Translate this page into:
Symptom Management among Patients with Chronic Kidney Disease
Address for correspondence: Dr. Dharshan Rangaswamy, Department of Nephrology, Kasturba Medical College and Hospital, Manipal Academy of Higher Education, Madhavanagar, Udupi, Manipal - 576 104, Karnataka, India. E-mail: dharshan.r@manipal.edu
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients with chronic kidney disease (CKD) experience high symptom burden, both physical and psychological, that is underrecognized and undertreated. The high symptom burden significantly impacts the quality of life for patients and their families. This review enumerates the various physical and psychological symptoms that patients with CKD often experience and guides in the management of these symptoms. This review follows the recommended international guidelines and has been tailored to suit the Indian context.
Keywords
End-stage kidney disease
pharmacological management
symptoms
INTRODUCTION
Patients with chronic kidney disease (CKD) experience high symptom burden, both physical and psychological, with low socioeconomic status negatively affecting the experience of symptoms.[1] Recent evidence suggests that symptom burden is not dependent on CKD stage, with patients in all stages experiencing symptoms that could potentially impact their quality of life (QoL).[2] CKD patients have a median of five to six symptoms.[3] Patients with end-stage kidney disease (ESKD) report a median number of 5.7–7.5 symptoms.[4] Those with Stage 5 CKD have physical and psychological symptom burdens similar or greater than that of advanced cancer patients.[5] Health-care providers frequently underrecognize and undertreat symptoms in CKD.[6] Assessment of symptom burden should be done at the first visit and at every follow-up visit for emergence of new symptoms and to assess the effectiveness of treatment.
Validated global symptom assessment tools are available and are appropriate for routine screening in renal programs, which will aid the identification of common and troublesome symptoms. Some of the commonly used validated tools include the Edmonton Symptom Assessment System (ESAS)-Revised: Renal,[78] Integrated Palliative Care Outcome Scale-Renal (IPOS-Renal),[910] and Dialysis Symptom Index.[6] The use of validated questionnaires will facilitate the assessment of symptoms in a systematic manner and should be incorporated into routine clinical practice to optimize symptom management.
SYMPTOM MANAGEMENT
Insomnia[11121314151617181920]
Sleep disorders are present from an early stage of CKD, affecting both duration and quality of sleep. Insomnia is among the top five troublesome symptoms reported in terms of intensity and severity. The prevalence ranges from 20% to 83% among CKD patients and 50%–75% among ESKD patients, with daytime sleepiness being the most troubling. Sleep disturbances decrease QoL, worsen health-related risks such as immune system impairment and cardiovascular disease, and increase mortality. It can also lead to impaired neurocognition with difficulties in attention and lowered work performance. The causes of insomnia in CKD are both physical and psychological, and the mode of treatment of insomnia is both pharmacological and nonpharmacological.
Nonpharmacological management
A systematic review in dialysis-dependent patients for insomnia identified four interventional modalities:
-
Cognitive behavioral therapy (CBT) (Level 2, Grade B): This includes sleep education, stimulus control, sleep restriction, relaxation training, and sleep hygiene. CBT for insomnia is carried out over 6–8-week sessions. Further studies are needed to assess the potential of other interventions
-
Acupressure (Level 2, Grade C)
-
Physical exercise: Aerobic and flexibility exercise (Level 2, Grade C)
-
Change of dialysis modality (Level 2, Grade C).
Pharmacological management (Level 2, Grade C)
-
Adequate management of concurrent symptoms such as pain, pruritus, depression, and obstructive sleep apnea
-
If symptoms of restless leg syndrome (RLS), neuropathic pain, or uremic pruritus (UP) are present along with insomnia, then low-dose gabapentin 50–300 mg at night can be prescribed
-
Doxepin 10 mg at night as second line to be used in presence of neuropathic pain or pruritus
-
Zopiclone 3.75–5 mg is third line
-
Melatonin 2–5 mg regulates and improves sleep–wake cycle in the short term but not at 1 year.
Anxiety and depression[2122232425262728]
Depression is common in adults with CKD, with a prevalence of depression being 3–4 times higher in patients with CKD and ESKD as compared to general population. The presence of depression is associated with adverse psychosocial and clinical outcomes and lower QoL. The mortality risk is 1.5 times higher in the presence of depressive symptoms, with increased emergency department visits, hospitalizations, prolonged stay in hospital, cardiovascular events, withdrawal from dialysis, and suicide. The etiology of depression in CKD/ESKD is multifactorial, both biological and behavioral. The recommended screening tools include a two-question approach modified from the Patient Health Questionnaire, Hospital Anxiety and Depression Scale, Patient Health Questionnaire-9, and General Anxiety Disorder-7.
Depression in patients with CKD is undertreated, with approximately only 35% of those with diagnosable depression receiving treatment. Although an improvement in depressive symptoms and psychosocial outcomes was observed when treated with a combination of antidepressants and psychotherapy, data regarding the benefits of pharmacotherapy are sparse and inconclusive.
Nonpharmacological management (not graded)
CBT, exercise therapy, and more frequent dialysis have been shown to be effective in improving depression in patients with CKD, however, the studies were of low quality and more research is required.
Pharmacological management
-
ESKD has no effect on the pharmacokinetics of selective serotonin reuptake inhibitors, and they are recommended as first-line treatment in all stages of CKD. Sertraline 50–200 mg/day, escitalopram 10–20 mg/day, or fluoxetine 20–60 mg/day is recommended (Level 2, Grade B)
-
For episodic anxiety, a short trial of benzodiazepines and beta-blockers may be tried (Level 2, Grade C)
-
Mirtazapine 15–45 mg/day in CKD 1–4. 50% dose reduction for patients in Stage 5 CKD (Level 2, Grade C)
-
Duloxetine 40–120 mg/day. Better avoided once estimated glomerular filtration rate (eGFR) <30 (Level 2, Grade C)
-
Tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors are not recommended in CKD.
A recent Cochrane review highlighted the lack of evidence for efficacy of antidepressants in CKD and emphasized the need for further research.
Uremic pruritus[2930313233]
In advanced kidney failure, UP is a common and burdensome symptom, often affecting multiple health-related QoL outcomes such as sleep, general mood, and social function and independently affects mortality. It is experienced in up to 46% of hemodialysis (HD) patients. UP is described as a daily or near-daily occurrence of itch involving large surface areas and is not restricted by dermatomal pattern. The pathophysiology of UP is often multifactorial. Uremic neuropathy, substance P, inflammation from chronic systemic inflammation of skin or nerves, and an increase in activity of µ-opioid receptors, all contribute to the pathogenesis of UP. There is growing evidence in favor of nonhistamine pathways in the pathogenesis of UP, and only a small proportion of cases have histamine as an implicating factor.
Management of patients with UP should involve assessment for any contributing and reversible factors. An associated fungal infection, drug hypersensitivity, allergic or contact dermatitis should be addressed prior to treatment of UP.
Nonpharmacological management
-
Appropriate skincare: To maintain short, smooth fingernails; wear gloves at night and avoid scratching. Moisturizers (e.g., baths with lukewarm water and gentle moisturizing soaps with no fragrances or additives; moisturize with aqueous emollients within 2 min of bath; the use of nonperfume coconut oil, Aloe vera cream, Vaseline lotion, and Lacto Calamine lotion are useful emollients to be used for managing UP (Level 2, Grade A)
-
Topical application: Capsaicin 0.025% or 0.03% ointment; pramoxine 1%; menthol/camphor/phenol 0.3% each either separately or in combination with each other; g-linolenic acid cream 2.2% (Level 2, Grade B)
-
Phototherapy (UVB) (Level 2, Grade C): 400–4800J/m2 three times weekly for a 3-week trial; impractical in Indian setting in view of availability of centers offering the therapy and the cost involved (opinion statement)
-
Modification in dialysis (Level 2, Grade B): High-flux HD and hemodiafiltration over a period of 12 weeks have been significantly found to reduce symptoms of UP compared to conventional HD.
PHARMACOLOGICAL MANAGEMENT
-
Gabapentinoids (Level 1, Grade A): Gabapentin or pregabalin has a significant benefit in reducing symptoms of UP. However, long-term effects have not been studied. Recommended dose: Gabapentin 100 mg daily (2 h before sleep) or 300 mg post HD thrice weekly; pregabalin 50 mg thrice weekly or 75 mg twice weekly post HD. Common side effects: somnolence, dizziness, and nausea
-
Antihistamine and mast cell stabilizer (Level 2, Grade B): Hydroxyzine 25 mg once daily or montelukast 10 mg daily
-
TCA: Doxepin 10 mg at bedtime, upon consultant prescription in patients on regular follow-up
-
Others: κ-opioid receptor agonist nalfurafine (Level 1, Grade B – 5 mcg – currently not available in India) and thalidomide (100 mg daily).
Restless leg syndrome (RLS)[29303435]
The prevalence of RLS ranges from 6.6% to 80% among dialysis population. The definition of RLS was revised during the National Institutes of Health consensus conference (2002), Bethesda, USA, to include:
-
Urge to move the legs usually with unpleasant sensations in the legs; arms and other body parts are occasionally involved
-
Symptoms begin or worsen during periods of rest or inactivity
-
Symptoms partially or totally relieved by movement and as long as the activity continues
-
Symptoms are worse during the evening or night than during the day.
Abnormalities in the dopamine pathway of the central nervous system (subcortical area) have been implicated. Other contributing factors include iron deficiency, anemia, hyperparathyroidism, elevated serum calcium and phosphorus, inadequate dialysis, and poor sleep hygiene. RLS is associated with decreased QoL and increased cardiovascular morbidity and mortality.
Nonpharmacological management
Removal of stimulants such as alcohol, caffeine, nicotine; optimizing sleep hygiene; aerobic exercises; pneumatic compressions, and regular mental alerting activities such as solving puzzles and board games were found to be useful in reducing RLS. Other contributing and reversible factors such as concurrent use of dopaminergic antagonists, iron deficiency, anemia, hyperparathyroidism, and elevated serum phosphorus must be addressed and corrected.
Pharmacological management
-
Gabapentin (Level 1, Grade A): As the drug is excreted by the kidney (77%), dose must be reduced owing to its long half-life in CKD patients. It modulates various receptor sites and alters dopamine, serotonin, and norepinephrine release. Recommended dose: Gabapentin 100 mg daily (2 h before sleep) or 300 mg post HD thrice weekly
-
Synthetic dopamine agonist (Level 1, Grade B): Ropinirole; increased selectivity to D3 and D4 receptors. Advantages: short plasma half-life with primary liver metabolism. Recommended dose: To initiate with 0.25 mg, taken 1 h before bedtime with slow titrating to a maximum of 4 mg per day. Pramipexole: Only immediate-release tablet is approved for use in RLS. Recommended dose: To initiate with 0.25 mg, taken 1 h before bedtime with slow titrating to a maximum of 0.75 mg per day
-
Antioxidants Vitamin C and E (Level 2, Grade A): Found to be effective when compared to placebo with minimal side effects
-
Others: Levodopa (Improves symptoms but not routinely used owing to increased vomiting, agitation, headaches, dry mouth, and gastrointestinal symptoms), benzodiazepines (increased risk of frequent falls but considered for use in patients who cannot take oral drugs), and iron dextran (less likely to have added benefit beyond correction of iron deficiency and anemia).
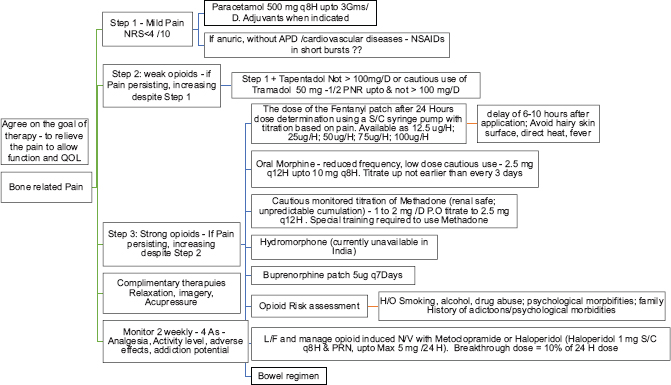
Chronic pain[29303637383940]
Pain has been recognized as one of the most common symptoms, affecting up to one-third of CKD patients. Majority of them experience moderate-to-severe pain. Pain often is musculoskeletal in origin, and chronic pain in CKD is often a mix of nociceptive and neuropathic pain. Management should involve patient assessment including physical examination, medical history, and patient's primary diagnosis and determine the effects of the pain on the patient's psychologic status, social functioning, functional status, and QoL.
-
Determine pain intensity: Modified Edmonton Symptom Assessment Version 2 (m-ESAS v.2). Convenant Health, Alberta, Canada and IPOS-Renal (POS-renal) are screening tools to be used A separate visual pain scale to be used for follow-up assessments, among patients in whom chronic pain forms the predominant symptom
-
Identify chronicity and possible reversible causes for the pain: Patients on dialysis experience recurrent pain from arteriovenous fistula cannulation, leg cramps, steal syndrome, etc., Adequate and effective pain control during episodes of acute pain reduces the incidence of chronic pain. As pain becomes more chronic, the original triggers become less important and somatosensory/psychologic mechanisms assume relevance
-
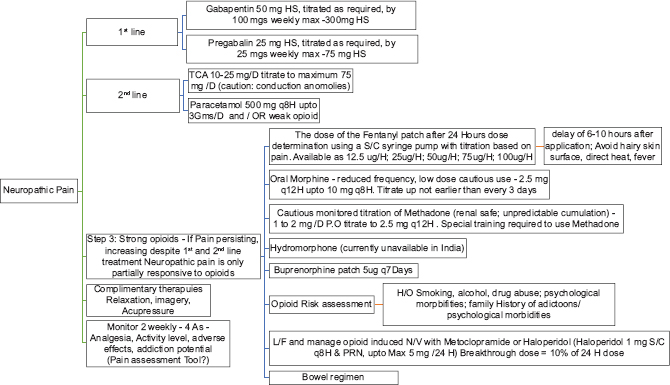
Type of pain – nociceptive, neuropathic, or combined: Neuropathic pain has a poor response to nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids and often requires adjuvant drug (drug that has a primary indication other than pain). In contrast, nociceptive pain responds well to nonopioid and opioid analgesics, at least in the short term. Since chronic pain in CKD is often mixed, addressing the neuropathic component first with adjuvant therapy avoids inapt use of opioids
-
Plan treatment goals: The goal of therapy should be realistic and aim to reduce pain to a tolerable level (at least by 30% on the pain scale), thereby allowing for routine day-to-day work and improving QoL. Slow introduction with upward titration of analgesics, starting with nonopioids followed by weak-to-strong opioids as per the WHO analgesic ladder, should form the basis of treatment plan. Before starting opioids, one needs to assess the risk for substance abuse and strategy to reduce opioid dependence should be part of the treatment plan. There should be an ongoing assessment and titration of drug doses, and any side effects should be actively managed.
Nonpharmacological management (not graded)
Aerobic exercise, acupressure, acupuncture, physiotherapy, and local heat application help a minority of patients and reduce the use of pharmacological agents in them. Cognitive-behavioral therapy, psychotherapy, and relaxation reduce drug dependency among patients with neuropathic pain.
Pharmacological management [Table 2]
| Grade | Strength of recommendation | Grade | Quality of evidence# |
|---|---|---|---|
| Level 1 | Most patients should receive the recommended course of action | A | High |
| Level 2 | Different choices for different patients based on the patient’s values and preferences | B | Moderate |
| Not graded* | Based on common use and individual experience or where adequate application of evidence is not possible | C | Low |
*The ungraded statements are simple declarative statements based on experience of their use among clinicians and should not be interpreted as stronger recommendation than Level 1 or 2, #The general hierarchical pyramid for quality of evidence which includes meta-analysis, systemic/ Cochrane reviews, randomized control trials, cohort studies, and case series
| Type of pain* | Drug class recommendation (step ladder) | Alternate drugs with caution |
|---|---|---|
| Nociceptive pain | Step 1: Acetaminophen | NSAIDs: Caution in the elderly and patients with good residual kidney function |
| Step 2: Tramadol; tramadol+acetaminophen | ||
| Step 3: Methadone/fentanyl | Buprenorphine/nalbuphine | |
| Neuropathic pain | Step 1: Start with adjuvant therapy | Duloxetine: Dose reduction with a maximum dose of 30 mg daily in patients with eGFR of>30 ml/min. In patients with eGFR<30 ml/min use with caution |
| First line: Gabapentin (50-100 mg daily at night), Pregabalin (25-50 mg at night or 75 mg post HD) | ||
| Second line: Tricyclic antidepressants, amitriptyline starting at 10-25 mg daily or doxepin starting at 10 mg at bedtime | ||
| Step 2: For additional analgesia add a weak opioid or opioid-like analgesic to step 1; tramadol 25-50 mg | ||
| Step 3: Add a strong opioid to step 1; transdermal fentanyl/methadone/nalbuphine |
*For mixed type of pain: Address neuropathic pain with an adjuvant drug first with addition of opioids for additional pain relief. NSAIDs: Nonsteroidal anti-inflammatory drugs, HD: Hemodialysis, eGFR: Estimated glomerular filtration rate
-
Acetaminophen (Level 1, Grade A): Maximum of 3 g daily, preferably oral route. Mild anti-inflammatory agent, it is an analgesic of choice for mild-to-moderate pain
-
NSAIDs (Level 1, Grade B): Only for acute pain and short-term use. Avoid in patients with good residual kidney function/urine output. No change in dose for majority of NSAIDs even when used in patients on HD. Commonly used NSAIDs such as ibuprofen, indomethacin, naproxen, and rofecoxib should be accompanied by a short course of proton-pump inhibitors. Topical diclofenac (5% or 10%) gel for local musculoskeletal pain is preferred to reduce systemic NSAID exposure ex: For pain localized to a small joint.
-
Tramadol (Level 1, Grade B): Half-life is prolonged in patients with kidney failure. Recommended dose: eGFR 10–30 mls/min = 50–100 mg twice daily. For eGFR <10 mls/min (and on dialysis): 50 mg twice daily. To be administered post dialysis as the drug is dialyzable
-
Tapentadol: The drug is not recommended in CKD patients with eGFR <30 ml/min. Recommended dose: Start at 50 mg twice daily, principally as an extended-release form, and titrate further upward based on tolerance
-
Fentanyl (Level 2, Grade B): As transdermal patch (12 mcg/h to 100 mcg/h) or parental route (i. v. or i. m.; 50–100 mcg as an initial dose followed by 50 mcg of additional supplemental dose if required). Advised to start with the lowest dose and then titrate upward to avoid excessive sedation. To use in opioid-tolerant patients with persistent moderate-to-severe chronic pain requiring continuous or prolonged opioid administration
-
Buprenorphine (Level 2, Grade C): Transdermal 5 mcg/h/sublingual 0.2–0.4 mg two to three times a day; parental (i. v./i. m./s. c.) 0.3–0.6 mg per day. Advise patients to report for possible side effects such as constipation and urinary retention
-
Methadone (Level 2, Grade A): Methadone available in India is a mixture of R and S racemic forms. It is effective both in nociceptive and neuropathic pain. It is also efficient to treat central sensitization, reduce tolerance, and in opioid-induced hyperalgesia. Recommended dose: 2.5 mg tablet twice daily oral, to further titrate upward up to a maximum of 20 mg per day
-
Nalbuphine (not graded): Parenteral (s. c or i. v.) 10–30 mg per day. For acute pain.
Drugs currently not available or available only on special request in India: hydromorphone and oxycodone. Not recommended for patients with advanced kidney failure: codeine and morphine.
Gastrointestinal symptoms[2941]
Gastrointestinal symptoms are common, especially in advanced stage of CKD. If not yet initiated on HD (CKD ND), patient symptoms could be related to uremia and initiation of dialysis will improve most of the symptoms and helps in increasing the appetite. If already on dialysis, adequacy of the same should be looked at. Measures should be taken to correct factors contributing to inadequate dialysis and improve uremia. If still there is no improvement, then appropriate additional steps as described should be implemented.
NAUSEA AND VOMITING
The reported prevalence of nausea in advanced kidney failure is approximately 46%.[29] Nausea in patients could be multi-factorial. Reasons including uremia, drugs (e.g., opioids, antibiotics, and anticonvulsants), diabetic gastroparesis and/or uremic gastropathy further worsenthese symptoms. There could be delayed gastric emptying more so in diabetic CKD. A short course of proton-pump inhibitors should be used, if gastritis is contributory. Prokinetic agents such as metoclopramide (10 mg q 8th hourly) with 50% dose reduction for patients with eGFR <40 ml/min (extrapyramidal symptoms due to metoclopramide should be watched for) and itopride can be tried for gastroparesis with close monitoring for any side effects (Level 2, Grade C). Haloperidol (0.5–2 mg q 12 h) or levomepromazine (12.5–25 mg q 8 h) is commonly used for nausea and GI symptoms but requires dose reduction due to their propensity for cerebral sensitivity.
Xerostomia is commonly seen in advanced kidney failure, which could be due to uremia. Patients are often suggested to try lozenges such as xylitol, mints, and ice chips. No specific medication is approved.
Constipation
Dietary restriction and poor appetite in advanced kidney failure can be contributory. It could be quite severe in a few patients needing inhospital intervention. Drugs such as opioids, iron supplements, phosphate binders, when on can contribute or worsen constipation. Nonpharmacological strategies include consuming fiber (20–38 gm/day) and fluid intake within allowed dietary restriction and physical activity. Pharmacological recommendations include oral osmotic laxatives (lactulose 15–30 ml once daily), stimulant laxatives (bisacodyl 5–15 mg PO once daily), sodium picosulfate, and stool softeners. Psyllium fiber husk/roughage powder is not commonly recommended as often patients are on strict fluid restriction.
Dyspnea
Dyspnea is very common in CKD Stage 5. It is commonly due to volume overload (pulmonary edema), cardiovascular cause, or anemia. If it is volume related, a trial of loop diuretics at the higher end of recommended dosing, initiation and intensification of dialysis, slow continuous ultrafiltration will help in alleviating the symptoms. If any cardiac cause is identified and corrected, it will also relieve the symptoms. Anemia should be corrected promptly, with iron supplementation and erythropoietin. In end stages, general measures such as propped-up position, inhaled oxygen (if there is hypoxia), and medications to reduce anxiety can be tried. A small dose of opioids can be used for dyspnea end-of-life care. Short-acting benzodiazepines like lorazepam 0.5–1.0 mg oral or sublingual at 6–8-h interval can be tried. Especially at the end of life, midazolam at a dose of 1.25–2.5 mg (Level 2, Grade B) can be given subcutaneously with close monitoring. It could be very difficult for the patient and family to manage severe dyspnea at home and may need hospital admission for the same. Especially at the end of life, a combination of low-dose opioids and benzodiazepines either as intermittent doses or continuous infusions helps in great relief.
CONCLUSION
There is an increased prevalence of unpleasant physical and psychological symptoms in patients with CKD. These symptoms impact the QoL adversely. Routine assessment and management of these symptoms throughout the disease trajectory should be a priority. While managing symptoms, toxicity of medications which impair residual kidney function, delayed drug clearance, and the effect of dialysis on drug clearance should be considered. This paper addresses the commonly experienced symptoms in patients with CKD and its management [Appendix 1]. The general rule is to preserve cognitive, physical, and kidney function and address the symptom burden with awareness that management priorities may change with time. Clear communication and shared decision-making should guide choosing conservative kidney management, advance care planning, and psychosocial support along with dialysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Symptom burden in chronic kidney disease: A review of recent literature. J Ren Care. 2013;39:140-50.
- [Google Scholar]
- Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J. 2017;10:788-96.
- [Google Scholar]
- Level of symptom relief and the need for palliative care in the hemodialysis population. J Hosp Palliat Nurs. 2007;9:50-8.
- [Google Scholar]
- Symptoms in the month before death for stage 5 chronic kidney disease patients managed without dialysis. J Pain Symptom Manage. 2010;40:342-52.
- [Google Scholar]
- Development of a symptom assessment instrument for chronic hemodialysis patients: The Dialysis Symptom Index. J Pain Symptom Manage. 2004;27:226-40.
- [Google Scholar]
- Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: A simple assessment of symptom burden. Kidney Int. 2006;69:1621-5.
- [Google Scholar]
- Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3189-95.
- [Google Scholar]
- Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract. 2009;111:c74-80.
- [Google Scholar]
- Development and validation of a core outcome measure for palliative care: The palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care. 1999;8:219-27.
- [Google Scholar]
- Symptom burden and quality of life in end-stage renal disease: A study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23:111-9.
- [Google Scholar]
- The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis. 2007;14:82-99.
- [Google Scholar]
- Association of insomnia symptoms with kidney disease quality of life reported by patients on maintenance dialysis. Psychol Health Med. 2013;18:70-8.
- [Google Scholar]
- Insomnia in maintenance haemodialysis patients. Nephrol Dial Transplant. 2002;17:852-6.
- [Google Scholar]
- Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998-1004.
- [Google Scholar]
- Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35:1052-60.
- [Google Scholar]
- Diagnosis and management of insomnia in dialysis patients. Semin Dial. 2006;19:25-31.
- [Google Scholar]
- Symptoms in renal disease: Their epidemiology, assessment, and management. In: Supportive Care for the Renal Patient. USA: Oxford University Press; 2011.
- [Google Scholar]
- The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: A randomized, placebo-controlled, cross-over study (EMSCAP study) Br J Clin Pharmacol. 2009;67:68-75.
- [Google Scholar]
- Non-pharmacological interventions for improving sleep quality in patients on dialysis: Systematic review and meta-analysis. Sleep Med Rev. 2015;23:68-82.
- [Google Scholar]
- Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179-91.
- [Google Scholar]
- A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012;81:247-55.
- [Google Scholar]
- Depression in chronic kidney disease and end-stage renal disease: Similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2:94-107.
- [Google Scholar]
- Association between depression and mortality in patients receiving long-term dialysis: A systematic review and meta-analysis. Am J Kidney Dis. 2014;63:623-35.
- [Google Scholar]
- Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis. 2009;54:741-52.
- [Google Scholar]
- Depressive symptom severity, contributing factors, and self-management among chronic dialysis patients. Hemodial Int. 2016;20:286-92.
- [Google Scholar]
- Antidepressants for treating depression in adults with end-stage kidney disease treated with dialysis. Cochrane Database Syst Rev 2016 CD004541
- [Google Scholar]
- Antidepressants for depression in stage 3-5 chronic kidney disease: A systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP) Nephrol Dial Transplantat. 2012;27:3736-45.
- [Google Scholar]
- Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: Developing a roadmap to improving quality care. Kidney Int. 2015;88:447-59.
- [Google Scholar]
- Recommendations for the care of patients receiving conservative kidney management: Focus on management of chronic kidney disease and symptoms. Clin J Am Soc Nephrol. 2019;14:626-34.
- [Google Scholar]
- Acupuncture for treating uremic pruritus in patients with end-stage renal disease: A systematic review. J Pain Symptom Manage. 2010;40:117-25.
- [Google Scholar]
- Treatment of uremic pruritus: A systematic review. Am J Kidney Dis. 2017;70:638-55.
- [Google Scholar]
- A promising therapy for uremic pruritus in hemodialysis patients: A randomized-controlled trial and review of literature. J Dermatolog Treat. 2016;27:515-9.
- [Google Scholar]
- Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101-19.
- [Google Scholar]
- Interventions for chronic kidney disease-associated restless legs syndrome. Cochrane Database Syst Rev. 2016;11:CD010690.
- [Google Scholar]
- Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin J Am Soc Nephrol. 2019;14:917-31.
- [Google Scholar]
- Pain management in CKD: A guide for nephrology providers. Am J Kidney Dis. 2017;69:451-60.
- [Google Scholar]
- WHO's Cancer Pain Ladder for Adults. 2016. Available from: http://www.who.int/cancer/palliative/painladder/en/
- [Google Scholar]
- 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J. 2017;10:688-97.
- [Google Scholar]
- Murtagh chapter ends stage kidney disease. In: Oxford Text Book of Palliative Medicine. (5th ed). USA: Oxford University Press; 2015. p. :1-25.
- [Google Scholar]
APPENDIX 1
APPROACH TO SYMPTOM MANAGEMENT IN END-STAGE KIDNEY DISEASE PATIENTS
Empathetic communications, active listening Assess proactively Document symptoms with scores – monitor until relief Encourage questions and clarify Assess the symptom; acknowledge the distress, and address both Routine use of validated Tools – Integrated Palliative Care Outcome Scale Renal Evaluate and explain the contributors thoroughly Discuss and agree upon goals of care with the patient, e.g., improvement in function Correct the correctable contributors Integrate nonpharmacological options early Employ MDT expertise Assist, arrange for support devices, aids for symptom relief, where indicated Consider availability, accessibility, and pharmacoeconomics of prescribed drugs Improve compliance by using the simplest prescription regime Monitor relief, and improved functions, prescription adherence, compliance, adverse effects.

UREMIC PRURITUS
Ref: Am J Kidney Dis 2017;70:638-55.
PRURITUS PATIENT HAND-OUT (BRITISH ASSOCIATION OF DERMATOLOGISTS)
Minimize scratching. Keep short fingernails. Try patting the skin instead of scratching. Try to avoid situations where you tend to scratch by observing habits Avoid hot water baths for long periods as hot water may remove the natural oils from your skin. Bathe or shower quickly in tepid (not hot) water no more than once daily. Use soak baths in cool water Use gentle soaps, limit soaping to axilla, groin. Avoid using soaps or foaming body washes even if they claim to be good for dry itchy skin. Many of these contain detergents which remove natural oil from the skin Pat dry the skin, quickly moisturize while still damp. Use airy, cool cotton to wear Keep your bedroom cool and do not sleep with heavy or heat retaining bedclothes. Room moisturizer is helpful during dry seasons for example winter. If you are hot and itchy during the day a fan can help to cool the skin down Aggressive moisturization – People with pruritus usually have dry skin, and therefore, moisturizers may help to soothe the dry skin. They ease itching, reduce scaling, soften cracked areas, and help the penetration of other topical treatments. Use moisturizers which do not have perfumes, preservatives. Hydrate orally, as per urine output For quick relief – You may find that laying a cool towel soaked in moisturizing cream on the skin can reduce the feeling of itch. A cooled moisturizer which has been kept in the refrigerator may also help.
RESTLESS LEG SYNDROME
Ref: CKMCare.com, International Restless Legs Syndrome Study Group

*** Dopamine antagonists – Haloperidol, metoclopramide, risperidone, quetiapine, olanzapine, anti-depressants – SSRIs, SNRIs, TCAs, opioids, Ca blockers, carbamazepine, lithium
Lifestyle
Avoid substances that can trigger symptoms, such as alcohol, tobacco. Limit caffeine intake Show all the drugs that you take, to the doctor Moderate aerobic exercise during the day can also help Sleep Hygiene (vide infra) If you need to travel for long periods of time, consider traveling at a time when your symptoms are least severe (usually in the morning). Try to plan for breaks or periods of time when you can walk around and stretch.
Activities that can reduce symptoms
Walking, stretching, massaging the affected area Taking a warm or cool bath, applying hot or cold packs, soaking in a warm bath can be helpful Relaxation techniques Mentally distracting activities, such as crossword puzzles, crochet, or talking to someone.
NAUSEA/VOMITING
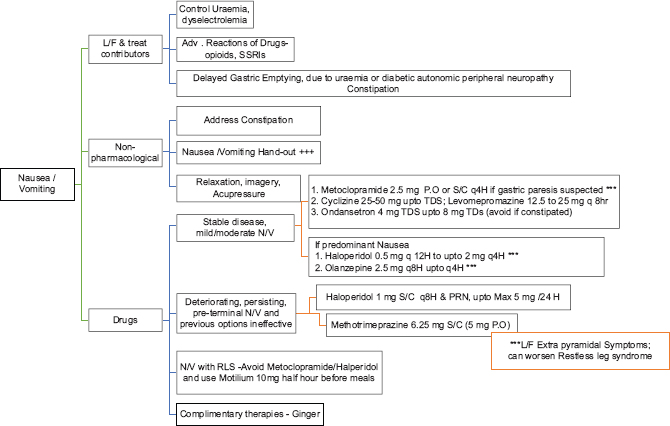
NAUSEA/VOMITING HANDOUT
Oral hygiene Small volume, frequent meals, chewed slowly No Alcohol Advice to drink water before/after, but not with meals Minimize aromas Use ginger Use Loose fitting clothes Avoid spicy, greasy, excessively sweet foods, patient choice Relax in up-right position after meals, to facilitate gastric phase of the digestion Apply cool damp cloth on forehead/nape of the neck.
NOCICEPTIVE PAIN
Ref: Safe and Effective Management of Pain in People with CKD. Sara N. Davison et al; CJASN 14: 1551–1553, 2019.

BONE PAIN
NEUROPATHIC PAIN
BREATHLESSNESS
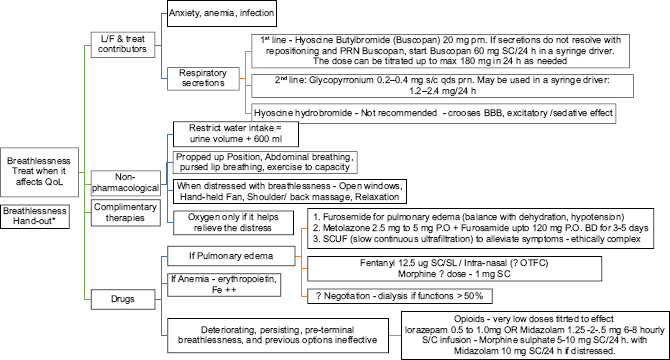
*Breathlessness – Non-pharmacological therapies
Explore triggers and alleviators Help experience awareness when symptom is under control Educate belly breathing, pursed-lip breathing Music, meditation, mindfulness, relaxation techniques Diet review During an attack.
Open windows, open space around patient, loosen clothes Reassurance, calmness in the responses Handheld fan, ceiling fan – Trigeminal V2 stimulation Oxygen – if it helps relieve symptom Shoulder-back massage
SLEEPLESSNESS
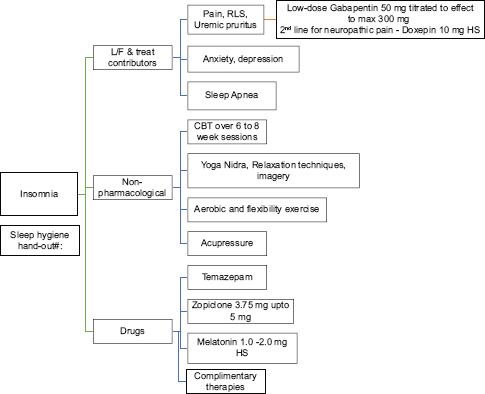
#Sleep Hygiene Handout: Ref: www.cci.health.wa.gov.au;
Get regular. Train your body to sleep well by going to bed and getting up at the same time every day, no matter how well or how poorly you have slept, even on weekends and days off! Get Some Natural Light. Try to spend some time outdoors or in natural light every day. Getting some sunlight early in the day can be helpful for setting your body's natural wake and sleep cycle Sleep when sleepy. Only try to sleep when you feel tired or sleepy, rather than spending too much time awake in bed Get out of bed when not sleeping and try again. If you haven't been able to get to sleep after about 20 min or more, get up and do something calming or boring until you feel sleepy, then return to bed and try again. Sit quietly on the couch with the lights off (bright light will tell your brain that it is time to wake up) or read something boring like the phone book. Avoid doing anything that is too stimulating or interesting, as this will wake you up even more Avoid caffeine and nicotine. It is best to avoid consuming any caffeine (in coffee, tea, cola drinks, chocolate, and some medications) or nicotine (cigarettes) for at least 4–6 h before going to bed. These substances act as stimulants and interfere with the ability to fall asleep Avoid alcohol. It is also best to avoid alcohol for at least 4–6 h before going to bed. Many people believe that alcohol is relaxing and helps them to get to sleep at first, but it interrupts the quality of sleep Use bed for sleeping. Try not to use your bed for anything other than sleeping and sex, so that your body comes to associate bed with sleep. If you use bed as a place to watch TV, eat, read, work on your laptop, pay bills, and other things, your body will not learn this connection No napping during the day. This makes sure that you are tired at bedtime. If you can't make it through the day without a nap, make sure it is for less than an hour and before 3 pm Sleep rituals/routines. You can develop your own rituals of things to remind your body that it is time to sleep. Establish a set routine that you follow every night. For example, have a hot bath, put on your pajamas, brush your teeth, and then listen to soft music and read on the couch until you start to feel sleepy and then go to bed. Some people find it useful to do relaxing stretches or breathing exercises for 15 min before bed each night or sit calmly with a cup of caffeine-free tea Relax. Try doing something to relax your body and mind before going to bed. Try taking a hot bath 90 min before you plan to go to bed. Or, try a relaxation exercise (see Calm Breathing and Progressive Muscle Relaxation), meditation, or listening to calming music Bathing. Having a hot bath 1–2 h before bedtime can be useful, as it will raise your body temperature, causing you to feel sleepy as your body temperature drops again No clock-watching. Frequently checking the clock during the night can wake you up (especially if you turn on the light to read the time) and reinforces negative thoughts such as “Oh no, look how late it is, I'll never get to sleep” or “it's so early, I have only slept for 5 h, this is terrible” Don't worry. Leave your worries about work, school, health, relationships, etc., out of the bedroom. Try scheduling a “worry time” earlier in the evening to deal with your worries. Write a to-do list for the next day/week. Maintain a journal and jot down the events, thoughts of the day, plans for the next day is helpful. If you wake up in the middle of the night worrying, try writing down your worries and tell yourself that you will address them in the morning Exercise during the day. Regular exercise is a good idea to help with good sleep, but try not to do strenuous exercise in the 4 hours before bedtime. Morning walks are a great way to start the day feeling refreshed! Eat right. A healthy, balanced diet will help you to sleep well, but timing is important. Some people find that a very empty stomach at bedtime is distracting, so it can be useful to have a light snack, but a heavy meal soon before bed can also interrupt sleep. Some people recommend a warm glass of milk, which contains tryptophan, which acts as a natural sleep inducer The right space. It is very important that your bed and bedroom are quiet and comfortable for sleeping. A cooler room with enough blankets to stay warm is best, and make sure you have curtains or an eye mask to block out early morning light and earplugs if there is noise outside your room. Make sure that you have a supportive mattress and fresh, comfortable bedding. Also, try to ensure that your room is not too hot or cold, minimize noise, and block out light Keep daytime routine the same. Even if you have a bad night sleep and are tired it is important that you try to keep your daytime activities the same as you had planned. That is, don't avoid activities because you feel tired. This can reinforce the insomnia Start Small! Making small changes can have a large impact on your sleep. Don't try to do everything all at once. Instead, pick one or two strategies and try them consistently. When you're ready, try adding a new strategy. The goal is to slowly start increasing behaviors that can help you sleep, while reducing the things that are interfering with your sleep Be Consistent. Pick a strategy and use it consistently. Try to do the same thing every night Be Patient. These strategies can take time to improve your sleep. In fact, sometimes things can get worse before they get better. Hang in there and stick with it! Chart Your Progress. Use the Sleep Diary form to keep track of the strategies you're using and your weekly progress.
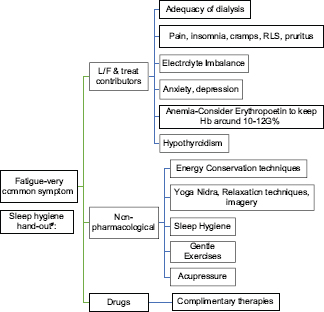
FATIGUE
NUTRITIONAL CONCERNS
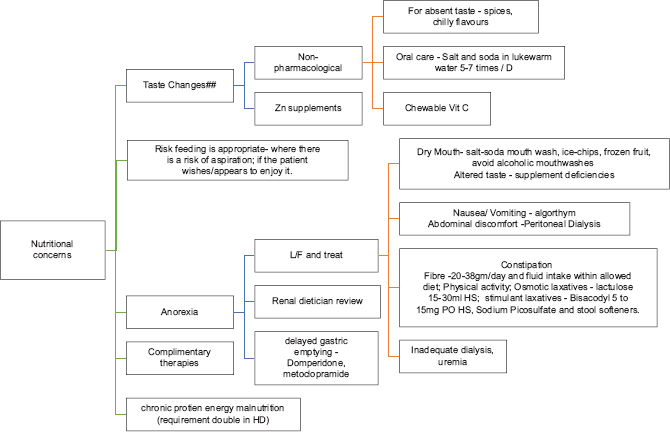
## Urea → bitter; Sodium → salty; Potassium → metallic; reduced Zn→ absence of taste
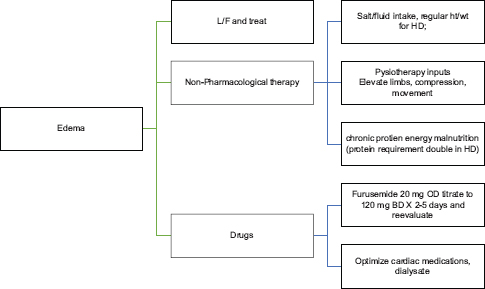
GENERALIZED EDEMA
DEPRESSION







