Translate this page into:
Practical Pharmacology of Methadone: A Long-acting Opioid
Address for correspondence: Dr. MM Sunilkumar, Department of Pain and Palliative Medicine, Trivandrum Institute of Palliative Sciences, WHO Collaborating Centre for Training and Policy on Access to Pain Relief, Arumana Hospital Building, Vallakadavu, Thiruvananthapuram, Kerala, India. E-mail: sunil@palliumindia.org
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Methadone is a naturally long-acting analgesic with unique pharmacodynamic and pharmacokinetic properties compared to other opioids, available now in India, to treat severe pain. It has the potential to dramatically relieve suffering among patients with serious illness who are living with persistent physical pain. However, clinicians must appreciate its unique pharmacologic properties and its use in clinical practice safely and effectively. The available formulation in India is a racemic mixture of the S- and R-enantiomers, and as such, it will have a propensity for drug-drug and drug-genetic interactions that can increase the risk of Torsades de Point and respiratory depression. Appropriate patient selection, careful dosing and thorough monitoring of methadone will mitigate these risks.
Keywords
Drug interactions
India
methadone
pharmacology
INTRODUCTION
Pain is one of the most common symptoms experienced by patients living with serious illness and is often a combination of persistent, around-the-clock pain with additional intermittent or “breakthrough” pain, which is sometimes incidental to routine body movements, such as coughing, or more strenuous physical activity. In cancer pain, experts recommend using long-acting opioids to control persistent pain and short-acting opioids dosed as-needed for breakthrough pain.[1] This combination improves pain control and quality of life.[2] From a pharmacokinetic perspective, using as-needed or scheduled short-acting, immediate-release opioids to control severe pain results in wide fluctuations in plasma levels, causing a roller-coaster of pain severity, and pain-related anxiety. In addition, this dosing strategy results in higher peak plasma levels, which are theoretically associated with a higher risk of side effects [Figure 1].[3] In the United States and Canada, elegant, controlled-release solid-dosage formulations of naturally short-acting opioids, such as morphine and oxycodone, have been engineered to provide sustained, steady analgesia over 8–12 h. However, there are limitations in using these formulations in patients with serious illness, as these long-acting oral preparations can be difficult to ingest. Methadone is a naturally long-acting analgesic, with a duration of analgesic effect of 8–12 h after repeated dosing.[4] Therefore, it can provide sustained analgesia through a variety of dosage forms (tablet, liquid, subcutaneous, and intravenous), allowing versatility in administration through the oral, buccal, rectal, and intravenous routes. This makes it an ideal analgesic to treat persistent pain in patients with the advanced serious illness.
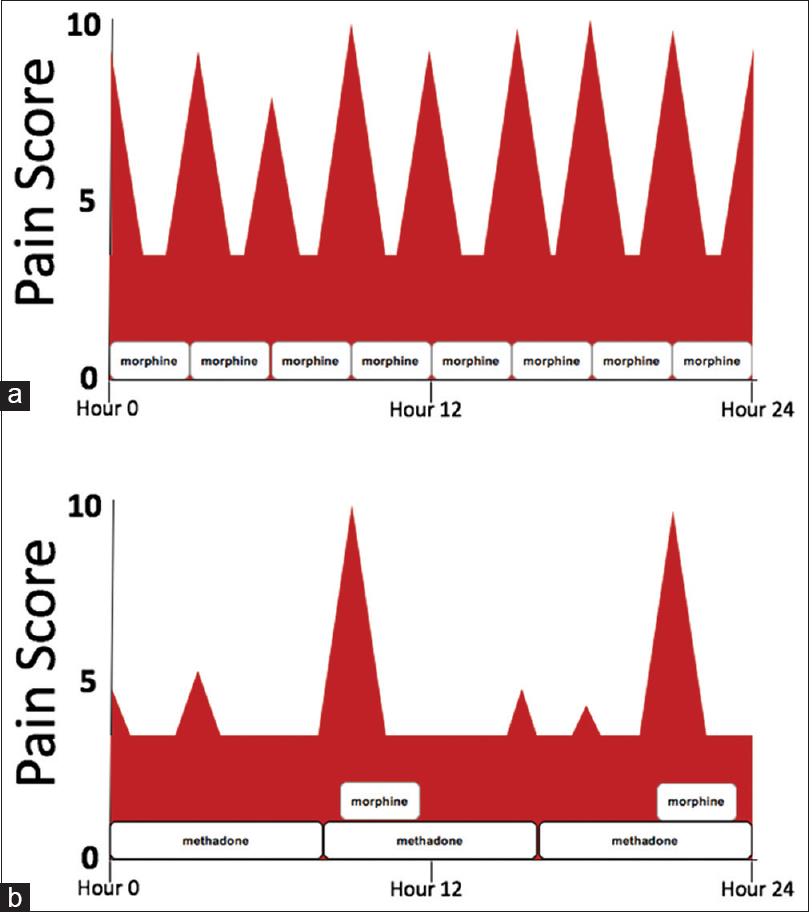
- Using schedule short-acting opioids such as morphine versus methadone with rescue morphine available. (a) Pain score pattern for a patient with severe, persistent pain taking scheduled morphine every 3 h. (b) Pain score pattern for a patient with severe, persistent pain taking scheduled methadone every 8 h with morphine available every 3 h as needed for moderate-to-severe pain
PHARMACODYNAMICS: WHAT METHADONE DOES TO THE BODY
Therapeutic effects
Methadone formulations in India include 5 mg oral tablets and 5 mg/ml syrup which contain racemic mixtures of R-methadone and S-methadone. The R-enantiomer is approximately 50 times more potent than the S-enantiomer for analgesia, in part due to 10-fold greater affinity for the mu opioid receptor. Methadone also activates the delta and kappa opioid receptors.[56] In addition to opioid receptor agonism, both R-methadone and S-methadone equally antagonize the N-methyl-D-Aspartate (NMDA) receptor. Since activation of NMDA leads to central sensitization, windup, and tolerance, methadone's blockage of this receptor can prevent and reverse acquired tolerance to pure opioid agonists such as morphine. Methadone improved pain control in patients with severe pain who were taking high doses of opioid agonists such as morphine and oxycodone.[789101112] S-methadone also prevents the reuptake of serotonin and norepinephrine.[46] This, combined with NMDA antagonism, makes methadone more effective for neuropathic pain than other opioids such as morphine and fentanyl.[1314]
Adverse effects
The action of methadone at opioid receptors can lead to opioid-related side effects including sedation, respiratory depression, and constipation. These effects are additive with other medications that cause these effects as well as with concomitant medications that increase methadone levels. In particular, most patients in the United States who experienced serious respiratory depression resulting in death while taking methadone were also taking benzodiazepines, which have an additive effect on respiratory depression.[4] Benzodiazepines, barbiturates, and alcohol should be avoided in patients taking methadone. Sleep apnea, central nervous system injury, and severe lung disease can increase the risk of respiratory depression in patients taking methadone, perhaps the “deep sleep” as a result of neuropathic pain, amelioration. Initiation in such patients should weigh safety risks with the patient's goals of care and should only be done with conservative dosing and careful monitoring.
Methadone can cause serotonin syndrome when given with other serotonergic medications such as monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotnin and norepinephrine reuptake inhibitors (SNRI), and certain tricyclic antidepressants (TCAs).[1516171819] Methadone should not be used with MAOIs. When methadone is used with SSRIs, SNRIs, or TCAs, it should be dosed conservatively with careful monitoring for serotonin syndrome, which can range in severity from agitation, clonus, and hyperreflexia to confusion, lethargy, coma, and seizures and is accompanied by autonomic instability, particularly hyperthermia.
In addition to its activity at the NMDA receptor and serotonin and norepinephrine reuptake transporters, S-methadone binds to the human Ether-a-go-go (hERG) to cause QT-prolongation, which can lead to Torsades de Point (TdP) and sudden cardiac death. Drug interactions that also prolong the QT interval or result in increased levels of S-methadone increase the risk of QT prolongation. Risk of TdP begins at corrected QT interval of 450 ms and is significant at a corrected QT interval (QTc) >500 ms or an increase of QTc >60 ms from baseline.[20] In outpatient palliative care patients, QTc >500 ms only occurred in 1 of 64 patients after initiation of methadone.[21] Patient risk factors for QT prolongation must also be considered for initiation and ongoing methadone therapy. These include hypokalemia and hypomagnesemia.[20] A recent case–control study among palliative care patients identified congestive heart failure, peptic ulcer disease, hypokalemia, rheumatologic disease, malignancy, hypokalemia, total daily dose of methadone >45 mg, concomitant QT-prolonging medications, and concomitant medications that increase methadone levels as significant risk factors for QT-prolongation in this patient population.[22] The Heart Rhythm Society and American Pain Society recommend obtaining ECGs before starting methadone in patients with the following risk factors: electrolyte abnormalities, Impaired liver function, age >68, female gender, structural heart disease, genetic predisposition, concomitant QT-prolonging drugs, history of syncope, and previous QTc >450 ms. Follow-up ECGs are recommended in 2–4 weeks after initiating methadone for patients with these risk factors, and again if the total daily dose of methadone reaches 30–40 mg and 100 mg.[20]
PHARMACOKINETICS: WHAT THE BODY DOES TO METHADONE
Methadone's basicity and high lipophilicity largely contribute to its long half-life. On oral ingestion, it is absorbed by the stomach and is 36%–100% (mean 75%) bioavailable, with a peak plasma concentration 2.5–4 h postdose.[5] In addition to commercially available 5 mg/mL syrup, methadone can be compounded into an oral syrup or solution.[23] 34% of methadone can be absorbed sublingually from a concentrated oral solution or syrup.[24] In addition, methadone oral solution administered rectally has approximately 80% bioavailability equivalent to the oral route. In contrast, methadone suppositories have a lower absorption efficacy of 35%–58%.[25] Absorbed methadone quickly moves out of the blood compartment, where it can act on its target receptors, and into lipophilic tissues (alpha-phase). An equilibrium of methadone concentration between the tissues and blood compartment is reached after saturation of lipophilic tissues, and the slow beta-phase of elimination begins, with a variable elimination half-life of 5–130 h (average of 22 h).[2627] For this reason, it can take 5–14 days to reach steady state and see the full impact of a dose initiation or increase. While methadone's half-life is long, the duration of analgesic effect after repeated dosing is 8–12 h.[28] The risk of side effects, however, including respiratory depression, persists beyond this analgesic window.
As methadone is released into and circulates in the blood, it is 90% bound to alpha-1 acid glycoprotein, with 4-fold variation among individual patients, further contributing to variability in dosing needs among patients.[5] Methadone and its metabolites are excreted into the urine and feces, with excretion rate increasing and shifting to the renal route when urinary pH is <6. Typically, 11% of a methadone dose is renally eliminated, but this can increase up to 57% in urine acidification.[56] Even so, methadone is a preferred opioid in end-stage renal disease. Whereas morphine is metabolized into active glucuronides that accumulate and increase risk of toxicity, including neurotoxicity, methadone is safe even in severe renal impairment.[29] It is not appreciably removed by dialysis.[30]
Metabolism adds additional variability to safe and effective doses of methadone among patients. Due to its extensive hepatic metabolism, conservative dosing is warranted in liver failure. Methadone is primarily metabolized to inactive metabolites, including 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, by cytochrome P (CYP) 2B6, followed by CYP 3A4 and CYP 2C19. Methadone is metabolized to a lesser extent by CYP 2D6, 2C9, and 2C8.[31323334] S-methadone, which causes 3.5-fold blockade of hERG, is preferentially metabolized by CYP 2B6, and R-methadone, which has up to 50x more analgesic activity, is preferentially metabolized by CYP 2C19. Both are equally metabolized by CYP 3A4.[3133] Methadone's extensive metabolism by CYP enzymes can lead to drug interactions with allopathic and ayurvedic medications that inhibit or induce these enzymes. In addition, consumption of grapefruit juice could increase methadone levels. Depending on the drug interaction, levels of one or both of S-methadone or R-methadone may be impacted and could result in decreased analgesia or increased toxicity. Furthermore, CYP 2B6, CYP 2C19, and CYP 2D6 are polymorphic, with some genetic variants resulting in increased metabolism of methadone and some resulting in decreased metabolism. In India, Ancestral North Indians (ANI) and Ancestral South Indians (ASI) have distinct pharmacogenetics from each other as well as from ethnic groups in whom methadone has been used to date, including Caucasians. Whereas 13.3%of Caucasians have a poor metabolizer genotype (CYP2C19 * 2) of CYP 2C19, 33.1% of ANI and 36.8% of ASI do. The poor metabolizer genotype CYP2D6 * 3 was found in 9.2% of ANI and 0% of ASI compared to 2.8% of Caucasians. More ANI (29.3%) and ASI (34.8%) have the fully functional CYP2D6 * 2 allele than do Asians (14%) and Caucasians (4%).[35] A poor metabolizer phenotype for CYP2B6 was discovered in 20.56% of ANI and 40% of ASI, compared to 6%–39% of Caucasians.[3637] These genetic variants in our population make it paramount to exercise caution when using this new medication.
HOW PHARMACOLOGY IMPACTS METHADONE USE
Given methadone's complicated pharmacodynamic and pharmacokinetic properties, “starting low and going slow” with close follow-up is central to safely using methadone. Numerous methods and conversion ratios are used for initiating methadone in patients already taking opioids, such as short-acting morphine.[38] Recent data indicate lower doses than those calculated with the most conservative conversion methods may be effective.[39] It is unknown if this may be due to patient-related factors or drug interactions not captured in the study. Case reports indicate frail; elderly patients should be initiated on much lower doses of methadone, even as low as 0.5 mg daily.[14] This could be due to age-related changes including increased fat composition and age-related decreases in activity of phase I CYP enzymes responsible for methadone metabolism, both of which would contribute to a prolonged half-life for methadone. Due to methadone's long and variable half-life, it can take 5 days or more to see the full effect of a given dose. Therefore, the starting dose of methadone should not be increased for at least 5 days in ambulatory patients to avoid early dose stacking. Readers are referred to the manuscript by Palat et al. within this issue for a comprehensive discussion of dosing methadone.
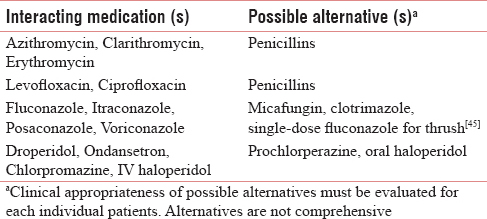
As outlined above, methadone has numerous pharmacodynamic and pharmacokinetic drug interactions. Consultation with a pharmacist knowledgeable about methadone may be helpful in identifying and managing drug interactions, including selecting therapeutic alternatives or adjusting doses. Whenever possible, avoid drug interactions with methadone by deprescribing the interacting medication or selecting a therapeutic alternative to the interacting medication when possible. It is particularly important to avoid using methadone with medications which have previously been shown to cause TdP if taken with methadone. Such medications are highlighted with an asterisk (*) in Table 1.[4041] Many medications can increase or decrease levels of methadone in the body [Table 2].[424344] Therapeutic alternatives may be selected when clinically appropriate [Table 3]. If a patient is taking a medication that lowers methadone levels, monitor carefully and adjust the dose of methadone as needed based on short-acting opioid use. It may take 2 weeks to see the full effect of a drug interaction that decreases methadone levels. If a medication increases methadone levels, the dose of methadone should be preemptively reduced by 25%–33% and the patient should be carefully monitored. When a patient needs methadone and another serotonergic medication together, choose a medication with lower serotonergic effect when possible. In any case, if a drug interaction is present, it is important to gain informed consent from the patient after communicating the risk of the drug interaction and how patient safety will be maximized and monitored.


SUMMARY
Methadone requires training, knowledge of its unique pharmacology, and careful clinical consideration. When knowledgeable clinicians judiciously add it to short-acting opioids for severe pain in a patient with serious illness, pain control, and quality of life improve without decreasing overall survival.[46]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Canadian recommendations for the management of breakthrough cancer pain. Curr Oncol. 2016;23:96-108.
- [Google Scholar]
- Effects of controlled-released morphine on quality of life for cancer pain. Oncol Nurs Forum. 1989;16:521-6.
- [Google Scholar]
- Controlled-release of opioids for improved pain management. Mater Today. 2016;19:491-502.
- [Google Scholar]
- Interindividual variability of the clinical pharmacokinetics of methadone: Implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153-93.
- [Google Scholar]
- Methadone for relief of cancer pain: A review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73-83.
- [Google Scholar]
- Dose ratios between high dose oral morphine or equivalents and oral methadone. J Palliat Med. 2013;16:947-50.
- [Google Scholar]
- Addition of methadone to another opioid in the management of moderate to severe cancer pain: A case series. J Palliat Med. 2013;16:305-9.
- [Google Scholar]
- Switching from oxycodone to methadone in advanced cancer patients. Support Care Cancer. 2012;20:191-4.
- [Google Scholar]
- Switching methadone: A 10-year experience of 345 patients in an acute palliative care unit. Pain Med. 2012;13:399-404.
- [Google Scholar]
- Methadone initiation and rotation in the outpatient setting for patients with cancer pain. Cancer. 2010;116:520-8.
- [Google Scholar]
- The use of very-low-dose methadone and haloperidol for pain control in the hospital setting: A preliminary report. J Palliat Med. 2015;18:114-9.
- [Google Scholar]
- Methadone is superior to fentanyl in treating neuropathic pain in patients with head-and-neck cancer. Eur J Cancer. 2016;65:121-9.
- [Google Scholar]
- Use of methadone as an adjuvant medication to low-dose opioids for neuropathic pain in the frail elderly: A Case series. J Palliat Med. 2016;19:1351-5.
- [Google Scholar]
- Serotonin syndrome due to co-administration of linezolid and methadone. Infez Med. 2017;25:263-6.
- [Google Scholar]
- Case scenario: Opioid association with serotonin syndrome: Implications to the practitioners. Anesthesiology. 2011;115:1291-8.
- [Google Scholar]
- Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95:434-41.
- [Google Scholar]
- Serotonin syndrome in a chronic-pain patient receiving concurrent methadone, ciprofloxacin, and venlafaxine. Psychosomatics. 2009;50:638-9.
- [Google Scholar]
- A case of serotonin syndrome associated with methadone overdose. Proc West Pharmacol Soc. 2008;51:42-4.
- [Google Scholar]
- Methadone safety: A clinical practice guideline from the American pain society and college on problems of drug dependence, in collaboration with the heart rhythm society. J Pain. 2014;15:321-37.
- [Google Scholar]
- The effect of oral methadone on the QTc interval in advanced cancer patients: A prospective pilot study. J Palliat Med. 2010;13:33-8.
- [Google Scholar]
- Methadone and corrected QT prolongation in pain and palliative care patients: A Case-control study. J Palliat Med. 2017;20:722-8.
- [Google Scholar]
- Oral methadone for management of regional sympathetic dystrophy syndrome: A case revisited. Int J Pharm Compd. 2007;11:187-92.
- [Google Scholar]
- Sublingual absorption of selected opioid analgesics. Clin Pharmacol Ther. 1988;44:335-42.
- [Google Scholar]
- Methadone for pain: What to do when the oral route is not available. J Pain Symptom Manage. 2015;49:e4-6.
- [Google Scholar]
- Cytochrome P450 2D6 genotype and methadone steady-state concentrations. J Clin Psychopharmacol. 2001;21:229-34.
- [Google Scholar]
- Revisiting methadone: Pharmacokinetics, pharmacodynamics and clinical indication. Rev Dor. 2015;16:60-6.
- [Google Scholar]
- Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28:497-504.
- [Google Scholar]
- Methadone is poorly removed by haemodialysis. Nephrol Dial Transplant. 1999;14:254-5.
- [Google Scholar]
- Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36-44.
- [Google Scholar]
- Implications of opioid analgesia for medically complicated patients. Drugs Aging. 2010;27:417-33.
- [Google Scholar]
- Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: A reconciliation. Basic Clin Pharmacol Toxicol. 2011;108:55-62.
- [Google Scholar]
- Distribution of genetic polymorphisms of genes encoding drug metabolizing enzymes & drug transporters - a review with Indian perspective. Indian J Med Res. 2014;139:27-65.
- [Google Scholar]
- Prevalence of poor and rapid metabolizers of drugs metabolized by CYP2B6 in North Indian population residing in Indian national capital territory. Springerplus. 2012;1:34.
- [Google Scholar]
- CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009;53:863-8.
- [Google Scholar]
- A review of common methods to convert morphine to methadone. J Community Hosp Intern Med Perspect. 2013;2(4) Epub ahead of print
- [Google Scholar]
- Methadone dose selection for treatment of pain compared with consensus recommendations. J Palliat Med. 2017;20:1385-8.
- [Google Scholar]
- Methadone, QTc interval prolongation and torsade de pointes: Case reports offer the best understanding of this problem. Ther Adv Psychopharmacol. 2013;3:219-32.
- [Google Scholar]
- QT Drugs List. Oro Valley, AZ, USA 85755: AZCERT, Inc. 1822 Innovation Park Drive. 2017. Available from: http://www.CredibleMeds.org
- [Google Scholar]
- Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine. 2007. Available from: http://www.medicine.iupui.edu/clinpharm/ddis/clinical-table
- [Google Scholar]
- Drug Interactions. Micromedex Healthcare Series. Thomson Micromedex. Available from: http://www.thomsonhc.com
- Current concepts in methadone metabolism and transport. Clin Pharmacol Drug Dev. 2017;6:125-34.
- [Google Scholar]
- Single-dose fluconazole therapy for oral thrush in hospice and palliative medicine patients. Am J Hosp Palliat Care. 2017;34:645-9.
- [Google Scholar]
- Overall survival among cancer patients undergoing opioid rotation to methadone compared to other opioids. J Palliat Med. 2017;20:656-61.
- [Google Scholar]






