Translate this page into:
Translation and Linguistic Validation of the Multidimensional Dyspnoea Profile into Telugu in a Palliative Care Setting
*Corresponding author: Mikael Segerlantz, Department of Clinical Sciences Lund, Respiratory Medicine, Allergology and Palliative Medicine, Institute for Palliative Care, Faculty of Medicine, Lund University, Lund, Sweden. mikael.segerlantz@med.lu.se
-
Received: ,
Accepted: ,
How to cite this article: Dufberg L, Kökritz M, Palat G, Ekström M, Brun E, Segerlantz M. Translation and Linguistic Validation of the Multidimensional Dyspnoea Profile into Telugu in a Palliative Care Setting. Indian J Palliat Care. 2025;31:48-51. doi: 10.25259/IJPC_244_2024
Abstract
Objectives:
Dyspnea, or breathlessness, is a frequent and distressing symptom among patients with heart and lung diseases, particularly in advanced cancer stages, where it affects up to 90% of lung cancer cases. This symptom considerably diminishes quality of life, leading to physical deconditioning, increased levels of anxiety and depression, repeated hospitalizations, and elevated mortality rates. The Multidimensional Dyspnea Profile (MDP), developed in 2011, allows assessment of both the sensory experience and emotional response to dyspnea. While the MDP has been translated into multiple languages, a Telugu version has not been developed, underscoring the need for a validated tool in this under-resourced and primarily illiterate patient population in palliative care. Our aim was to translate and linguistically validate the MDP for use in Telugu-speaking populations in an Indian palliative care setting, where illiteracy rates are high.
Materials and Methods:
The MDP was translated and adapted into Telugu through collaboration with the Mapi Institute, which specializes in culturally relevant translation and validation of patient-reported outcome (PRO) measures. A structured translation process included both forward and backward translations by two certified independent translators. The translated version was refined through feedback from two Indian palliative care physicians and four healthcare workers. In-depth interviews with 24 Telugu-speaking cancer patients were conducted to evaluate the tool’s clarity and suitability for this patient population.
Results:
The Telugu version of the MDP was adapted specifically for palliative care settings that serve socioeconomically disadvantaged populations with high levels of illiteracy. The translation adhered closely to international PRO standards set by the Mapi Institute. The MDP facilitated healthcare providers’ understanding of dyspnea’s impact on this group of palliative care patients.
Conclusion:
Applying the MDP in a palliative care context improved clinicians’ insights into factors that contribute to dyspnea. However, given the instrument’s length, selective use of its sections may be more practical in time-constrained settings.
Keywords
Breathlessness
Dyspnoea
Multidimensional
Palliative care
Telugu
INTRODUCTION
Dyspnoea, or breathlessness, is the subjective experience of breathing discomfort, characterised by distinct sensations of varying intensity as described by the American Thoracic Society.[1] As the severity of cardiac and pulmonary diseases progresses, the frequency of dyspnoea increases,[2,3] yet this distressing symptom often remains inadequately managed.[4]
Dyspnoea not only limits physical activity, exacerbating breathlessness through physical deconditioning, but it also establishes a cycle that severely affects patients’ quality of life. Its presence is linked to heightened anxiety and depression, frequent hospitalisations and increased mortality.[5] This burdensome symptom is especially prevalent in palliative care, with rates of 50–70% among patients with advanced cancer and reaching as high as 90% in those with lung cancer.[6]
To address the multifaceted nature of dyspnoea, the Multidimensional Dyspnoea Profile (MDP) was introduced by Banzett et al. in 2015. This tool is structured to assess both the sensory and emotional dimensions of breathlessness, providing a comprehensive evaluation of the symptom’s impact.[7] Since its development, the MDP has been translated into several languages, such as Swedish,[8] French, Japanese, Spanish and, more recently, Hindi.[9]
However, no validated Telugu version of the MDP currently exists, despite Telugu being spoken by approximately 75 million people in Andhra Pradesh and Telangana. Given the high illiteracy rates in this population and the critical need for accessible tools within palliative care, a Telugu adaptation of the MDP is both timely and essential.
Aim
The aims of this study were to translate, culturally adapt and linguistically validate the MDP for effective use among Telugu-speaking populations in Indian palliative care settings, with consideration for high illiteracy rates.
MATERIALS AND METHODS
Translation and linguistic validation process
The MDP was systematically translated from American English to Telugu in partnership with the Language Services Unit at Société par Actions Simplifiée [Figure 1]. Mapi is known for its expertise in translating and validating patient-reported outcome (PRO) instruments.
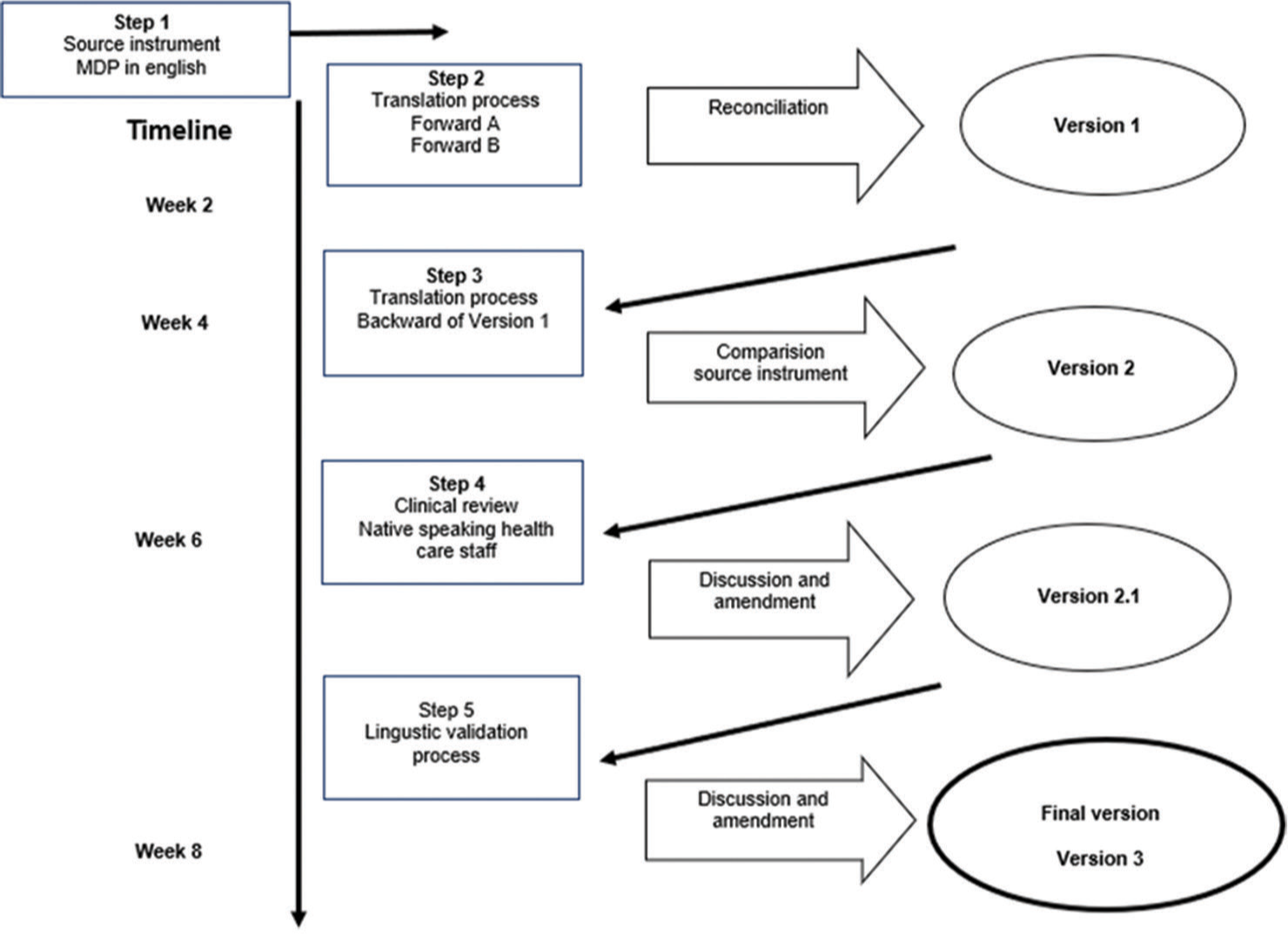
- Flowchart of the translation and lingustic validation process of the Multidimensional Dyspnoea Profile (MDP).
Ethical review and consent
The Ethics Committee at Mehdi Nawaz Jung Institute of Oncology and Regional Cancer Center (MNJIORCC) in Hyderabad, India (2022), approved this study. Patients and their families were verbally briefed by healthcare providers and written or fingerprint-signed consent was obtained from all participants, with witnesses and healthcare providers also signing the consent forms.
Methodology
Following the translation guidelines of Mapi Research Trust (accessible online at Clinical Outcome Assessments (COA) Translation and Linguistic Validation | Mapi Research Trust, mapi-trust.org), the MDP underwent a rigorous, multistage translation and validation process in accordance with international standards.[10,11] After approval for translation, two certified Telugu translators from Translated S.R.L., Rome, Italy, produced initial Telugu versions independently. A third translator then reconciled these versions to create Version 1, which was back-translated into English and reviewed by an Indian linguistic consultant. After necessary modifications, Version 2.0 was produced.
Review by clinicians
The second version (Version 2.0) was evaluated by two native Telugu-speaking physicians (authors GP and SR) at MNJIORCC, who assessed its clarity and relevance for Telugu-speaking patients with dyspnoea, leading to a refined Version 2.1 for use in the study.
Participant criteria
Patients in palliative care, either at home or at Kumudini Devi Hospice, were selected based on specific criteria: Age 18 or older, native Telugu speakers, experiencing recent significant breathlessness and capable of answering questions. Patients with cognitive impairments or those who were non-native Telugu speakers were excluded from the study.
Linguistic validation process
Version 2.1 of the translated MDP was administered orally to patients by healthcare staff in a single session, with responses recorded by experienced palliative care nurses or physicians according to a standardised protocol. Cognitive interviews were conducted to assess clarity, comprehensibility and suitability of the MDP. Appendix 1 includes the validated questions for patients, and participants provided feedback on their understanding of each item, suggesting alternative wording for any unclear phrasing [Appendix 2].
Healthcare staff conducting patient interviews were later interviewed to discuss any issues or misunderstandings observed in patient responses. Appendix 3 contains the validated questions for staff, who commented on areas where patients may have experienced confusion and offered alternative phrasing suggestions [Appendix 2]. After careful review and adjustments, the finalised Telugu MDP (Version 3) was completed and is detailed in Appendix 4. The sample size is determined by reaching data saturation.
RESULTS
Study outcomes
Patient profile
The study involved 24 cancer patients diagnosed with various types, including head and neck (n = 5), breast (n = 5), gynaecological (n = 4), lung (n = 4), haematological (n = 3), gastrointestinal (n = 2) and prostate (n = 1) cancers. The group included nine males and 15 females, with an average age of 49.5 years. Dyspnoea severity, measured using the Shortness of Breath Questionnaire (SQ) with a scale from 0 to 10, showed varying intensities among participants. Fourteen participants received in-home care, and the rest were in hospice care. More demographic details are shown in Table 1.
| Variable | Number (%) |
|---|---|
| Literate | |
| Yes | 14 (58) |
| No | 10 (42) |
| Education | |
| No education | 9 (37.5) |
| 1–10 years | 11 (45.9) |
| 11+ years | 4 (16.6) |
| Employment | |
| Unemployed | 13 (54.2) |
| Daily labourer | 3 (12.5) |
| Employed | 4 (16.6%) |
| Retired | 2 (8.3%) |
| Household income (per/month, INR) | |
| 5000–10000 | 1 (4.2) |
| >10000 | 1 (4.2) |
| White card | |
| Yes | 5 (20.9) |
| No | 4 (16.6) |
Data listing the demographics of the patient group included, ranging from educational level to household income. Provides an overview of the socioeconomic status of the patients. INR: Indian rupee
Translation review and revisions
Following clinician feedback, additional changes were made to the initial translation, with a focus on enhancing the clarity of terms in the MDP’s second domain.
Validation results
The validation process engaged 24 Telugu-speaking patients, with 14 in palliative home care (58%) and 10 at Kumudini Devi Hospice (42%). A majority (83%) completed the entire questionnaire, and all responded to validation questions. In addition, three doctors and one nurse evaluated the instrument’s applicability. Most patients (88%) reported that the MDP questions were easy to understand, although 3 (12%) encountered difficulties. Four patients (17%) gave inconsistent responses in the second SQ step, and 9 (38%) selected ‘does not apply’ for some SQ symptoms while rating them higher on the scale.
All healthcare staff (4/4) indicated that the MDP helped them better understand patients’ dyspnoea and found it useful for both home and hospice care. However, some items were challenging for patients with limited education, potentially causing response inconsistencies. Staff also noted that the MDP’s comprehensiveness could be time-intensive, particularly for critically ill patients.
Final modifications
Based on staff feedback, the ‘radio metaphor’ from the American English MDP was removed due to its complexity for patients with limited education [Appendix 5]. While initially included in Version 2.1, patient feedback revealed misunderstandings, leading to its exclusion in the final version. Further refinements included simplifying terms and using symbols (e.g. replacing ‘or’ with ‘/’). The term ‘depression’ was also modified for clarity, with a Telugu equivalent added alongside the English term in Telugu script. With these adjustments, the final Telugu MDP (Version 3) was approved by the Mapi Institute on 28 August 2023, and is available in their database for clinical and research use.
For details on specific revisions made, please refer to Appendix 2.
DISCUSSION
This study presents a linguistically validated Telugu version of the MDP, designed to support a comprehensive assessment of breathlessness in a palliative care context serving a largely underserved population with high illiteracy rates.[12-14] The translation followed international guidelines for PROs as outlined by the Mapi Institute, recognised for its expertise in cross-cultural validation of such tools.
This research is among the initial efforts to adapt the MDP for a palliative care setting, where specific adaptations were necessary. During earlier work translating the MDP into Hindi, challenges were identified that helped inform the Telugu adaptation process.[9] A significant issue noted with both translations is the MDP’s length and complexity, which may be demanding for critically ill patients. In high-intensity care settings, using selected sections of the MDP, rather than the entire tool, may help reduce demands on patients and healthcare providers alike.
The linguistic validation involved Telugu-speaking patients from socioeconomically disadvantaged backgrounds, many with limited formal education, which influenced their ability to engage with certain rating scales and abstract concepts.[15,16] For example, the numeric rating scale (NRS) was challenging for some patients when evaluating symptom severity. To address this, local healthcare staff suggested using a familiar concept – the Indian Rupee – as a reference point, where 10 rupees indicated high symptom severity, and half a rupee indicated a moderate level. Further simplifying the language in the MDP could improve accessibility for patients with limited educational backgrounds.
CONCLUSION
This study resulted in a linguistically validated Telugu MDP, offering a useful tool for assessing dyspnoea in Telugu-speaking patients in palliative care. The MDP provided helpful insights into factors contributing to breathlessness in this population. However, given the time-intensive nature of the tool, selectively using sections as needed may help reduce the burden. Simplifying the language further may also be essential to ensure that patients with limited education fully comprehend the MDP questions, maximising its utility in this context.
Acknowledgments
The authors wish to thank Vineela Rapelli for administrative support regarding the study and Dr Hrudai, Dr Wajid, Dr Prajwell and Mr Swarup, who contributed to the clinical review of the MDP.
Authors’ contributions
Conception, design: ME, MS and EB. Local adjustment of study design: MS, EB, GP and SR. Participated in the translation and revision of important intellectual content: GP and SR. Interviews, validation: MK and LD. Conception of the article and continuous revision of the article: MK, LD, ME, MS and EB. All authors approved the version to be published.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study, waiver number MNJ 2022/09.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: I was supported by an unrestricted grant from the Swedish Research Council (Dnr: 2019-02081).
References
- An official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185:435-52.
- [CrossRef] [PubMed] [Google Scholar]
- Underlying Contributing Conditions to Breathlessness among Middle Aged-Individuals in the General Population: A Cross Sectional Study. BMJ Open Resp Res. 2020;7:e000643.
- [CrossRef] [PubMed] [Google Scholar]
- Dyspnea as an Independent Predictor of Mortality. Clin Respir J. 2016;10:142-52.
- [CrossRef] [PubMed] [Google Scholar]
- Is Chronic Breathlessness Less Recognized and Treated Compared with Chronic Pain?: A Case-Based Randomised Controlled Trial. Eur Respir J. 2018;52:180088.
- [CrossRef] [PubMed] [Google Scholar]
- The Effect of Anxiety on Respiratory Sensory Gating Measured by Respiratory-related Evoked Potentials. Biol Psychol. 2012;91:185-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dyspnea in Palliative Care Treasure Island, FL: StatPearls Publishing; 2022. 1 Version
- [Google Scholar]
- Multidimensional Dyspnea Profile-An Instrument for Clinical and Laboratory Research. ERJ. 2015;45:1681-91.
- [CrossRef] [PubMed] [Google Scholar]
- Swedish Translation and Linguistic Validation of the Multidimensional Dyspnoea Profile. Eur Clin Respir J. 2016;3:32665.
- [CrossRef] [PubMed] [Google Scholar]
- Translation and Linguistic Validation of the Multidimensional Dyspnea Profile into Hindi in a Palliative Care Setting. Indian J Palliat Care. 2024;30:252-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability and Validity of the Multidimensional Dyspnea Profile. Chest. 2012;141:1546-53.
- [CrossRef] [PubMed] [Google Scholar]
- Test-retest Reliability of Multidimensional Dyspnea Profile Recall Ratings in the Emergency Department: A Prospective, Longitudinal Study. BMC Emerg Med. 2012;12:6.
- [CrossRef] [PubMed] [Google Scholar]
- Real-life Assessment of the Multidimensional Nature of Dyspnea in COPD Outpatients. Eur Respir J. 2016;47:1668-79.
- [CrossRef] [PubMed] [Google Scholar]
- Patient Reported Outcome Measures in Chronic Obstructive Pulmonary Disease: Which to Use? Expert Rev Respir Med. 2016;10:351-62.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Assessment of Breathlessness in the Clinical Setting. Expert Rev Respir Med. 2014;8:151-61.
- [CrossRef] [PubMed] [Google Scholar]
- Multinational Trials Recommendations on the Translations Required, Approaches to Using the Same Language in Different Countries, and the Approaches to Support Pooling the Data: The ISPOR Patient-Reported Outcomes Translation and Linguistic Validation Good Research Practices Task Force report. Value Health. 2009;12:430-40.
- [CrossRef] [PubMed] [Google Scholar]
- Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8:94-104.
- [CrossRef] [PubMed] [Google Scholar]







