, RD, and analgesia. Naloxone is indicated for use in both adults and pediatrics and will reverse both natural and synthetic opioids as well as some mixed agonist-antagonist analgesics such as nalbuphine, pentazocine, and butorphanol.[21] Naloxone is available as injections, autoinjectors, and nasal sprays.[1222]
Various studies have proved efficacy and beneficial effect of naloxone for RD.[2324] Due to its short duration of action, observation period for the possibility of recurrence of opioid toxicity varies. In cases of short-acting opioid overdoses, patients may be discharged once respiration and mental status return to normal, and an observation period of 2 h can be considered after naloxone administration. For long-acting or sustained-release opioid overdoses, hospital admission with continuous infusion of naloxone may be warranted. The observation period in these cases may extend to 4–6 h after naloxone infusion and until the patient is awake and alert with no signs of RD.[21252627] In these cases, naloxone doses should be diluted in 9 mL of normal saline and administered at 0.5 mL/min.[282930] Therefore, we recommend regular and frequent assessment of toxicity in patients who are on opioids, especially in frail elderly and patients with renal impairment.
CONCLUSION
Opioid-induced toxicity should be regularly assessed and aggressively treated to improve the quality of life of the patient and to decrease distress in both patient and their caregivers. Frequent monitoring is required especially when other treatments are likely to reduce analgesic requirements.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18:1437-49.
- [Google Scholar]
- The prevalence of severe pain, its etiopathological characteristics and treatment profile of patients referred to a tertiary cancer care pain clinic. Indian J Palliat Care. 2015;21:148-51.
- [Google Scholar]
- 2016. N.E.C.N. Palliative and End of Life Care Guidelines: North Engl Clin Networks. Available from: http://www.nescn.nhs.uk/wp-content/uploads/2016/09/NECNXPALLIATIVEXCAREX2016.pdf
- Pain Management in Palliative Care Originally produced in New Zealand for bpacNZ with Content Provided by Pain Management in Palliative Care. Available from: http://www.palliativecarebridge.com.au/resources/PainManagementInPalliativeCare.pdf
- 2010. Cancer Pain Management. Available from: https://www.britishpainsociety.org/static/uploads/resources/files/book_cancer_pain.pdf
- Respiratory function during parenteral opioid titration for cancer pain. Palliat Med. 2007;21:81-6.
- [Google Scholar]
- Experimental pain stimulates respiration and attenuates morphine-induced respiratory depression: A controlled study in human volunteers. Pain. 1996;64:123-8.
- [Google Scholar]
- Use of naloxone for reversal of life-threatening opioid toxicity in cancer-related pain. J Oncol Pharm Pract. 2016;22:114-20.
- [Google Scholar]
- Scottish Palliative Care Guidelines – Choosing and Changing Opioids. Available from: http://www.palliativecareguidelines.scot.nhs.uk/guidelines/pain/choosing-and-changing-opioids.aspx
- Prescribing naloxone to prevent opioid overdose in the community setting. J Nurse Pract. 2017;13:100-1.
- [Google Scholar]
- Opioid overdose and naloxone: The antidote to an epidemic? Drug Alcohol Depend. 2016;163:265-71.
- [Google Scholar]
- Actions of opioids on respiratory activity via activation of brainstem mu-, delta- and kappa-receptors; an in vitro study. Brain Res. 1997;778:233-41.
- [Google Scholar]
- Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218-30.
- [Google Scholar]
- Birmingham Cancer Network Pan. Guidelines for the Use of Naloxone in Palliative Care in Adult Patients. Available from: https://www.uhb.nhs.uk/Downloads/pdf/CancerPbNaloxoneInPalliativeCare.pdf
- [Google Scholar]
- NICE opioids in palliative care (Clinical guideline 140) – A guideline summary. Ann Med Surg (Lond). 2012;1:44-8.
- [Google Scholar]
- Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-87.
- [Google Scholar]
- ABCDE Approach. Available from: https://www.resus.org.uk/resuscitation-guidelines/abcde-approach/
- Naloxone without the needle – Systematic review of candidate routes for non-injectable naloxone for opioid overdose reversal. Drug Alcohol Depend. 2016;163:16-23.
- [Google Scholar]
- Evaluation of the overdose education and naloxone distribution program of the baltimore student harm reduction coalition. Am J Public Health. 2016;106:1243-6.
- [Google Scholar]
- Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in massachusetts: Interrupted time series analysis. BMJ. 2013;346:f174.
- [Google Scholar]
- A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15:566-70.
- [Google Scholar]
- Early discharge of patients with presumed opioid overdose: Development of a clinical prediction rule. Acad Emerg Med. 2000;7:1110-8.
- [Google Scholar]
- Opioid toxicity recurrence after an initial response to naloxone. J Toxicol Clin Toxicol. 1998;36:11-7.
- [Google Scholar]
- Narcan use in the endoscopy lab: An important component of patient safety. Gastroenterol Nurs. 2004;27:20-1.
- [Google Scholar]
- Assessment and management of opioid side effects. In Textbook of Palliative Medicine 2006:392-3.
- [Google Scholar]
- Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain (6th ed). Glenville, IL: American Pain Society; 2008. p. :19-21.






