Translate this page into:
Efficacy of Chronomodulated Chemotherapy for Palliation of Hematemesis in Inoperable Gastric Cancer: A Single-Institutional Retrospective Study
Address for correspondence: Dr. Santanu Acharyya, Department of Radiotherapy, R G Kar Medical College and Hospital, 1, Khudiram Bose Sarani, Kolkata - 700 004, West Bengal, India. E-mail: drsantanuacharyya1@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Aside abdominal discomfort and pain, upper gastrointestinal bleeding (UGIB) significantly disgraces the quality of life (QoL), especially in inoperable gastric cancer patients. Although, in early stages, it is infrequent and often ignored, but in advanced stages, its aggressiveness often deteriorates patient's hemoglobin (Hb) level and performing status.
Aim:
The aim of this study is to correlate the change in (1) the frequency of episodes of UGIB, (2) its severity in terms of Common Terminology Criteria for Adverse Events (CTCAE) grade for UGIB, and (3) Hb level with the successful completion of successive cycles of palliative chemotherapy where it becomes invariably the only modality to palliate the cancer disease.
Setting and Design:
This single-institutional retrospective observational study included seventy gastric carcinoma patients with a chief complaint of frequent hematemesis. They were divided according to the cause behind inoperability or irresectability: (1) Metastatic disease, (2) locally advanced irresectable disease, (3) uncontrolled comorbidities, (4) poor GC (PGC), and (5) refused to give surgical consent.
Subjects and Methods:
Following baseline evaluation and prechemotherapy workups, patients were subjected to three-weekly chronomodulated modified EOX regimen. Relevant parameters, i.e., (1) average episodes per-week (AEP) score, (2) Hb, and (3) average CTCAE grade value for UGIB were recorded after every cycle.
Results:
At 12-week follow-up, there was a significant decrease in mean AEP score from baseline (from 2.6691 ± 0.7047 to 1.5033 ± 0.6272) for the entire cohort (P < 0.001). Maximum benefit in terms of mean Hb (increase by 1.0737% above baseline) took place for PGC group (P < 0.001). Mean CTCAE grade value for the entire cohort decreased from baseline by 0.6428, which was statistically significant with a P < 0.001.
Conclusions:
PGC group was maximally benefited considering all three parameters. Though surgery defines the mainstay of treatment for gastric carcinoma, yet in inoperable cases, only chronomodulated chemotherapy significantly affects the severity of UGIB and thus may improve QoL.
Keywords
Adenocarcinoma stomach
hematemesis
inoperable gastric ca
modified EOX
palliative chemotherapy
INTRODUCTION
Among all malignancies, gastric cancer dominates by establishing itself as the second-most common cause of cancer death (782,685 deaths annually).[1] If Japan is considered as an exception, worldwide, a 5-year survival rate is as low as 10%–30% only.[23] Almost half of the advanced-stage gastric cancer patients lack the opportunity of upfront curative surgery;[45] therefore, comprehensive modalities such as chemotherapy and radiotherapy (RT) take the lead.
In our clinical practice, it was noticed that hematemesis or colloquially “blood vomiting” not only affects the patient's haemoglobin (Hb) level or Eastern Cooperative Oncology Group performing status (ECOG PS), but also it has a deleterious effect on the psyche of the gastric cancer patient. This contributes a lot to the poor quality of life (QoL) of these patients. The aim of our study was to assess whether the well-established modified EOX (mEOX) regimen alone can improve hematemesis and thereby leave a positive effect on Hb level and Common Terminology Criteria for Adverse Events (CTCAE) v5.0[6] grade for upper gastrointestinal bleeding (UGIB) in these patients. We tried to correlate the changes in these parameters with the successful completion of successive cycles of this first-line palliative chronomodulated chemotherapy regimen. Lack of other options besides best supportive care (BCS) for these patients with persisting and progressing tumour-source for UGIB added profound relevance to this study.
SUBJECTS AND METHODS
Inclusion criteria
This single-institutional observational retrospective study was conducted over a 2 year period from January 1, 2017, to December 31, 2018. Patients of gastric carcinoma registered in our outpatient department were screened and thereafter included in the current study abide by the following inclusion criteria: (1) diagnosed as well/moderately/poorly differentiated adenocarcinoma of the stomach by endoscopic biopsy (hence other causes of hematemesis, for example, esophageal varices were ruled out), (2) at presentation, significant hematemesis constituted one of the chief complaints besides abdominal pain and discomfort, epigastric pain and vomiting, (3) decided as inoperable or irresectable by a multidisciplinary tumor board, (4) workup permitted initiation and administration of the mentioned mEOX regimen and continuation of the same up to six cycles, (5) recording of proposed parameters as a mandatory part of weekly follow-up at least up to 12 weeks after the completion of sixth i.e., the last cycle of chemotherapy, (6) age between 50 and 80 years, and (7) ECOG PS 1–3.
Regimen used
For our study, modified EOX regimen consisting of chronomodulated capecitabine (epirubicin 50 mg/m2 i.v., day 1; oxaliplatin 130 mg/m2 i.v. day 1; capecitabine at a twice-daily dose of 1000 mg/m2 PO for 2 weeks, followed by 1 week of rest) was used which is our institutional protocol. Modified EOX or mEOX regimen was repeated every 3 weeks for a total of six cycles.
Causes of inoperability: study population was divided according to the causes of inoperability as follows: (1) metastatic disease (MD), (2) locally advanced irresectable (LAI) disease, (3) uncontrolled comorbidities (UC), (4) poor GC (PGC), and (5) refused to give surgical consent (RSC).
Outcome measures
We formulated a scoring system to quantify UGIB and termed it average episodes per-week (AEP) Score defined as, AEP = (Number of total episodes of UGIB in last 6 weeks)/6.
According to our study protocol, two successive episodes of UGIB had to be separated by a 6-h gap; if not so, they were considered collectively as a single episode. Neither severity nor quantity of blood vomited was considered as a criterion, as reporting was depended on patients themselves and sometimes their close relatives. There was every chance of biased score if it was taken into account. Hence, the basis of AEP was just a “number” and not the severity. Rather blood level of Hb (gram/dl) was considered to understand and correlate to the severity of blood loss. Hb was measured weekly during the entire course of study period, and every time, the most recent level was reported for the statistical analysis. In our selected patients with a chief complaint of hematemesis, packed red blood cells (PRBC) units were transfused aiming maintenance of Hb >10 g/dl. According to our institutional protocol, chemotherapy cycle had to be deferred if Hb% was lower than the said level. Independent of these cases of time-delay in the administration of cycles, AEP was always calculated for the past 6 weeks only.
According to CTCAE v5.0,[6] average CTCAE grade value for UGIB was another data to be collected weekly. No doubt, a CTCAE grade is always an integer for any specific patient. Naturally, the median value is always an integer indeed. However, we realized that instead of the median value, specifically for this study, mean of the CTCAE grade was much more representative and transparent way to quantify the symptomatic betterment in question, i.e., UGIB. Again, our CTCAE data were almost devoid of outliers. Undoubtedly, its mean value for any group may come in fraction and not an integer always.
Statistical analysis
For the statistical analysis, the data were entered into a Microsoft excel spreadsheet and then analyzed by SPSS (version 25.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5. Data had been summarized as mean and standard deviation for the numerical variables and count and percentages for the categorical variables. Two-sample t-tests for a difference in mean involved independent samples or unpaired samples. Paired t-tests were a form of blocking and had greater power than unpaired tests. One-way analysis of variance was a technique used to compare the means of three or more samples for numerical data (using the F distribution). Unpaired proportions were compared by the Chi-square test or Fischer's exact test, as appropriate. P ≤ 0.05 was considered for statistically significant.
RESULTS
The study population comprising of total 70 patients was divided into five major groups reflecting the causes of inoperability. Eventually, LAI group and PGC group carried same weightage (27.1% in each). Fourteen patients (20%) had MD, whereas 11.1% were inoperable due to UC. Portion RSC was not at all negligible (14.3%). Median age for the entire cohort was 69 years. The oldest patient (77 years) belonged to the RSC, and the youngest (61 years) was from PGC group. The fact, that all patients of RSC group belonged to the eighth decade of life, which transparently reflected the geriatric psychology. Thirty-seven (52.9%) patients were male and 33 (47.1%) patients were female, showing a slight male preponderance. The entire UC was comprised male patients, whereas female patients occupied 90% of the RSC group. At first visit, majority of the patients (45.7%) presented with ECOG PS 2; 31.4% patients had ECOG PS 3 and only 16 out of 70 patients had ECOG PS 1 at presentation. No doubt, patients with PS 4 were beyond any definite oncological treatment, so excluded and sent for BCS. MD group was dominant (7 out of 16; 43.8%) to present with ECOG PS 1. Endoscopy revealed that, >65% patients of our inoperable study population had their primary disease originated from body of the stomach followed by fundus (24.3%) and pylorus (10%). Out of 14 MD group patients, 12 and 63.2% LAI patients had their primary bleeding lesion located at the body of the stomach.
The cN stage of the disease was considered instead of the standard pathological nodal staging i.e., pN (American Joint Committee on Cancer TNM staging; 8th Edition) on the basis of contrast-enhanced computed tomography of whole abdomen. Keeping in account the inoperable and/or irresectable status of our patients, we were compeled to accept this. However, percentage for N0, N1, N2, and N4 stages was 41.4%, 25.7%, 28.6%, and 4.3%, respectively. Stage N2 was evident in 63.2% of LAI patients, and all three patients detected with N3 disease eventually belonged to this that very group. Majority of the UC group and RSC group was N0 (87.5% and 70%), respectively, which was logically expected. Hepatic metastasis was evident in 14 patients; whereas 17.1% patients had disease metastasized to distal peritoneal sites. Nonregional peritoneal nodules were present in 52.6% of LAI patients. Exactly 50% of the entire cohort had moderately differentiated adenocarcinoma, whereas 31.4% and 18.6% patients had and poorly and well differentiated disease, respectively; 85.7% (i.e. 12 out of 14) of MD patients and 52.6% (10 out of 19) of LAI patients had moderately differentiated adenocarcinoma of the stomach.
At baseline evaluation, mean AEP scores were 2.46 ± 0.3589, 2.82 ± 0.7560, 2.28 ± 0.4107, 3.01 ± 0.7565, and 2.39 ± 0.8008 for NSC, LAI, MD, PGC, and UC, respectively, with the overall baseline mean of 2.6691 ± 0.7047 for the entire cohort. In fact, baseline median AEP score was also maximum, i.e., 3.17 for the PGC group. At the evaluation after the completion of second cycle of CT, the overall mean AEP decreased down to 2.5396 ± 0.5867 establishing a significant P < 0.001. At this stage, every group showed individual decrease in AEP value. However, benefits for different groups did not show that much variation. Decrease in AEP score was maximum (0.48) for UC group and minimum (0.050) for MD group. Evaluation after the fourth cycle of mEOX also showed the same trend. The decrease in AEP value for the entire cohort was 0.3175 which was statistically significant with a P < 0.001. After successful administration of the sixth and last cycle of CT, decrease in mean AEP score from the baseline was 0.8341 for the cohort. Maximum decrease was 0.5542 and minimum decrease was 0.3162 for PGC and UC group, respectively. At the follow-up after a span of 6 weeks on completion of chronomodulated chemotherapy, absolute values of mean AEP score became 1.5660 ± 0.6718, 1.8684 ± 0.6283, 1.5829 ± 0.7601, 2.2274 ± 0.5530, and 1.6013 ± 0.8544 for NSC, LAI, MD, PGC, and UC, respectively. At 12-week follow-up, the decrease in mean AEP score was 1.1691 for the entire cohort, while for maximum beneficiary PGC group from this aspect showed a decrease of 1.4926 in AEP from baseline. Minimum benefit was noticed in UC group (mean decreased by 0.815). The overall benefit was statistically significant with a significant P < 0.001. Figure 1 depicts the changing trend of mean AEP score over the entire study period.

- Changing trend of mean average episodes per-week score
The overall mean Hb was 9.4814 ± 0.9787% at baseline. Subsequently, the distribution of mean baseline Hb was considered among different groups. It was maximum (10.3875 ± 0.9493) for UC group and minimum (8.6421 ± 0.6727) for PGC group. After administration of CT2 with mEOX overall mean Hb was decreased by 0.1757%. At this almost initial stage of treatment by palliative chemotherapy, mean Hb was maximum (9.8250 ± 0.6065%) for UC group and minimum (8.9105 ± 0.4175) for PGC group. Only PGC group saw the face of an increase (0.2684) in mean Hb value. For other groups, Hb% was decreased at this level of evaluation. This result in one hand reveals the inevitable transient hematopoietic toxicity of CT, on the other hand an increasing value for PGC group evoked hope. After CT4, mean Hb for the entire cohort was further decreased to 9.3443±0.5623%. Hematotoxicity was apparent. Patients had to receive PRBC transfusions often at this stage to proceed for further cycles of chemotherapy. The 7 days' gap between the successive cycles was proved to be very useful. However, at the end of CT6, the increased mean Hb (9.5671 ± 0.4995) was evident for the entire cohort (P < 0.001). Absolute mean values for NCS, LAI, MD, PGC, and UC groups were 9.4900% ± 0.4175%, 9.4579% ± 0.4992%, 9.8571% ± 0.4603%, 9.4053% ± 0.4034%, and 9.8000% ± 0.6633%, respectively. After a follow-up period of 6 weeks, the mean Hb for the entire cohort was 9.7729% ± 0.5264%. The maximum beneficiary group at this follow-up was PGC patients with an increase of 0.9947 (P < 0.001). At the final step of response evaluation, i.e., at follow-up after 12 weeks, the mean Hb was 9.9157% ±0.5208% overall. Maximum benefit (increase by 1.0737%) took place for PGC group (P < 0.001). Decrease from baseline was noticed in UC group (0.1125% decrease though insignificant statistically). Figure 2 shows the changing trend of mean Hb over the entire study period.
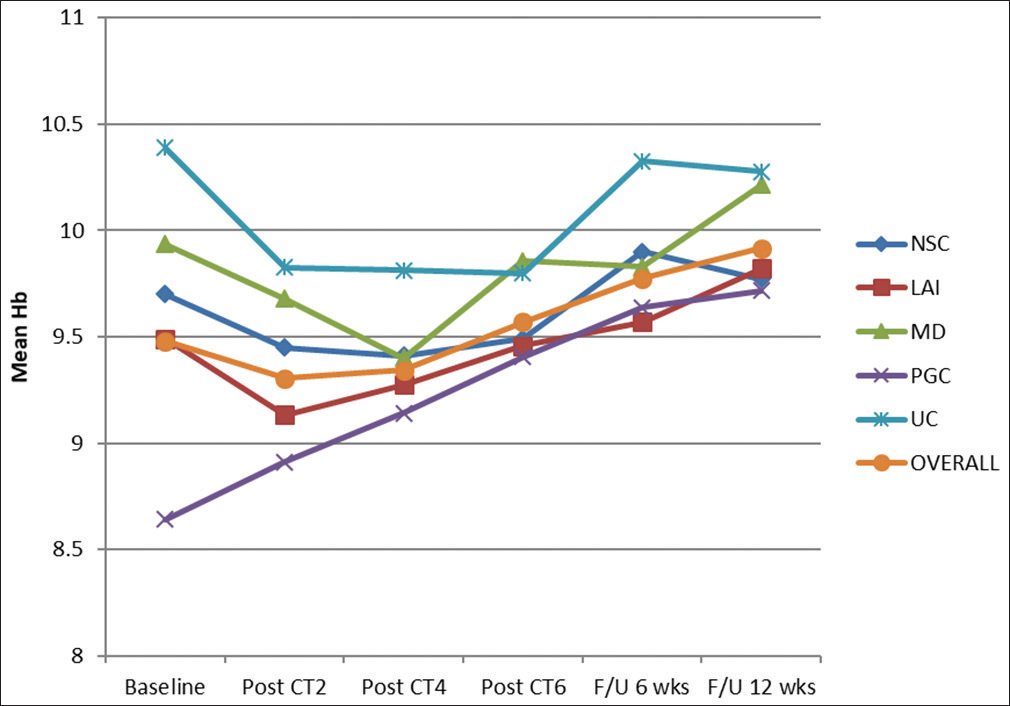
- Changing trend of mean hemoglobin
Next, the CTCAE grade was taken in account. The baseline mean CTCAE grade for the entire cohort was 2.0571 ± 0.6786. For different groups, i.e., NSC, LAI, MD, PGC, and UC, the values were 1.8000 ± 0.6325, 2.2105 ± 0.6306, 1.7143 ± 0.7263, 2.4737 ± 0.5130, and 1.6250 ± 0.5175, respectively. After the completion of the second cycle, mean CTCAE grade decreased down to 1.9857 ± 00.6018. There was a decrease (by 0.3684) in PGC group and on the contrary increase (0.105) in LAI group. However, after the fourth cycle of CT statistically significant change was visible with a decreased mean CTCAE grade of 1.9286 ± 0.5729 overall; P < 0.001. At the conclusion of all six cycles of mEOX, overall mean was further decreased to 1.7000 ± 0.461; LAI group and PGC group were equally benefited (mean decreased by 0.5263).
After 6 weeks of follow-up, the overall mean came down to 1.4857 ± 0.5580. At 12-week follow-up, the mean value for the entire cohort was further decreased to 1.4143 ± 0.4962, making it statistically significant with a P < 0.001. Absolute decrease in the mean CTCAE grade value from the baseline for different groups, i.e., NSC, LAI, MD, PGC, and UC were 0.600, 0.842, 0.214, 0.947, and 0.250, respectively. Clearly, the most beneficiary group from this aspect was again PGC group while minimum benefit was evident in MD group. Figure 3 shows the changing trend of mean CTCAE grade value over the entire study period.
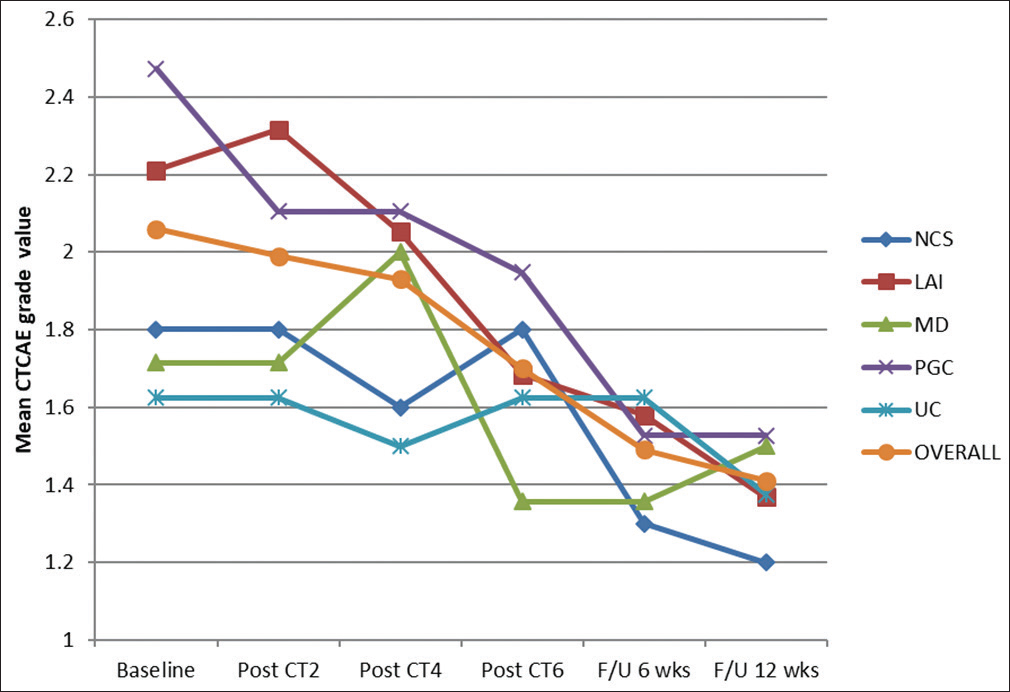
- Changing trend of mean Common Terminology Criteria for Adverse Events grade value
In order to reveal a reflection of blood loss, number of units of PRBC transfused over the overall treatment time was recorded as a passive surrogate. Out of a total 726 units of PRBC, 30.59% was transfused to PGC group. LAI, MD, UC, and RSC groups received 23.18%, 20.48%, 13.61%, and 12.12% PRBC units, respectively. Interestingly, these percentages roughly kept a pace with the proportion of patients in each group in relation to the entire cohort (P 0.527). It interpreted that the cycle-to-cycle relative change in parameters is important, whereas the record of overall transfusion did not clarify the difference.
As a by-product of our study median progression-free survival (mPFS) was calculated over a 12 months median follow-up period. It was 7.4 months for the entire cohort. While calculated differentially, mPFS was minimum for MD group (5.8 months) and maximum for RSC group (9.1 months).
DISCUSSION
Majority of our patient population was mainly constituted by “sporadic” gastric cancer patients, which is common in the sixth and seventh decades of life.[7] Naturally, at this stage of life, geriatric complications contribute to poor general condition along with the cancer disease itself.
Complete resection with a standardized D2 lymphadenectomy, which is the well-established curative surgical modality is not always possible due to several causes reflecting inoperability or irresectability.[8]
A study from the Indian subcontinent demonstrated that the curative resection rate is 56.33%.[4] After a robust analysis of 2280 cases of gastric cancer over 15 years, Ruiz et al. also confirmed that the operability and respectability rates (56.8% and 58.5%, respectively) are beyond negotiations.[5]
UGIB is defined as mucosal bleeding from the proximal part of the oesophagus to the ligament of Treitz. Among all causes of UGIB, peptic ulcers is just seconded by gastric cancer.[9]
In a study Sheibani et al. revealed that gastric cancer is the culprit for >73% cases of UGIB caused by malignancy. Moreover, in 79% patients, this hematemesis was the first manifestation of the cancer disease and 75% of these patients were metastatic at presentation, they concluded.[10] This data supported our one as in more than half of our patients (62.8%) had no complaint except multiple episodes of UGIB at their first visit.
UGIB remains a devastating occurrence for inoperable gastric cancers. Moreno-Otero et al. suggested that in a majority of patients where surgery was possible in gastric cancer, UGIB became self-limiting. However, 55.6% patients had persistent or massive hematemesis and literally all of them who were not operated, expired due to that very cause, i.e., UGIB.[11]
Glasgow Batchford Scores, Modified Early Waning Score, and preendoscopic Rockall scores are recommended scoring systems employed for the prognosis of patients at risk for hemodynamic crisis, need for transfusion, and hospitalization.[12]
In quest of relationships between change in blood parameters and UGIB, Hoffmann et al. demonstrated the changes in total and differential leucocyte count, Hb, platelets, C-reactive protein, alanine transaminase, and creatinine levels in association with UGIB.[13]
Although Dahlerup et al. concluded that UGIB in patients with gastric cancer is generally mild to moderate,[14] our patients' Hb levels often suffered continuous threats even in spite of transfusions and intravenous tranexaemic acid injections. The European Society of Gastrointestinal Endoscopy recommends maintaining Hb levels between 7.0 and 9.0 g/dl using blood transfusion to reduce mortality.[15] Tomizawa et al. established that a threshold value of 10.8 g/dl of Hb in the presence of UGIB in relation to malignancy identifies patients at risk for hemodynamic crisis.[16]
According to the literature review, preoperative RT in treating gastroesophageal junction (GEJ) cancer is much more well established while the application of preoperative RT still lacks evidence in form of large-scale phase III trials in advanced gastric cancer.[17] Further, advanced conformal techniques of RT can reduce toxicities to surrounding normal tissues while treating gastric cancer. Unfortunately, in our study period, intensity-modulated radiation therapy facility was not available in any RT department of state hospitals in West Bengal therefore making the objective of the study pragmatic. Further, while the intent is palliation, according to our institutional protocol, for gastric cancer patients with ECOG PS up to 2, palliative chemotherapy dominates over the option of palliative RT. The latter is kept as a last resort for patients who are incapable of tolerating mEOX (e.g., reduced left ventricular ejection fraction, deranged hepatic/renal function, etc.)
Considering the fact that combination chemotherapy for advanced gastric cancer is often associated with severe treatment-related toxicities Hwang et al. comment that most oncologists are reluctant to perform combination chemotherapy in patients with a poor clinical condition. They retrospectively investigated not only the efficacy but the tolerability of single-agent chemotherapy in patients with recurrent or metastatic gastric cancer with poor performance status.[18] However, combination palliative chemotherapy is well-established for locally advanced, inoperable, or metastatic cancer of the stomach or the GEJ. Multiple practice-changing studies have given evidence in favor of palliative chemotherapy while compared with BCS in terms of not only overall survival but also improvements in QoL.[192021] The regimen we used for our study was the well-established modified EOX regimen. This palliative chemotherapy regimen was introduced by Pluschnig et al. as a “better-tolerated modified version” of the EOX regimen.[22] One reason to choose this regimen over the standard EOX was the 7 days gap between two successive cycles. It was not only a matter of convenience but also provided a feeling of 7 days' vacation to these cancer patients, which in turn contributed to their psychological health and QoL.
In our study, the benefit for PGC group was robust. It evoked a hope for these patients who were sent for BCS almost in all cases, sometimes without adequate initiative to treat the cancer disease actively.
CONCLUSIONS
Bamias and Pavlidis may be recalled stating that, the role of systemic chemotherapy including chronomodulated administration of 5-FU (or capecitabine) was promising.[23] Our study established that even today chronomodulated capecitabine containing modified EOX regimen may stand as a strong wall against UGIB while treating inoperable gastric cancer patients with expectation for an elevated QoL.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [Google Scholar]
- Operability and curative resection rate in gastric carcinoma: An experience at Peshawar, Pakistan. Gomal J Med Sci. 2014;12:73.
- [Google Scholar]
- Operability and resectability of gastric cancer: Analysis of 2280 cases in 15 years. Rev Gastroenterol Peru. 1997;17:135-42.
- [Google Scholar]
- Department of Health and Human Services National Institutes of Health. National Cancer Institute; 2017.
- Gastric cancer: Global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-49.
- [Google Scholar]
- Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J Clin Gastroenterol. 2008;42:128-33.
- [Google Scholar]
- Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013;38:144-50.
- [Google Scholar]
- Acute upper gastrointestinal bleeding as primary symptom of gastric carcinoma. J Surg Oncol. 1987;36:130-3.
- [Google Scholar]
- Validity of modified early warning, Glasgow Blatchford, and pre-endoscopic Rockall scores in predicting prognosis of patients presenting to emergency department with upper gastrointestinal bleeding. Scand J Trauma Resusc Emerg Med. 2015;23:109.
- [Google Scholar]
- A novel easy-to-use prediction scheme for upper gastrointestinal bleeding: Cologne-WATCH (C-WATCH) risk score. Medicine (Baltimore). 2015;94:e1614.
- [Google Scholar]
- Diagnosis and treatment of unexplained anemia with iron deficiency without overt bleeding. Dan Med J. 2015;62:C5072.
- [Google Scholar]
- Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
- [Google Scholar]
- Low hemoglobin levels are associated with upper gastrointestinal bleeding. Biomed Rep. 2016;5:349-52.
- [Google Scholar]
- Progress of preoperative and postoperative radiotherapy in gastric cancer. World J Surg Oncol. 2018;16:187.
- [Google Scholar]
- First-line single-agent chemotherapy for patients with recurrent or metastatic gastric cancer with poor performance status. Exp Ther Med. 2012;4:562-8.
- [Google Scholar]
- Modified therapy with 5-fluorouracil, doxorubicin and methotrexate in advanced gastric cancer. Cancer. 1993;72:37-41.
- [Google Scholar]
- Randomized comparison of fluorouracil, epirubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with nonresectable gastric cancer. Br J Cancer. 1995;71:587-91.
- [Google Scholar]
- Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163-8.
- [Google Scholar]
- Modified EOX (Epirubicin, Oxaliplatin and Capecitabine) as palliative first-line chemotherapy for gastroesophageal adenocarcinoma. Anticancer Res. 2013;33:1035-9.
- [Google Scholar]
- Systemic chemotherapy in gastric cancer: Where do we stand today? Oncologist. 1998;3:171-7.
- [Google Scholar]






