Translate this page into:
Morphine May Contribute to Improving Respiratory Failure in Severe COVID-19: A Case Report
*Corresponding author: Yukako Tanaka-Yagi, Department of Palliative Care, Konan Medical Center, Kobe, Hyogo, Japan. yucaco125@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yagi Y, Yamaguchi T, Matsuda Y, Mori M, Ikari T, Miwa S, et al. Morphine may contribute to improving respiratory failure in severe COVID-19: A case report. Indian J Palliat Care 2022;28:221-3.
Abstract
At present, the world is undergoing successive waves of the COVID-19 pandemic. When COVID-19 becomes severe, it causes respiratory failure and symptoms of dyspnoea. The patient’s dyspnoea worsens to the IPOS of 3. One COVID-19 patient admitted to our medical institution developed severe illness characterised by hypoxaemia and dyspnoea. In addition to disease-modifying treatments such as remdesivir and dexamethasone, we administered morphine to relieve his dyspnoea. Surprisingly, we observed an improvement in both hypoxaemia and dyspnoea.
Keywords
COVID-19
Morphine
Dyspnoea
INTRODUCTION
Progressive respiratory failure in severe COVID-19 develops usually approximately 1 week after the onset of initial symptoms.[1] In severe COVID-19 cases, hypoxaemia is very common.[2,3] Dyspnoea is the most prevalent symptom in severe COVID-19 cases, with a reported prevalence of 57.5–84% in COVID-19 patients referred to specialist palliative care services.[4-8] Thus, not only disease-modifying treatments such as remdesivir[9] and dexamethasone[10,11] are indicated but also supportive care for relieving the patients’ distress. After administrating morphine for relieving dyspnoea from COVID-19, we observed improvement in both dyspnoea and hypoxaemia.
CASE REPORT
An 87-year-old non-smoking male with a medical history of subarachnoid haemorrhage was admitted to our hospital for the management of COVID-19 confirmed by RT-PCR from a nasopharyngeal swab [Figure 1]. He experienced fatigue and dyspnoea the week before the presentation. We considered him at high risk because his COVID-19 GRAM (a clinical risk tool) risk score[12] was 173 points and his probability of developing critical illness was 76.3%. Computed tomography showed widespread interstitial shadows in bilateral lung fields [Figure 2A]. We treated patients diagnosed as a high-risk group based on the COVID-GRAM risk score with remdesivir and dexamethasone. After admission, remdesivir and dexamethasone were administered. However, his respiratory status was getting worse and supplemental oxygen therapy could not sustain O2 saturation above 90%. He refused intubation because he was enough old and he clearly stated it to us. Therefore, we used high-flow nasal cannula oxygen therapy (oxygen flow 40 l, FiO2 50%) on day 2 to maintain O2 saturation above 90%. Despite these treatments, his condition deteriorated further and dyspnoea worsened. On day 5, we started the patient on 10 mg/day of continuous released oral morphine. Thereafter, we changed the administration route to a continuous subcutaneous infusion of 6 mg/day (12 mg/day of continuous released oral morphine) on day 7 because the patient developed difficulty swallowing. After starting morphine, dyspnoea intensity evaluated by the integrated palliative care outcome scale (IPOS) improved from 4 at baseline to 2. In this case, the patient could not rate the patient-reported outcome scale such as Numerical Rating Scale (NRS) due to his distressing symptom. Thus, we assessed his dyspnoea by healthcare provider rated IPOS which was listed as a secondary evaluation scale for the assessment of dyspnoea in research policy for dyspnoea in Japan. At the same time, FiO2 required to maintain oxygen saturation above 90% decreased (FiO2 90%→70%) and tachypnoea also improved (respiratory rate 28/min→(20/min). While the dyspnoea had been under control for several days, the shadows on his chest X-ray showed no improvement on day 8 [Figure 2B]. His chest X-ray showed bilateral lung shadow with a P/F ratio of <200. However, his condition worsened thereafter. We titrated his morphine dose up to 15 mg/day (30 mg/day of continuously released oral morphine), which was not effective anymore to relieve his dyspnoea. Therefore, palliative sedation with midazolam was started on day 16. After starting palliative sedation, his consciousness score sank from −2 to −4 on the Richmond Agitation-Sedation Scale with no signs of dyspnoea. He died 23 days after admission with no signs of distress. His clinical course is shown in Figure 1.
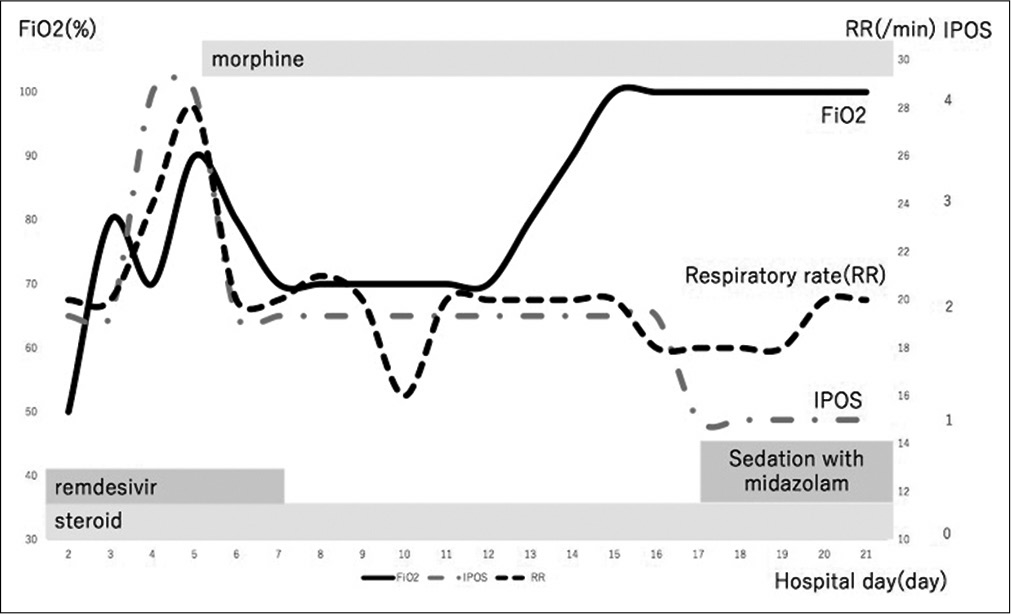
- Clinical course after starting morphine on day 5, dyspnoea intensity and FiO2 required to maintain oxygen saturation above 90% and tachypnoea were improved.
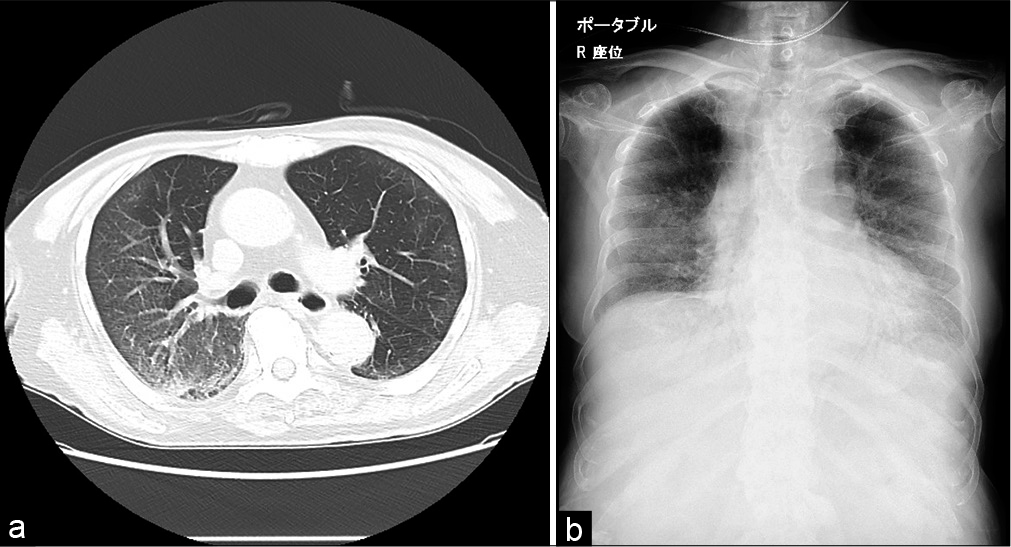
- (a) Lung CT on day 1. (b) Chest X-ray on day 8.
Following the ethical guidelines for human research of the Ministry of Health, Labour and Welfare in Japan, IRB approval was not required for the case report. Furthermore, we have obtained consent by general consent from the patients or their surrogates at the time of admission.
DISCUSSION
This is, to the best of our knowledge, the first reported case that showed an improvement in oxygenation after being administered morphine for relieving dyspnoea from COVID-19.
Morphine or other opioids are commonly prescribed to relieve dyspnoea in severe COVID-19 cases.[4-8,13] However, we observed not only an improvement in dyspnoea but also in hypoxaemia after administering morphine in the present case. Several hypotheses could explain this phenomenon. The previous study showed that opioids may reduce respiratory workload and myocardial oxygen consumption in patients with tachypnoea.[14] This reduction may improve hypoxaemia by reducing metabolic rate and oxygen demand in the respiratory and cardiac systems. Another hypothesis might be that improving ventilation efficiency affects a reduction in the proportion of dead space ventilation. Low lung compliance in severe COVID-19 patients has been described.[15] An increased breathing frequency may augment dead space ventilation/alveolar ventilation ratio in subjects with lung constraints.[16] In our present case, the respiratory rate was normalised from tachypnoea after morphine administration. The improvement of his rapid shallow breathing may have decreased dead space ventilation/ alveolar ventilation ratio and improved ventilation efficiency by increasing tidal volume, which resulted in improved oxygenation.[17]
Apart from relieving dyspnoea and possibly improving oxygenation in particular cases, there are other potential benefits of using opioids for dyspnoea in severe COVID-19 patients.
Patient self-induced lung injury is recognised as the underlying pathophysiology of acute respiratory distress syndrome in COVID-19.[2,18,19] Dyspnoea may stimulate inspiratory drive in spontaneously breathing patients. The vigorous inspiratory effort promotes increased inspiratory intrathoracic negative pressure, which together with an increased permeability due to lung inflammation contributes to the development of lung edema.[20] Thus, relieving dyspnoea in the early phase of COVID-19 may suppress an excess inspiratory drive, which may help prevent a worsening of lung damage in COVID-19 patients.
Although several hypothetical benefits of using opioids for dyspnoea in severe COVID-19 patients exist, there is a paucity of evidence. Thus, we should carefully evaluate severe COVID-19 patients who are given morphine and other opioids for dyspnoea in daily practice. Observational studies can help answer the question of whether morphine and other opioids improve dyspnoea and oxygenation and prevent a worsening of lung damage in severe COVID-19 patients, which may contribute to optimising care for severe COVID-19 patients.
CONCLUSION
Morphine may improve not only dyspnoea but also oxygenation in hypoxemic COVID-19 patients. Further studies of morphine and other opioids for severe COVID-19 cases are warranted to optimise the care for severe COVID-19 cases with dyspnoea and hypoxaemia.
Authors’ contributions
Conception and design: Yukako Yagi and Takashi Yamaguchi. Collection and assembly of data: Yukako Yagi, Kyosuke Nakata and Masayuki Kanatani.
Data analysis and interpretation: Yukako Yagi and Takashi Yamaguchi.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21:198.
- [CrossRef] [PubMed] [Google Scholar]
- Dyspneic and non-dyspneic (silent) hypoxemia in COVID-19: Possible neurological mechanism. Clin Neurol Neurosurg. 2020;198:106217.
- [CrossRef] [PubMed] [Google Scholar]
- An audit of end-of-life symptom control in patients with corona virus disease 2019 (COVID-19) dying in a hospital in the United Kingdom. Palliat Med. 2020;34:1249-55.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative care during COVID-19: Data and visits from loved ones. Am J Hosp Palliat Care. 2020;37:988-91.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and hospital palliative care a service evaluation exploring the symptoms and outcomes of 186 patients and the impact of the pandemic on specialist hospital palliative care. Palliat Med. 2020;34:1256-62.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics, symptom management, and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage. 2020;60:e77-81.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 End-of-life care: Symptoms and supportive therapy use in an australian hospital. Intern Med J. 2021;51:1420-5.
- [CrossRef] [PubMed] [Google Scholar]
- Remdesivir for the treatment of Covid-19 final report. N Engl J Med. 2020;383:1813-26.
- [CrossRef] [PubMed] [Google Scholar]
- Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute Respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;324:1307-16.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081-9.
- [CrossRef] [PubMed] [Google Scholar]
- End-of-life care in COVID-19: An audit of pharmacological management in hospital inpatients. Palliat Med. 2020;34:1235-40.
- [CrossRef] [PubMed] [Google Scholar]
- Remifentanil improves breathing pattern and reduces inspiratory workload in tachypneic patients. Respir Care. 2011;56:827-33.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am J Respir Crit Care Med. 2020;201:1560-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ventilatory constraints influence physiological dead space in heart failure. Exp Physiol. 2019;104:70-80.
- [CrossRef] [PubMed] [Google Scholar]
- West's Respiratory Physiology: The Essentials (10th ed). Netherlands: Wolters Kluwer; 2016.
- [Google Scholar]
- COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099-102.
- [CrossRef] [PubMed] [Google Scholar]
- Management of COVID-19 respiratory distress. JAMA. 2020;323:2329-30.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438-42.
- [CrossRef] [PubMed] [Google Scholar]






