Translate this page into:
A New Perspective of Neuromyopathy to Explain Intractable Pancreatic Cancer Pains; Dry Needling as an Effective Adjunct to Neurolytic Blocks
Address for correspondence: Dr. Lakshmi Vas; E-mail: lakshmi@paincareindia.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We present a new perspective of neuromyopathy in pancreatic cancer pain (PCP) referral to bodywall; proposal of new rationale to include ultrasound guided dry needling (USGDN) of body wall muscles as an effective adjunct to neurolytic coeliac plexus block (NCPB) or splanchnic nerve radiofrequency ablation (SRF) for comprehensive interventional management. Methods: PCP response to SRF in 2 patients and NCPB in 3 patients was documented on numerical rating scale (NRS) on post procedure days 3 and 15. If the residual pain was >5 NRS on day 15, USGDN of abdominal and back muscles was started on a thrice weekly basis. The response to USGDN documented on day 30 after approximately 6 sessions of DN, showed a significant pain reduction (0-2 NRS) with 50% reduction of pre-treatment opioid consumption. This was sustained at 6 months or till their demise. Convergence of visceral and somatic nerves at the dorsal horn (viscerosomatic neurons) causes referral of visceral pain to the back and abdominal muscles. This leads to formation of myofascial trigger points (MTrPs) in the muscles which sets up a parallel network of sensitized peripheral and central motor nociceptive processing (neuromyopathy). USGDN specifically addressed the MTrPs that develop as an epiphenomenon of self-perpetuating neuromyopathy while SRF/NCPB, analgesics and neuromodulators could address only visceral nociceptive afferents (pain mediated through celiac plexus) which forms a meagre 10% of the total spinal cord afferent input. Thus, we conclude that combination of neuromyopathy and viscerosomatic convergence in PCP indicate a specific role for DN as an adjunct to SRF/NCPB in our patients
Keywords
Myofascial trigger points
Neuromyopathy
Pancreatic cancer pain
Ultrasound guided dry needling
Viscerosomatic convergence
INTRODUCTION
The course of pancreatic cancer (PC) is characterized by severe intractable pain. As recommended by Christo and Mazloomdoost (2005),[1] Tay and Ho (2009),[2] Sharfman and Walsh (1990);[3] the treatment of advanced PC is palliative care with medications by World Health Organization's Pain Relief Ladder and if necessary, interventions such as neurolytic celiac plexus block (NCPB) or radiofrequency (RF) ablation of splanchnic nerves (SRF), which is considered as step 4 in the analgesic ladder. Yan and Myers (2007)[4] conducted a review of literature for RCTs conducted from 1996 to 2005 to compare the efficacy of NCPB with standard treatments in pancreatic cancer pain (PCP). They concluded that the data available on NCPB for PCP were insufficient to judge for efficacy, long-term morbidity, or cost-effectiveness, since NCPB shows only a minimal clinical significance in improving pain control, reduced narcotic usage, and constipation when compared with medical management.
The neural pathways in PCP involve celiac plexus, as well as body wall innervations.[5678] A variety of pathophysiological processes have been identified to explain the referral of pain to parietal somatic structures of the body wall. NCPB/SRF targets only the pain mediated through celiac plexus but has no effect on the referred pain to the body wall, which persists even after NCPB/SRF.
Our therapy protocol for all abdominal pains in the last 10 years is a combination comprising SRF/NCPB to address visceral nociceptive nerve supply and ultrasound guided dry needling (USGDN) of the abdominal wall and back muscles to address the referred pain to the muscles of the body wall. We report its efficacy in achieving a comprehensive management of PCP in 5 patients specifically documented for the purpose of this report.
CASE REPORTS
Table 1 shows a brief description of the case presentation, medications, Numerical Rating Scale (NRS) of the pain, and quality of life scores (Short form-12 version 2 of Short form- 36 (SF-36) of health survey as formulated by Ware et al.[9]) of our patients. Patients’ consent was taken for SRF/NCPB as well as USGDN (an established treatment to relieve myofascial trigger points [MTrPs] known to cause myofascial pains) of the pain in abdominal and back muscles, if the pain 15 days after SRF/NCPB was more than 5 NRS despite continued opioid and neuromodulator medications. A thorough explanation of risks and expected outcomes of all the three procedures were given.
METHODS
The patients were positioned prone on the radiolucent table in the operation theater for SRF/NCPB. An intravenous line was secured for administration of a liter of Lactated Ringers solution, as well as 100 ml of paracetamol 10 mg/ml and tramadol 50 mg.
SRF procedure
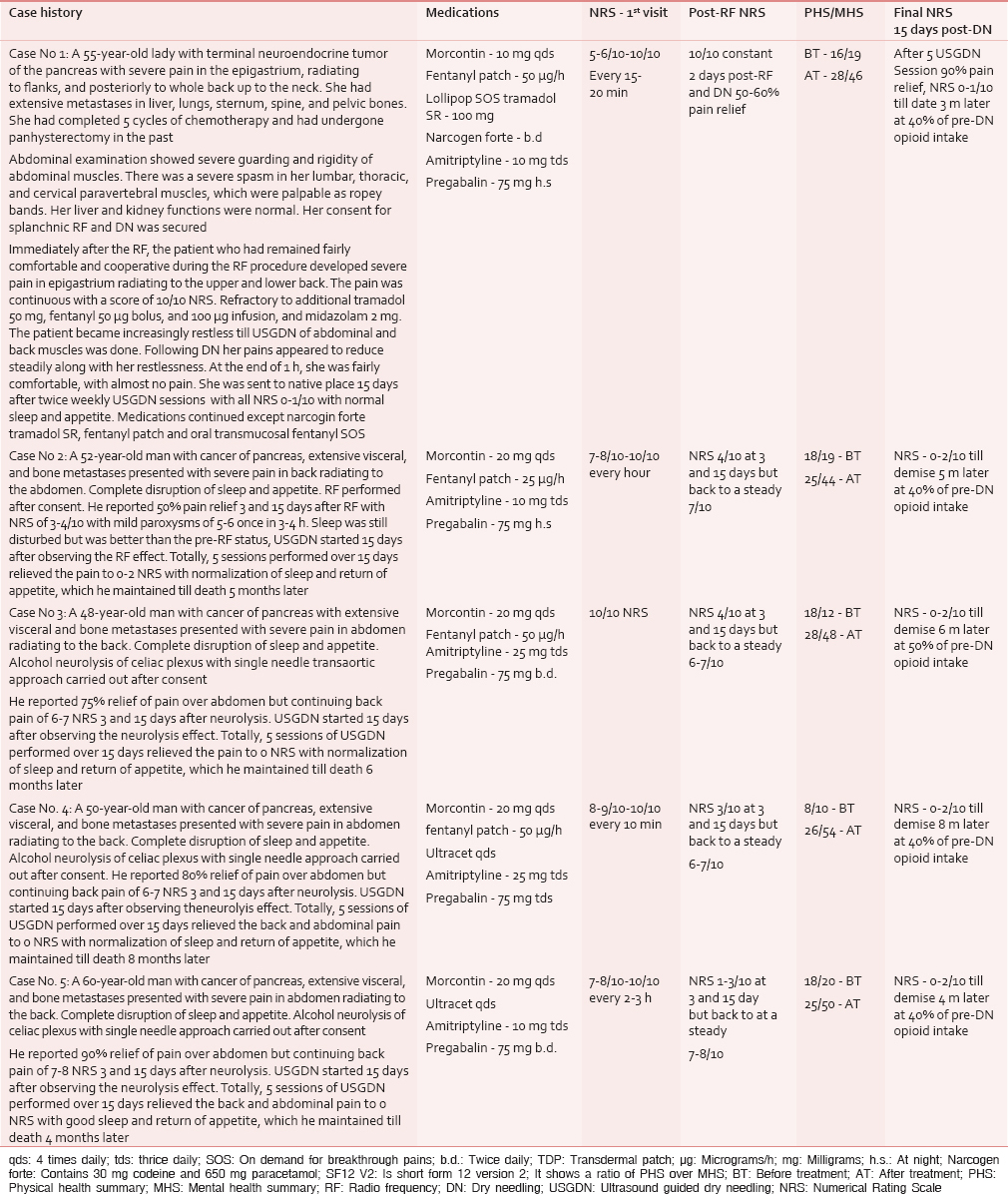
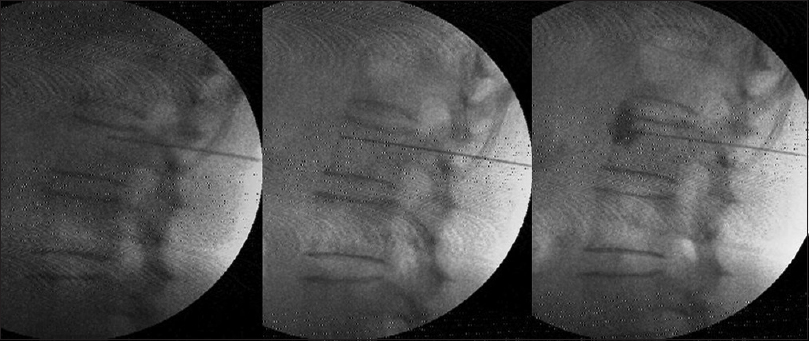
Under C-arm guidance, three 15 cm, 22-gauge RF cannulae (Cosman [cannula] RFK TM) were positioned so that the exposed curved active tip of 10 mm, lay at the anterior one-third of the vertebral bodies of 11th and 12th thoracic, and first lumbar vertebrae [Figure 1]. The tip position was reconfirmed with the spread of the nonionic dye Iohexol (Omnipaque® 300 mg l/ml GE Healthcare, Shanghai, China). A positive response to sensory stimulation at 0.6 V at 50 Hz (denoted by sensation of vibration in epigastrium) and a negative response to motor stimulation at 2.0 V at 2 Hz (denoted by the absence of visible twitches in abdomen or back or lower extremity) were documented. This was followed by injection of 2 ml of 2% lidocaine before activation of the RF generator (Cosman Medical Inc., Burlington, MA 01803, USA) for 2 min at 80°C. The procedure was repeated on the other side.
- Transaortic approach to celiac plexus
Transaortic neurolytic celiac plexus block procedure
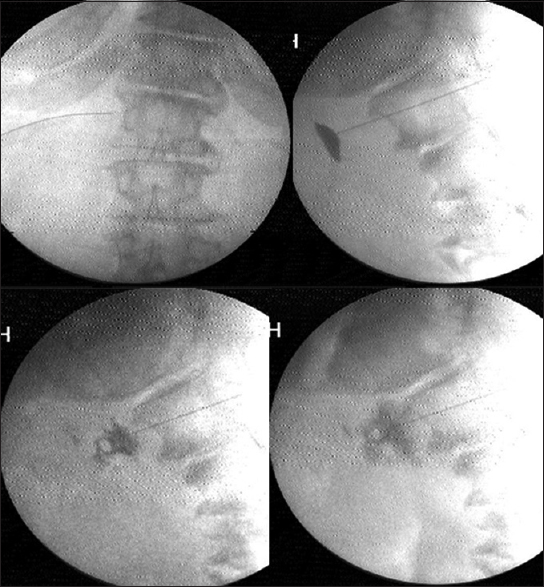
Under C-arm guidance a 15 cm, 22-gauge Quincke needle was positioned at the antero-lateral instead of anterior lateral aspect of the vertebral body of L1 on the left side. The needle was advanced anteriorly till the back flow of arterial blood through a three way extension tubing was visualized. The needle was advanced till the back flow stopped, just as the needle exited the aorta with a gentle pop. The tip position was reconfirmed with the spread of iohexol around the pulsating aorta in lateral view [Figure 2]. Once the predominantly anterior periaortic spread was confirmed, 5 ml of lignocaine was injected. Five minutes later, after confirmation of pain reduction and well-maintained vital parameters, 15–20 ml of absolute alcohol was slowly injected. The alcohol was flushed out with another milliliter of lignocaine prior to needle withdrawal through the aorta and the paravertebral tissues. The vital parameters, bowel movements, and pain relief were monitored for another 2 h prior to discharge.
- Splanchnic radiofrequency at T12
The response of pain to SRF/NCPB was assessed on post procedure days 3 and 15. The first assessment was done on day 3 when patients were able to perceive the effect of SRF/NCPB after the procedural pains had subsided. The second assessment was done at 15 days as presumably the neuritic phenomena from the thermal effects of RF would have subsided by then,[10] enabling the patient to appreciate the extent of relief from SRF.
On day 15, if the residual pain was >5 by NRS, USGDN of abdominal and back muscles was started on a thrice weekly basis. The rationale the 15 days wait was to confirm the effect of SRF/NCPB prior to starting another intervention like DN.
The patient was put in a lateral position with the painful side up, and the back and abdomen were cleaned with betadine. The linear 6–13 MHz probe of Sonosite TM MSK (USA) was used to guide solid 32-gauge needles of 25–50 mm length into all the abdominal and paravertebral muscles that form the ellipse of abdomen [Figures 3a and b]. The needle trajectory into the relevant muscle was visualized on the US as shown in Figure 3c–e. These needles can be clearly visualized in-plane with experience. The tip of the needle seen as bright spots in out-of-plane view was constantly kept in view to confirm entry into specific muscles, as well as avoid accidental needle injury to underlying peritoneum and viscera.
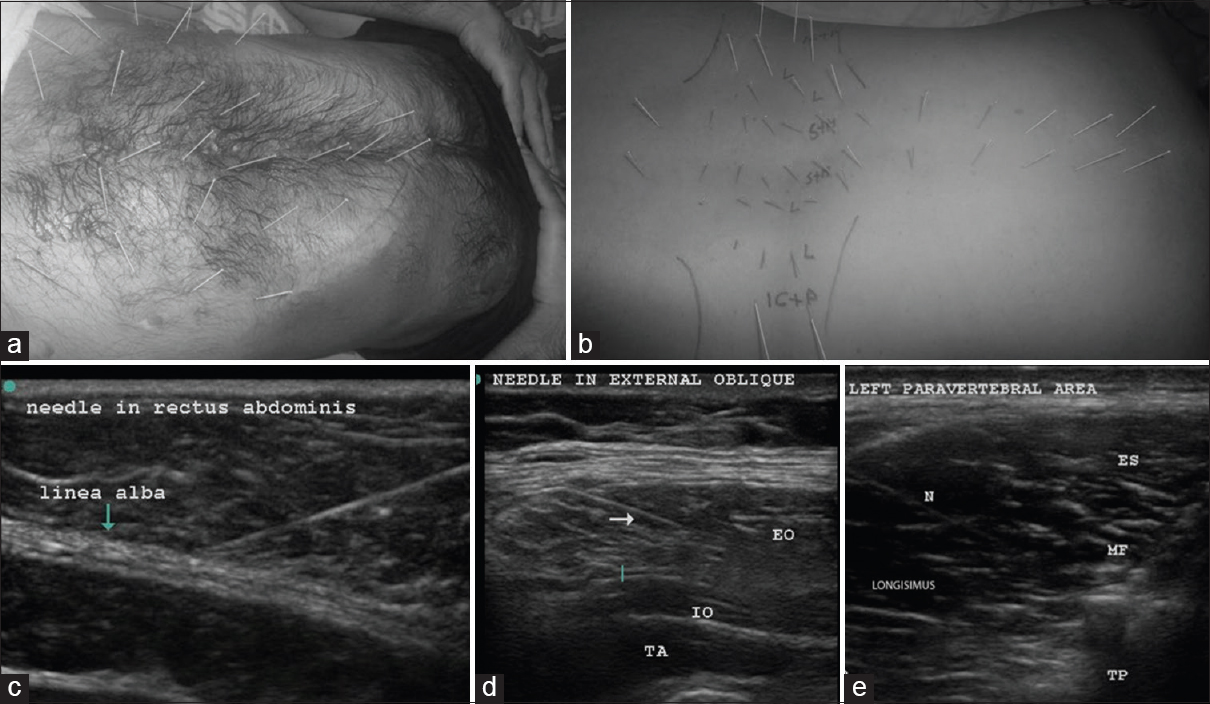
- Upper row; abdominal muscle DN (a); in the abdominal wall about 7 pairs of needles were placed in rectus abdominis of each side equidistantly between the xiphisternum to the pubis. Three pairs were supraumbilical, 3 pairs were infraumbilical, and 1 pair was on either side of the umbilicus. About 3–4 needles were placed at a distance of 3–4 cm lateral to the needles in rectus abdominis, and another 3–4 needles were placed a further 3–4 cm lateral to the latter to target external and internal oblique muscles, as well as transversus abdominis between the costal margin and the inguinal ligament. The dark stains are betadine used as a medium for USG probe. The costal margin is marked with a blue line. Paravertebral muscle DN (b); the point of insertion was about 1 cm lateral to the spinous process on either side and medial to the facet for the spinalis and multifidi. An out of plane USG was used to visualize the needle tips clearly in the spinalis. The Figures b and e shows the length of the needle “in plane” in the longissimus. Longissimus and iliocostalis part of erector spinae were needled 4–6 cm lateral to the spine below the costal margin. The psoas at L3-5 on either side was visualized to place 3–4 needles in the muscle mass by starting far laterally so that the needle could slip beneath the transverse process (6–8 cm from the spinous process). The muscles targeted by the needles are marked on the figure as follows: S + M – Spinalis + Multifidus, L – longissimus, IC + P – Iliocostalis + Psoas. The costal margin and the iliac crests are also marked, the second row; USGDN: Needles (indicated by N or arrow) are visualized in rectus abdominis (c) EO: External oblique; (d) IO: Internal oblique, TA: Transversus abdominis, ES: Spinalis part of erector spinae, MF: Multifidus, TP: Transverse process; N: Needle in the longissimus (e)
The paravertebral muscles including psoas were also visualized from a T3-4 level down to the origin of erector spinae on the sacrum to place needles in the muscle mass [Figure 3b].
In the abdominal wall, about 7 pairs of needles were placed in rectus abdominis equidistantly from the xiphisternum to symphysis pubis [Figure 3a]. Care was taken to visualize these needles in plane superficial to the easily visible peritoneum and gut. The needles were kept in situ for 20–30 min and then removed.
RESULTS
In Table 1, patient 1 [Table 1] complained of a severe exacerbation of pain immediately post-SRF. She was given intravenous tramadol 100 mg followed by fentanyl 50 μg as bolus along with midazolam 2 mg. A further 150 μg of fentanyl was added in the infusion, but her pain continued to escalate over the next 1 h. Hence, an emergency USGDN session was performed with the assumption that this pain crescendo was a myofascial pain syndrome (MPS) from an exaggerated referral to abdominal muscles, since the visceral component had just been addressed by SRF. This immediately reduced her pain to 3–4 NRS. Subsequently, she continued to receive thrice weekly USGDN for next 2 weeks when she reported 0–1 NRS pain.
Patients 2–4 [Table 1] reported a persistent guarding, rigidity, and pain of at/above 5 NRS 15 day post-SRF/NCPB, and hence, USGDN was started. Our patients reported significant pain relief (to 0–2 NRS), within a week (after 3 DN sessions). Opioid consumption was reduced to 50% of the pre-treatment intake. We did DN for another week to ensure that most/all MTrPs were resolved.
Thus, we started DN 15 days after SRF/NCPB and did 6 DN sessions over 15 days, thereby completing the whole treatment within 30 days. All the patients reported continued pain relief at 6 months or until their demise.
DISCUSSION
PCP is a complex syndrome caused by many mechanisms. About 70–85% patients report severe neuropathic pain resulting from PC.[1112] Metastases and treatments such as chemotherapy and radiation can also result in pain.[1314] Neuropathy in PC starts with tumor spread along pancreatic nerves causing nerve damage, loss of neural sheath, and stretching of neural tissue at local ganglia up to the celiac axis. The concomitant release of neurolytic pancreatic enzymes causes pancreatitis. The increase in interstitial and ductal pancreatic pressure induces pancreatic ischemia or “pancreatic compartment syndrome.” All these chemical and mechanical stimuli lead to peripheral and subsequent central sensitization.[15] Hitherto “silent” mechanically insensitive afferents acquire mechanosensitivity to further increase the nociceptive barrage.[16171819]
Nociceptive afferent A delta and C sensory fibers accompany both sympathetic and parasympathetic ramifications on the viscera into the celiac plexus, which acts as a relay station for the sympathetic, parasympathetic, and intrinsic visceral afferents. The greater (T5-T9), lesser (T10-T11), and the least splanchnic (T12) nerves carry the nociceptive fibers from celiac plexus to their cell bodies in laminae 1 and 5 of the dorsal horn through the white rami.[2021]
“Phenotype switch” described by Sengupta,[18] and Regan and Peng[22] is a second mechanism, whereby there is axonal sprouting of A beta fibers into nociceptive C-fiber locations with an expression of nociceptive mediators such as substance Pfrom A beta fibers. Consequently, the low-threshold information from large A beta afferents that is normally perceived as touch may now be misinterpreted by the nervous system as pain.[2223] This leads to a state of hyperexcitability in the “wide dynamic range” neurons of dorsal horn with multisegmental spread of noxious input, which eventually overwhelms the “gate control” allowing the sensitization to proceed to cortical centers.[212324] The altered supra-spinal facilitatory and inhibitory impulses also modulate the dorsal horn, further aggravating the symptoms.[252627] Thus, in “centrally sensitized” visceral pain states, there is a generalized amplification of pain even in the reciprocally innervated body wall structures.[5192223252628]
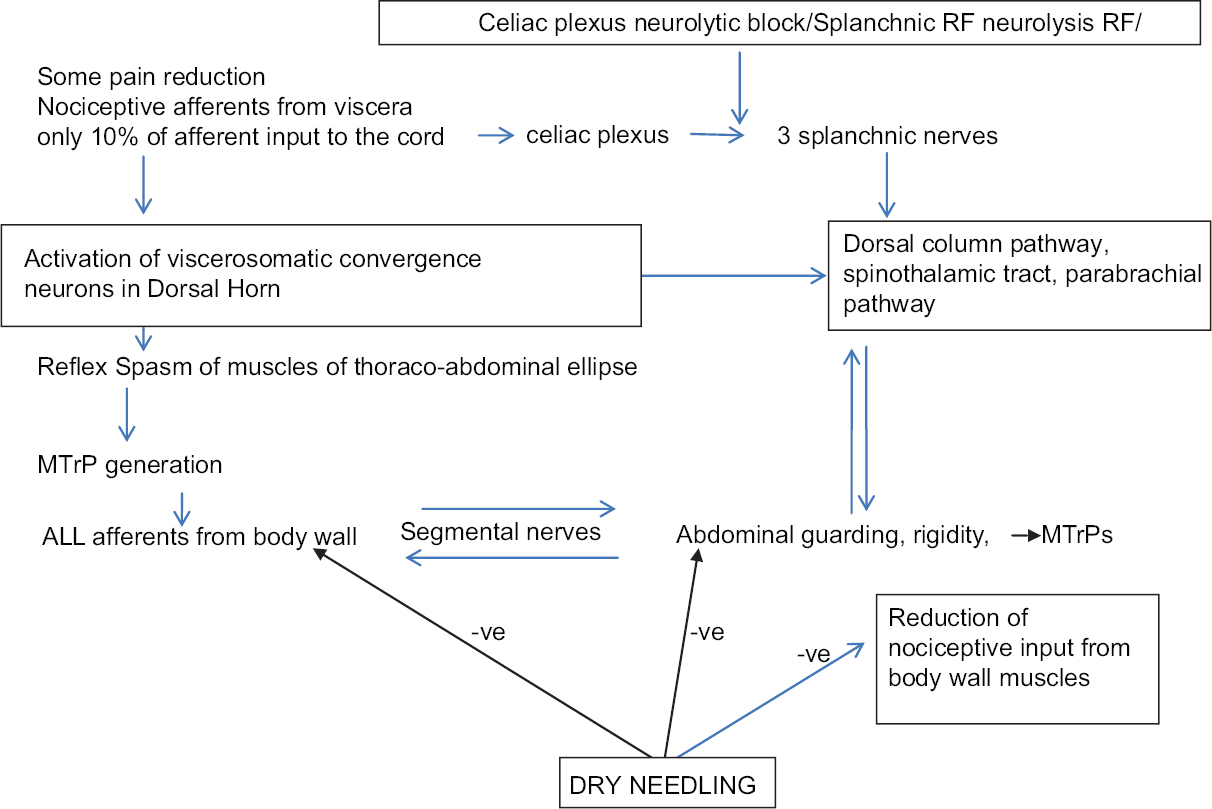
The third and the most important mechanism in PCP is “viscerosomatic convergence,” which is the rule in visceral pain. All dorsal horn cells that receive input from the viscera also receive input from receptors in the body wall (viscerosomatic neurons).[516171825] Further, the relative contribution of visceral afferent fibers to the total spinal cord afferent input is < 10% while somatic afferent fiber contribution from the body wall muscle is abundant, paving the way for visceral nociception to be referred to the body wall. Thus, true visceral pain from pancreas forms only a small component of the severe pain experienced by the patient. The larger component of the pain perceived by the patient results from the referred pain to the muscles of abdomen and back, or extremities, and creates a regional MPS. As described by Sikandar and Dickenson,[5] and Gerwin,[25] the genesis of MPS starts with the formation of MTrPs in corresponding somatic segments of referred visceral pain. Mechanisms such as dichotomizing or split sensory fibers, afferent-afferent interactions with orthodromic and antidromic impulses, and sympathetic reflexes to the skin causing fluid extravasation and edema have been proposed to explain the trophic changes and formation of MTrPs. Initial sustained muscle contraction/tightness (which may be visible as a taut band), causes latent MTrPs, which are not necessarily painful. However, the latent MTrPs once formed progress to active MTrPs,[29] which are painful and respond with severe exacerbation of pain with each triggering wave of visceral nociception. Active MTrPs are the areas of increased spontaneous electrical activity (SEA) or end-plate noise (EPN).[30] The increase in EPN results in a reduced pain threshold and increased pain intensity as described by Kuan et al. (2007).[31] MTrPs become an independent source of persistent nociceptive input to the spinal cord, which is reported to be especially effective in inducing neuroplasty in the dorsal horn.[2329] This muscle nociception is independent of the viscerosomatic convergence and maintains a parallel central sensitization.[24262932] In PCP, the shared innervation between pancreas and muscles leads to MPS in these muscles. The fascia covering abdominal muscles is in anatomical continuity with thoraco-dorsal fascia enveloping the back muscles.[20] Tightening of the abdominal ellipse formed by muscles, fascia, and skin is perceived as abdominal guarding and rigidity [Figure 4]. Sensitized muscle nociceptors in the abdominal and back muscles with a lowered stimulation threshold manifest with symptoms such as allodynia and mechanical hyperalgesia with muscle movement (on breathing, coughing, movements, etc.). Thus, a vicious cycle of combined visceral and parietal nociception sets in, leading to abdominal and back muscle spasm, which in turn leads to more pain, and more sensitization (diagrammatically represented in Figure 5). Descriptions by Sikandar and Dickenson,[5] Chen and Wai,[15] Regan[22], and Gerwin[25] confirm our observation that the referred pain and muscle hyperalgesia in PCP occurs early, is accentuated by repeated episodes of colic, and remains long after the original visceral nociceptive stimulus has resolved.

- a) Anterior nerve root; (b) Cadaveric dissection, lateral lamina removed; (c) Main nerve and anterior ramus sandwiched between psoas and intertransversarius muscles; (d) MRI - Muscles sandwiching the nerve root
- Diagrammatic representation of the way body wall muscles become the expressor organs of visceral pain
We have proposed the concept of “neuromyopathy” as a novel perspective of neuropathy in nerves responsible for both muscle pain, sensation, and motor action. We have proposed that neuromyopathy mediates the secondary MPS, which causes the pain in various diverse pain syndromes. Some examples are knee pain, presently referred to as pain of knee osteoarthritis[33] and postsurgical neuropathic pain after knee replacement surgery,[34] and in a case of refractory camptocormia occurring as a complication of RF ablation of medial branch to facet.[35] We believe that it plays a major role in the causation of complex regional pain syndrome as well.[36373839] We have also proposed neuromyopathy in the pain of interstitial cystitis[40] and in “writer's cramp.”[41]
We have described results in all these conditions with dry needling. Pain in abdominal cancer is yet another condition that appears to involve not just visceral neuronal pathways but also a global activation of body wall muscle innervation causing a secondary MPS with severe spasm, guarding, and rigidity through the viscerosomatic convergence making it a neuromyopathic condition.
The concept of neuromyopathy explains why efficacy of SRF/NCPB has been reported as minimal in relieving PCP by Sharfman and Walsh (1990),[3] Wyse et al. (2014),[7] and Wiechowska-Kozłowska et al. (2014).[12] It also explains immediate post-RF pain crisis in patient 1 where we surmised that the SRF had eliminated the sympathetic action on the gut leading to un-opposed vagal propulsive action on bowel movement. Second, the existing pain was probably magnified after the fresh thermal injury to the nerves from SRF which cut off only the small visceral component (10%) of the viscerosomatic convergence, whereas the dominant somatic input continued unabated. These two mechanisms presumably led to the sudden escalated barrage of afferent nociceptive impulse traffic to and from the active MTrPs in the already spasmodic abdominal and paravertebral muscles immediately after SRF. According to Dommerholt,[29] Kuan et al.,[31] and Dommerholt et al.,[42] an increase in SEA and EPN results in a reduced pain threshold and increased pain intensity, which explains the excruciating pain experienced by patient 1. The USGDN led to the deactivation of the MTrPs presumably by reducing the EPN at the region of the MTrPs with consequent relief of the vicious circle of pain-spasm-more pain, which had remained unresponsive to intravenous opioids. Our findings confirm the observations made by Dommerholt et al.,[42] Chou et al.,[43] Chen et al.,[44] Srbely et al.,[45] and Lewit et al.,[46] that dry needling restores muscle activation patterns and exerts an anti-nociceptive effect resulting in a reduction of local, referred, and widespread pain.
In patients 2–5, NCPB/SRF had provided only partial pain relief probably because of elimination of only the visceral contribution to pain. However, the numerous active MTrPs and their well-established network of sensitized peripheral and central motor nociceptive processing remained unabated, resulting in persistent pain > 5 NRS 15 days after SRF/NCPB.
We also suggest another mechanism to explain the severe pain crisis in patient 1, as well as the persistent pain in others. The main nerve roots are sandwiched between the psoas and the intertransversarius muscles, which firmly encircle the nerve root foramen [Figure 4c]. In fact, we found in cadaveric dissections that it is difficult/impossible to visualize the nerve root unless the intertransversarius muscle has been removed [Figure 4b]. Spasm of these muscles could exert a pincer-like effect causing nerve entrapments at the main nerve roots causing yet another vicious cycle of pain-spasm-more pain. The iliocostalis, longissimus, and spinalis components of erector spinae are supplied by the lateral branch of the posterior rami of spinal nerves and the multifidi and the small muscles of the back are supplied by the medial branch of the posterior rami of the corresponding thoracic spinal nerves [Figure 4a and c]. The anterior abdominal wall muscles are supplied by the anterior rami of inferior six thoracic nerves.[20] All these nerves could suffer further entrapment by the muscles of abdomen tautened by the neuromyopathy [Figure 4]. Relaxation of back and anterior abdominal muscles probably reduced these nerve entrapments at neural foramina and along the course of the nerves, as well as diminishing the compressive effect of the muscle ellipse on the abdominal viscera.
Thus, it is clear that by the time a patient is referred for palliative care, there is a dysfunctional but self-sustaining feedback system of complex, interlinked somatic, sensorimotor, visceral, and sympathetic pathways responsible for viscerosomatic convergence and neuromyopathy. Woolf and Mannion,[47] and Planjar-Prvan et al.[48] recommend that the treatment of neuropathic pain in cancer should target not the etiological factors or the symptoms, but the mechanisms that operate to produce those symptoms. These mechanisms need to be understood, defined, and addressed separately by a multimodality approach. We suggest that USGDN is one such approach that can be used along with neurolytic blocks and medications for a comprehensive pain relief in PCP.
CONCLUSION
The referral of visceral pain to the body wall leads to the formation of MTrPs in the muscles as an epiphenomenon of neuromyopathy. This referred pain and muscle hyperalgesia in PCP occurs early and is accentuated by repeated episodes of colic, and remains long after the original visceral nociceptive stimulus has resolved or has been eliminated by neurolysis. USGDN appeared to be the only modality that specifically addressed the myopathy in our 5 patients to achieve complete pain relief. A study of DN in a large number of patients with residual pain after neuroablative procedures for visceral cancer would provide confirmation of this surmise.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Interventional pain treatments for cancer pain. Ann N Y Acad Sci. 2008;1138:299-328.
- [Google Scholar]
- The role of interventional therapies in cancer pain management. Ann Acad Med Singapore. 2009;38:989-97.
- [Google Scholar]
- Has the analgesic efficacy of neurolytic celiac plexus block been demonstrated in pancreatic cancer pain? Pain. 1990;41:267-71.
- [Google Scholar]
- Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430-8.
- [Google Scholar]
- Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care. 2012;6:17-26.
- [Google Scholar]
- Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol. 2014;20:2186-92.
- [Google Scholar]
- Pain management in patients with pancreatic carcinoma. Cancer. 1996;78(3 Suppl):639-53.
- [Google Scholar]
- How to Score the SF-12 Physical and Mental Health Summary Scales. (2nd ed). Boston: The Health Institute; 1995.
- [Google Scholar]
- Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6:405-24.
- [Google Scholar]
- Ultrasonic interventional analgesia in pancreatic carcinoma with chemical destruction of celiac ganglion. World J Gastroenterol. 2006;12:3288-91.
- [Google Scholar]
- The efficacy and safety of endoscopic ultrasound-guided celiac plexus neurolysis for treatment of pain in patients with pancreatic cancer. Gastroenterol Res Pract 2012 2012:503098.
- [Google Scholar]
- Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575-80.
- [Google Scholar]
- Pain management in advanced pancreatic cancer: Local experience in caritas medical centre. HKSPM Newsl. 2009;1:24-6.
- [Google Scholar]
- Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2-5.
- [Google Scholar]
- Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;194:31-74.
- [Google Scholar]
- Grant's Atlas of Anatomy. (11th ed). Philadelphia: Lippincott, Williams & Wilkins; 2005.
- [Google Scholar]
- Autonomic innervation of the viscera in relation to nerve block. Anesthesiology. 1968;29:793-813.
- [Google Scholar]
- Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717-26.
- [Google Scholar]
- Referred muscle pain: Clinical and pathophysiologic aspects. Curr Rev Pain. 1999;3:489-98.
- [Google Scholar]
- Myofascial and visceral pain syndromes: Visceral-somatic pain representations. J Musculoskelet Pain. 2002;10:165-75.
- [Google Scholar]
- Referred muscle pain/hyperalgesia and central sensitisation. J Rehabil Med. 2003;41(Suppl):85-8.
- [Google Scholar]
- Travell and Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. Upper Half of Body. Vol 1. (2nd ed). Baltimore, MD: Williams & Wilkins; 1999.
- [Google Scholar]
- Viscerosomatic convergence onto spinal neurons responding to afferent fibers located in the inferior cardiac nerv. Brain Res. 1977;137:164-8.
- [Google Scholar]
- Dry needling – Peripheral and central considerations. J Man Manip Ther. 2011;19:223-7.
- [Google Scholar]
- Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12:371-84.
- [Google Scholar]
- The myofascial trigger point region: correlation between the degree of irritability and the prevalence of endplate noise. Am J Phys Med Rehabil. 2007;86:183-9.
- [Google Scholar]
- Pulsed radiofrequency of the composite nerve supply to the knee joint as a new technique for relieving osteoarthritic pain: a preliminary report. Pain Physician. 2014;17:493-506.
- [Google Scholar]
- Successful management of chronic postsurgical pain following total knee replacement. Pain Med. 2014;15:1781-5.
- [Google Scholar]
- Painful camptocormia as a complication of radiofrequency denervation of lumbar facet joints. Pain Physician. 2014;17:E654-7.
- [Google Scholar]
- Successful reversal of complex regional pain syndrome type 1 of both upper extremities in five patients. Pain Med. 2012;13:1253-6.
- [Google Scholar]
- Ultrasound appearance of forearm muscles in 18 patients with complex regional pain syndrome 1 of the upper extremity. Pain Pract. 2013;13:76-88.
- [Google Scholar]
- Reversal of complex regional pain syndrome type 2 and the subsequent management of complex regional pain syndrome type 1 occurring after corrective surgery for residual ulnar claw. Pain Med. 2014;15:1059-63.
- [Google Scholar]
- Musculoskeletal Ultrasonography to distinguish muscle changes in complex regional pain syndrome type 1 from those of neuropathic pain: An observational study. Pain Pract 2015 DOI: 10.1111/papr.12338
- [Google Scholar]
- Treatment of a patient with interstitial cystitis/painful bladder syndrome as a neuropathic pain condition with a combination of caudal epidural analgesia followed by Botox injection of perineal muscles. Indian J Urol. 2014;30:350-3.
- [Google Scholar]
- Myofascial trigger points as a cause of abnormal cocontraction in writer's cramp. Pain Med. 2015;16:2043-6.
- [Google Scholar]
- An evidence-informed review of the current myofascial pain literature – January 2015. J Bodyw Mov Ther. 2015;19:126-37.
- [Google Scholar]
- Probable mechanisms of needling therapies for myofascial pain control. Evid Based Complement Alternat Med 2012 2012:705327.
- [Google Scholar]
- Inhibitory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2001;80:729-35.
- [Google Scholar]
- Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med. 2010;42:463-8.
- [Google Scholar]
- Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959-64.
- [Google Scholar]
- Pathophysiologic basis of the treatment of neurogenic pain. Acta Med Croatica. 2004;58:197-205.
- [Google Scholar]






