Translate this page into:
Early Competing Deaths in Locally Advanced Head-and-Neck Cancer
Address for correspondence: Dr. Sandeep Muzumder, Department of Radiation Oncology, St. John's Medical College Hospital, Bengaluru, Karanataka, India. E-mail: sandeepmuzumder@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The competing (noncancer) deaths have increased with aggressive treatment approach and better disease control in locally advanced head-and-neck cancer.
Aim:
The aim of this study is to find incidence, cause and predictors of early competing mortality in locally advanced head-and-neck cancer patients undergoing combined modality therapy.
Subjects and Methods:
In this retrospective study, a total of 125 locally advanced head-and-neck patients treated from January 2013 to June 2017 were analyzed. The total number of deaths, cause, and the time of death from the start of therapy was recorded. To study the risk factors of competing deaths, univariate and multivariate logistic regression was applied. Data were analyzed using SPSS v. 24 software.
Results:
A total of 51 deaths (31 cancer deaths and 20 competing deaths) recorded at a median follow-up of 16 months (1–62 months). The incidence of early competing mortality was 12% (n = 15) with a median time of 2.7 months from treatment initiation. Sepsis was major cause of early competing death (n = 13). On univariate and multivariate logistic regression analysis, competing death was significantly associated with pharyngeal (oropharynx, hypopharynx, and larynx) site primary (odds ratio [OR] = 3.562; 95% confidential interval [CI] = 1.207–10.517; P = 0.016), and Stage IVA/IVB disease (OR = 5.104; 95% CI = 1.123–23.202; P = 0.021).
Conclusion:
Competing deaths is one of the multifaceted problems in locally advanced head-and-neck cancer patients. Sepsis being single most cause of early competing deaths in Stage IVA/IVB pharyngeal and laryngeal cancer.
Keywords
Combined modality therapy
competing death
head-and-neck cancer
noncancer death
sepsis
INTRODUCTION
Cancer-directed therapy has become more aggressive over time. Combine modality therapy (CMT) is the standard of care in locally advanced head and neck cancer, especially concurrent chemoradiation therapy (CCRT).[1] The toxicities may be under-reported in randomized controlled trials (RCTs).[2] The incidence of mucositis increases by two-fold when chemotherapy was given concurrently with radiation therapy (RT).[34] Dysphagia and aspiration are invariable accompaniments of mucositis. These leads to decreased quality of life, increased cost, unscheduled treatment break, incomplete treatment, and probable increase in toxic deaths.[5]
In meta-analyses of chemotherapy in head-and-neck cancer study, the benefit of chemotherapy was due to decrease in deaths related to head-and-neck cancer without effecting noncancer deaths.[1] Noncancer deaths, also known as competing deaths, are due to treatment-related acute or late toxicities, comorbidities, second cancer, or unknown causes.[67] Competing deaths can be categorized as early (deaths within 6 months of treatment initiation) versus late (death after 6 months of treatment initiation).[7] Competing deaths have been well documented in long-term survivors of testicular cancer,[8] Hodgkin's lymphoma[9] and early breast cancer.[10] However, little has been written about competing deaths in head-and-neck cancer.[67] Therefore, we conducted the study to find incidence, cause, and predictors of early competing deaths in locally advanced head-and-neck cancer patients undergoing CMT.
SUBJECTS AND METHODS
Study design and setting
After Institute Ethical Clearance, the present retrospective study was conducted in the Department of Radiation Oncology at St. John's Medical College and Hospital, Bengaluru, India. We analyzed all locally advanced head-and-neck cancer patients treated radically from January 2013 to June 2017. AJCC 7th (2010) was used for staging. Patients received RT and chemotherapy as per standard guidelines and practice. Patient received 54–60 Gy in adjuvant and 66–70 Gy in definitive setting. Patients were treated either with intensity-modulated RT (IMRT) or three-dimensional conformal radiotherapy technique as per treating radiation oncologist. Concurrent chemotherapy was given when indicated. Concurrent chemotherapy was given either 3 weekly or weekly. All patients were reviewed twice a week during RT. Patients were followed up weekly till acute reactions subsided, then monthly till 3 months, 3 monthly till 2 years and then yearly.
The study being retrospective, we have included the whole cohort of locally advanced head-and-neck cancer patients receiving radical or adjuvant therapy during the defined period to eliminate selection bias. The study population size was based on convenience sampling. The data collection was done by mining the radiotherapy review and follow-up records. Toxicity grading was done with CTCAE 4.03.
Primary outcome of the study is incidence, cause, and predictors of early competing deaths in head-and-neck cancer undergoing curative intent therapy. Early competing deaths were defined that occurred within 6 months of treatment initiation.[7] Infection, dehydration, aspiration pneumonia, thromboembolism, renal failure, and multiorgan failure were clubbed in sepsis as a cause of death.
Statistical methods
Data were analyzed using SPSS v. 24 software (SPSS, Chicago, IL). All categorical data were summarized using frequency and percentages. All continuous data were described using mean and standard deviation or median and interquartile range based on the distribution. To study the risk factors of competing deaths in head-and-neck patients, univariate logistic regression was applied and factors which were significant was included for multivariate logistic regression. The various predictors compared were as follows: age, gender, Charlson comorbidity index (CCI), primary site, T Stage, N Stage, Stage group, RT/postoperative RT (PORT) versus RT/PORT + chemotherapy, Mucositis (Grade max), and Dysphagia (Grade max). P value is considered statistically significant at 5% level of significance for all comparisons. Kaplan–Meier method was used to plot actuarial overall survival. Time taken to overall survival was taken from date of diagnosis to date of death or last follow-up.
RESULTS
A total of 166 patients received head-and-neck irradiation from January 2013 to June 2017. One pleomorphic adenoma parotid, 14 palliative RT, and 26 early Stage (I and II) cancer were excluded from the study. A total of 125 locally advanced head-and-neck cancer were analyzed. The follow-up data were available for 117 (93.6%) patients. The median follow-up period for all surviving patients was 16 months (range 1–62 months). The median age of the study population was 58 years, and more than two-thirds were male. Cancer of oropharynx, hypopharynx, larynx and nasopharynx were clubbed into pharyngeal group and constituted half (63/125) of study cohort. Two-third of patients belong to Stage IV. A total of 82 patients received chemotherapy: 79 CCRT, 2 neoadjuvant chemotherapy followed by CCRT and 1 patient received neoadjuvant chemotherapy followed by radiation alone. Twenty-nine (23.2%) patients underwent postoperative RT and 16 (12.8%) patients received adjuvant concurrent chemoradiation. Cisplatin was used in all 81 patients receiving concurrent chemotherapy. Approximately half of the patients received weekly and half received 3 weekly concurrent chemotherapy. One-hundred sixteen patients (92.8%) received IMRT. The median radiation dose received was 6400 cGy. Grade ≥3 mucositis and dysphagia was recorded in 40% and 60% of patients, respectively. The baseline characteristics of patients, tumor, and treatment are displayed in Table 1.

A total of 51 patients died in the study population. Thirty-one patients (61%) died due to disease progression or recurrence and 20 patients (39%) died due to competing causes at a median follow-up of 16 months. Fifteen competing deaths occurred within 6 months of initiation of RT ± chemotherapy. The incidence of early competing deaths was 12% and occurred at a median time of 2.7 months from initiation of Therapy. Treatment-related toxicity was responsible for early deaths in the majority of cases. Sepsis being documented as the leading cause of early deaths (n = 13). Two patients died of cardiac event. The median time to overall survival was 33.1 months (95% confidential interval [CI]; 15.8–20.4 months) [Figure 1]. A total of five late competing death was recorded (cause: Aspiration pneumonia; 3 and Suicide; 2).
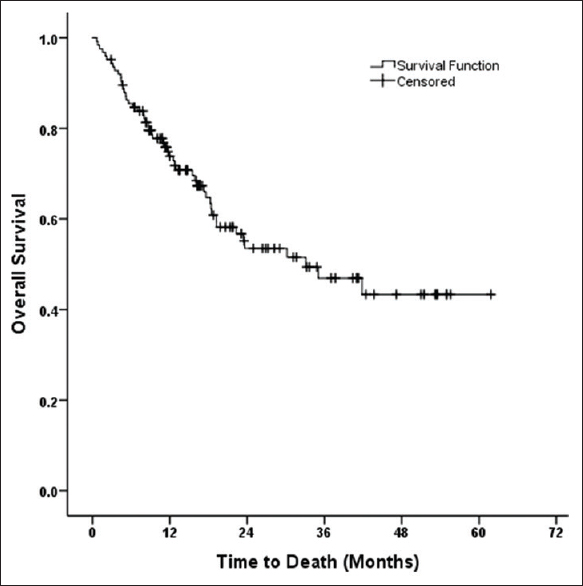
- The median time to overall survival was 33.1 months (95% confidential interval; 15.8–20.4 months)
On univariate logistic regression analysis, competing mortality was significantly associated with pharyngeal (oropharynx, hypopharynx, larynx, and nasopharynx) site primary and nonmetastatic Stage IV [Table 2]. There was a trend of association with use of concurrent chemotherapy (P = 0.120). The pharyngeal site primary (odds ratio [OR] = 4.502; 95% CI = 1.475–13.737; P = 0.008) and Stage IVA/B disease (OR = 6.645; 95% CI = 1.416–31.191; P = 0.016) maintained statistical significance with multivariate analysis.

Two patients developed second primary. One patient was diagnosed with carcinoma buccal mucosa after CRT of carcinoma oropharynx. Another patient developed carcinoma cervix after CRT of carcinoma hard palate. No deaths were recorded in 26 patients of early-stage head-and-neck cancer (excluded from study population).
DISCUSSION
In this analysis, the incidence of early competing deaths was 12% at a median follow-up of 16 months. Thirty-nine percent (20/51) of total deaths were related to competing causes. Early deaths are contributed by treatment-related toxicity at a median time of 2.7 months from initiation of RT/chemotherapy. Sepsis was the single most important cause of noncancer death in our study population. Patients with pharyngeal site primary and Stage IV A/B disease are more likely to die from competing causes. There were more deaths in concurrent chemoradiation group (n = 16) versus radiation alone group (n = 4) but was not statistically significant. We report treatment-related toxicity; namely, sepsis is the major competing cause of early deaths. The authors hypothesize that radiation-induced mucositis, dysphagia and consequent aspiration is the cause of sepsis in advanced pharyngeal primary site and probably aggravated by concurrent chemotherapy use.
Convenience sampling and retrospective setting are the main limitations of the study. The follow-up in our study is short (median 16 months) to record late deaths due to toxicity, comorbidities, or second primary. The literature is scarce regarding competing mortality in head-and-neck cancer patients receiving CCRT. The 5-year cumulative incidence of competing mortality was 19.6% in locally advanced head-and-neck cancer.[6] Most treatment-related deaths are early, i.e., during the first 6 months from treatment initiation. Out of all deaths, 15% were due to treatment-related complications, of which 9% were early deaths and 6% were late.[7] Due to poor understanding of interaction between various treatment modality, ascertaining the cause of competing mortality is another challenging task during CMT. In a recent review, early mortality of chemoradiotherapy is between 2% and 9.3%, and sepsis is the major cause of deaths occurring within 30 days from the end of treatment.[5] The present study also follows this pattern. Competing mortality was associated with female sex, increasing age, increasing CCI, decreasing body mass index, and decreasing distance to treating center.[6] Due to limited sample size of the present study, the association of these factors with competing death was not demonstrable.
Cancer patients (all cancer combined) are likely to have 50% more risk of noncancer deaths compared with general population.[11] With aggressive combined modality approach and resulting better local control, the author expects noncancer deaths to be a raising problem in locally advanced head-and-neck cancer. As CCRT is standard, direct comparison with radiation alone cannot be done at present time. In an RCT, comparing RT alone versus CCRT, overall survival did not improve. This reflects effective salvage surgery and competing deaths, where 47% (27 out of 58 deaths) died of noncancer causes.[12] The 10-year update of RT oncology group 91–11 showed similar overall survival but more cancer deaths in RT group and more noncancer deaths in CCRT group.[13] Even though more competing deaths are associated with CMT, there are no real alternative to CCRT in locally advanced head-and-neck cancer. A systemic review and meta-analysis concluded that no form of alternate fractionation can compensate for lack of concurrent chemotherapy.[14] Therefore, research should focus on decreasing competing deaths especially treatment-related toxicities. This can be achieved by better selection of patients, active surveillance of sepsis and implementation strategies for CCRT in real-world setting. Pretreatment patient factors like age >60 years, female gender, Charlson comorbidity score ≥3, low body mass index, the Karnofsky performance status ≤80, weight loss >5%, and abnormal renal function are more prone for competing deaths and must be considered before deciding for CCRT.[615] In carcinoma of larynx, patient with extensive T3, T4 lesion, nonfunctional larynx and documented aspiration should be offered surgery and postoperative RT instead of CCRT.[16] Monitoring of systemic inflammatory response syndrome, active surveillance for sepsis and tissue hypoperfusion during and after CCRT, can decrease sepsis-related death in a potentially curative patient.[5]
CONCLUSION
Competing mortality is one of the multifaceted problems in locally advanced head-and-neck patients. Sepsis being single most cause of early deaths in locally advanced pharyngeal and laryngeal cancer undergoing CMT. Patient selection and active surveillance of sepsis might be key to decrease early competing causes of deaths.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4-14.
- [Google Scholar]
- Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25:4096-103.
- [Google Scholar]
- Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-52.
- [Google Scholar]
- Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-44.
- [Google Scholar]
- Sepsis in head and neck cancer patients treated with chemotherapy and radiation: Literature review and consensus. Crit Rev Oncol Hematol. 2015;95:191-213.
- [Google Scholar]
- Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol. 2010;28:15-20.
- [Google Scholar]
- Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004;10:1956-62.
- [Google Scholar]
- Second malignant neoplasms and cause of death in patients with germ cell cancer: A Danish nationwide cohort study. JAMA Oncol. 2016;2:1624-7.
- [Google Scholar]
- Analysis of competing risks of causes of death and their variation over different time periods in Hodgkin's disease. Clin Cancer Res. 2008;14:5300-5.
- [Google Scholar]
- Apopulation-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2:88-93.
- [Google Scholar]
- Non-cancer mortality among people diagnosed with cancer (Australia) Cancer Causes Control. 2006;17:287-97.
- [Google Scholar]
- Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer. 2000;88:876-83.
- [Google Scholar]
- Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845-52.
- [Google Scholar]
- Systematic review and meta-analysis of conventionally fractionated concurrent chemoradiotherapy versus altered fractionation radiotherapy alone in the definitive management of locoregionally advanced head and neck squamous cell carcinoma. Clin Oncol (R Coll Radiol). 2016;28:50-61.
- [Google Scholar]
- Predictive factors of survival and treatment tolerance in older patients treated with chemotherapy and radiotherapy for locally advanced head and neck cancer. Oral Oncol. 2015;51:521-8.
- [Google Scholar]
- Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2018;36:1143-69.
- [Google Scholar]






