Translate this page into:
Asymptomatic Cardiac Metastasis in a Diagnosed Case of Squamous Cell Carcinoma of the Middle Third of Esophagus
Address for correspondence: Dr. Sarbani Ghosh Laskar, Room 1125, 13th Floor, Homi Bhabha Block, Department of Radiation Oncology, Tata Memorial Centre, Mumbai - 400 012, Maharashtra, India. E-mail: sarbanilaskar@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A lady walks in with complaints of dysphagia mostly to solids to begin with and gradually progressive to liquids requiring naso-gastric tube feeding, with history of vomiting after taking food and weight loss of 20kilograms over 2months. Upper gastrointestinal endoscopy showed an ulceroproliferative growth starting at 28cms from the central incisor and extending upto 36 cms with luminal compromise. Biopsy from the lesion was found to be moderately differentiated squamous cell carcinoma. As part of metastatic work-up a PET-CT (Figure 1 shows lesion in the left ventricle) was done which revealed a metabolically active lesion involving the lower third of esophagus and a metabolically active lesion in the wall of the left ventricle which was the only site of metastatic diseae(Figure 2. Maximum intensity projection). Further investigations were done for characterisation of the cardiac lesion. 2-D Echo cardiography was done and was normal with an ejection fraction of 60%. A cardiac MRI was done which showed a soft tissue mass in the wall of the left ventricle which was isointense with normal myocardium and hyperintense on double inversion recovery sequence which measured 3.2 x 3 cms with post contrast enhancement. She was planned for palliative radiotherapy. A total dose of 30Gy in 10 fractions over 2 weeks was delivered. She tolerated the treatment well with Grade I mucositis (as per RTOG grading) and on follow-up after 4 weeks she had 40% relief in dysphagia and could take semi-solid food with little difficulty.
Keywords
Cancer
cardiac metastasis
esophagus
INTRODUCTION
Metastatic squamous esophageal cancer has a poor prognosis with median overall survival of 12 months.[1] Palliative radiotherapy and/or chemotherapy are not known to provide any advantage in terms of survival but may improve quality of life in selected patients, owing to their palliative qualities.[2] Cardiac metastasis from squamous carcinoma of esophagus is a rare event. Heart metastases are mostly clinically silent and are mostly diagnosed at autopsy.[34] A tumor could spread to the heart through different routes such as direct cardiac extension, bloodstream, lymphatic system, or intracavitary diffusion. Identification of the path of diffusion can be made on the basis of which cardiac structures are primarily affected: for example, pericardial involvement is due to lymphatic spread or even direct extension, while myocardial and endocardial metastases are the result of the heart chambers’ being invaded through the bloodstream. The distinction regarding the metastatic pathway is based on a clinical evaluation of the structure of the heart involved or postmortem examination.[4] Considering that most studies on the incidence of cardiac metastases are based on autopsies, yet no in vivo evidence exists as to a possible correlation between hematogenous spread and cardiac metastases.
We report the case of a 38-year-old female who presented with squamous cell carcinoma of the middle third of esophagus with cardiac metastasis who was asymptomatic for the same. She was treated with palliative intent to relieve dysphagia.
CASE REPORT
A female walks in with complaints of dysphagia mostly to solids to begin with and gradually progressive to liquids requiring nasogastric tube feeding, with history of vomiting after taking food and weight loss of 20 kg over 2 months. On further history taking, it was found that she neither had any comorbidities nor any habits (e.g., tobacco chewing/smoking). She did not have any complaints of chest pain/breathlessness/syncope. Upper gastrointestinal endoscopy showed an ulceroproliferative growth starting at 28 cm from the central incisor and extending up to 36 cm with luminal compromise. Biopsy from the lesion was found to be moderately differentiated squamous cell carcinoma. As part of metastatic workup, a positron emission tomography-computed tomography (PET-CT) [Figure 1] was done which revealed a metabolically active lesion involving the lower third of esophagus with a distinct fat plane between the growth and descending aorta and few enlarged metabolically active lymph nodes in gastrohepatic region and subcarinal region, and a metabolically active lesion in the wall of the left ventricle which was the only site of metastatic disease [Figure 2]. Further investigations were done for characterization of the cardiac lesion. Two-dimensional (2D) echocardiography was done and was normal with an ejection fraction of 60%. A cardiac magnetic resonance imaging (MRI) was done which showed a soft-tissue mass in the wall of the left ventricle which was isointense with normal myocardium and hyperintense on double inversion recovery sequence which measured 3.2 cm × 3 cm with postcontrast enhancement. Invasive procedure to prove the metastatic lesion was not done since the intent of treatment was palliative and cardiac MRI was conclusive [Figure 3]. She was planned for palliative radiotherapy. She was simulated in the supine position with intravenous contrast and thoracic arm rest, and CT scan of 5 mm thickness was taken. Gross tumor volume (as per ICRU 50) included the entire visible lesion of the middle-third esophagus and the involved subcarinal and gastrohepatic nodes [Figure 4]. Clinical target volume (as per ICRU 50) included the gross tumor volume and the cardiac lesion, with a 1 cm margin. Planning target volume consisted of clinical target volume with a 5 mm margin [Figure 5]. A total dose of 30 Gy in 10 fractions over 2 weeks was delivered [Figure 6] using anteroposterior portals with lung blocks on a Telecobalt machine (ELITE-80). She tolerated the treatment well with Grade I mucositis (as per RTOG grading), and on follow-up after 4 weeks, she had 40% relief in dysphagia and could take semisolid food with little difficulty. She was due for her follow-up after 2 months but did not turn up, on telephonic contact with the family members; it was found that she had expired 3 months after treatment completion.
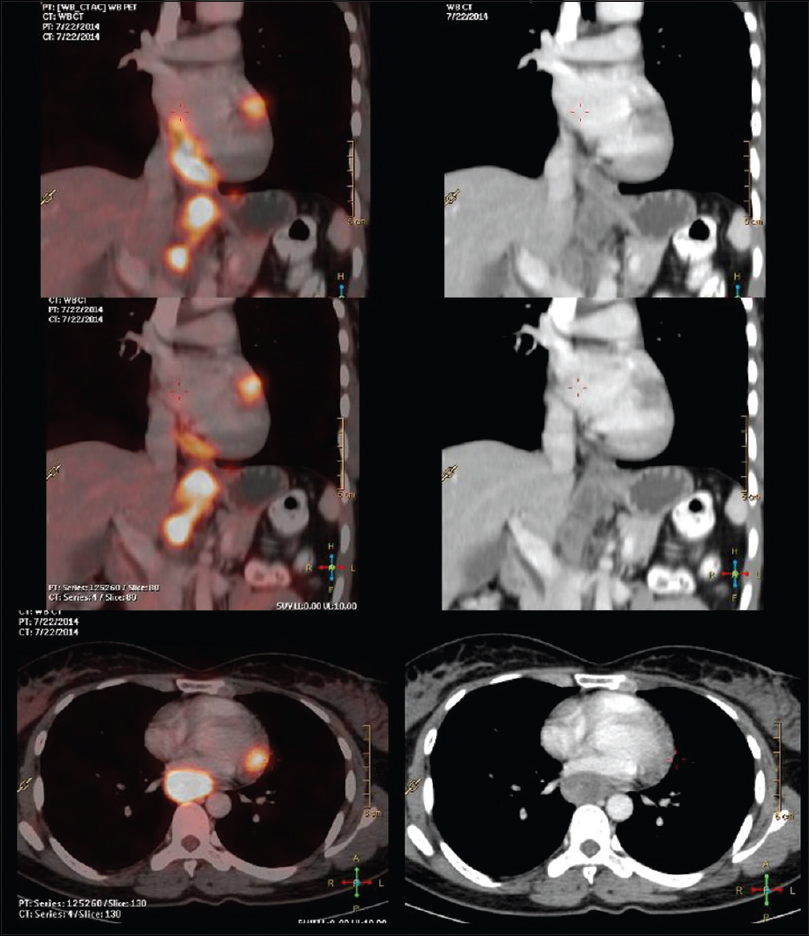
- Positron emission tomography-computed tomography shows lesion in the left ventricle

- Maximum intensity projection

- Cardiac magnetic resonance imaging

- Planning computed tomography

- Beams eye view

- Anteroposterior-posteroanterior portals with corner blocks showing 95% dose wash
DISCUSSION
Primary tumors of the heart are rare, occurring at a frequency of 0.02% in pooled autopsy series.[5] Histologically, three-quarters of primary heart tumors turn out to be benign, almost half of them being myxomas.[6] Whether benign or malignant, the majority of primary cardiac tumors are intracavitary and preferentially develop in the left atrium, thereby leading to left ventricular inflow obstruction. Embolism is also common. Cardiac metastases were found in up to 25% of postmortem patients who had died of malignancies.[7] Secondary or metastatic heart tumors occur comparatively more frequently, with an at least 100 times higher incidence than primary tumors of the heart.[8] Intracavitary growth of secondary heart tumors, however, is unusual. Therefore, despite their frequency, metastatic heart tumors only rarely gain clinical attention, may be because they are asymptomatic. However, signs of cardiac involvement are often overlooked since the symptoms of disseminated tumor disease prevail. Thus, like primary tumors of the heart, metastases may imitate valvular heart disease or cause cardiac failure, ventricular or supraventricular heart rhythm disturbances, conduction defects, syncope, embolism, or, quite often, pericardial effusion. Not infrequently, cardiac tumor invasion contributes to the mechanism of death in affected patients.[9] The most common tumors which metastasize to heart are carcinomas of the lung, breast, esophagus, malignant lymphoma, leukemia, and malignant melanoma.[710] Malignant melanomas frequently metastasize to the heart and represent the tumor with the highest rate of cardiac metastases (in more than half the cases).[11] Lymphatic spread tends to give rise to pericardial metastases; hematogenous spread preferentially gives rise to myocardial metastases. Only rarely are endocardial tumor deposits found. Secondary heart tumors that have partial or total intracavitary growth are very rare; when they do occur, they are often covered by thrombotic material.[12] Extracardiac tumors may also reach the atria and even the chambers of the heart by transvenous extension. Intraluminal growth of renal cell carcinoma (hypernephroma) through the vena renalis and vena cava inferior into the right atrium (in 1% of these tumors) has been reported.[13] Cardiac metastases usually remain clinically silent, particularly as the vast majority of cardiac metastases are small. Only about one-tenth of patients who died of disease with cardiac spread at postmortem examination presented with symptoms or findings indicative of cardiac involvement.[14] Heralds of metastases to the heart are a rapid increase in heart size by pericardial effusion, new signs of heart failure or valve disease, conduction defects, and atrial or ventricular heart rhythm disturbances.[14] Symptoms such as dyspnea or tachypnea and clinical findings such as systolic heart murmur, peripheral edema, pleural or pericardial effusion, or ascites, however, may also be the result of tumor-associated anemia and hypoproteinemia, or of lung metastases. Hypotension, dyspnea and peripheral cyanosis, pulsus paradoxus (systolic blood pressure decreases by >10 mmHg during inspiration), and venous congestion are also hallmarks of clinical diagnosis. The method of choice to detect cardiac metastases and their complications, however, is 2D echocardiography,[15] with advances in imaging cardiac MRI has become one of the sensitive tests for detecting cardiac metastasis. PET-CT has also emerged as a sensitive modality for detecting cardiac metastasis.[16] Differential diagnosis must include thrombus, vegetation, and a foreign body.
Surgical resection is only indicated in exceptional cases of solitary intracavitary heart metastases, leading to obliteration of cardiac chambers or valve obstruction if the tumor of origin was surgically resected in toto and the patient appears to have a good prognosis; valve replacement and pace maker implantation are other options. Few respond well to radiotherapy and chemotherapy. Chemotherapeutics or radioisotopes may be instilled through the catheter to prevent recurrence of the effusion (by direct cytostatic and/or by sclerosing activity with adhesion of the visceral and the parietal pericardium as a result of inflammatory reactions).[17] However, instillation of antitumor agents or tetracycline derivatives can result in severe pain, arrhythmias, and bone marrow toxicity, and in unpredictable sclerotic processes. Radioisotopes have been shown to be safe, without noteworthy side effects and of high efficacy, but the technical expenditure is high.[318] There are no guidelines for radiotherapy to cardiac metastasis regarding volume, dose, and fractionation.
CONCLUSION
Cardiac metastases are rare events and are silent in most of the cases. In the absence of any other metastatic sites, its diagnosis has an impact on intent of treatment. 2D echocardiography, cardiac MRI, and PET-CT are investigations with high sensitivity and specificity, but it is important to choose one among them keeping in view the economics. Treatment in the presence of cardiac metastasis is dictated both by the profile of the locoregional disease and the pattern of metastatic disease in the patient. In the presence of locoregionally advanced disease with multiple systemic metastases, the intent is usually palliative, directed toward palliation of symptoms. Rarely in the event of solitary metastases to the heart with low volume locoregional disease, more aggressive protocols employing multimodality treatment strategies maybe carried out. Despite this, the prognosis is poor with a short life expectancy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122:1118-29.
- [Google Scholar]
- Survival and symptom relief after palliative radiotherapy for esophageal cancer. J Cancer. 2016;7:125-30.
- [Google Scholar]
- Myocardial metastasis from squamous cell carcinoma of the esophagus. Gen Thorac Cardiovasc Surg. 2009;57:440-5.
- [Google Scholar]
- Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027-31.
- [Google Scholar]
- Tumors of the Cardiovascular System, Atlas of Tumor Pathology. 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology; 1996.
- Metastatic and invasive tumours involving the heart in a geriatric population: A necropsy study. Virchows Arch A Pathol Anat Histopathol. 1991;419:183-9.
- [Google Scholar]
- The heart in malignant melanoma. A study of 70 autopsy cases. Am J Cardiol. 1968;21:555-71.
- [Google Scholar]
- The diagnosis of intracardiac metastasis of colon carcinoma by radioisotopic and roentgenographic studies. Am J Cardiol. 1970;26:300-4.
- [Google Scholar]
- Successful extraction of intracardiac tumor thrombus of renal carcinoma. J Urol. 1977;118:474-5.
- [Google Scholar]
- Metastatic tumors of the heart detected by two-dimensional echocardiography. Am Heart J. 1985;109:343-9.
- [Google Scholar]
- Asymptomatic myocardial metastasis from cancers of upper aero-digestive tract detected on fdg pet/ct a series of 4 cases. Cancer Imaging. 2014;14:16.
- [Google Scholar]
- Malignant pericardial diseases: Diagnosis and treatment. Am Heart J. 1987;113:785-90.
- [Google Scholar]
- Treatment of malignant pericardial effusion with 32P-colloid. Br J Cancer. 1999;80:1955-7.
- [Google Scholar]






