Translate this page into:
Efficacy and Safety of Celiac Plexus Neurolysis in the Treatment of Chronic Pain Secondary to Oncological Pathology of the Upper Hemiabdomen: A Systematic Review and Meta-Analysis
*Corresponding author: Jose Percy Amado-Tineo, School of Medicine, Universidad Nacional Mayor de San Marcos, Lima, Peru. jpamadot@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pacheco-Feijoó GM, Amado-Tineo JP, PlancarteSánchez R, Contreras Valdivia C, López-Millán JM. Efficacy and Safety of Celiac Plexus Neurolysis in the Treatment of Chronic Pain Secondary to Oncological Pathology of the Upper Hemiabdomen: A Systematic Review and Meta-Analysis. Indian J Palliat Care 2023;29:394-406.
Abstract
Objectives:
The management of chronic pain among patients with abdominal cancer is complex; against that, the neurolysis of the celiac plexus (CPN) is the best technique at the moment to determine the efficacy and safety in the treatment of chronic pain secondary to oncological pathology of the upper abdomen.
Material and Methods:
This was a systematic review of controlled clinical trials between 2000 and 2021, in the sources MEDLINE/PubMed, Cochrane, Scopus, Web of Science, and Google Scholar. Three independent evaluators analysed the results of the bibliographical research. The quality of the studies was assessed with the Jadad scale and the mean difference (95% confidence interval) and heterogeneity of the studies (I2) were calculated with Review Manager 5.3.
Results:
Seven hundred and forty-four publications were identified, including 13 studies in the qualitative synthesis and three studies in the quantitative synthesis. No difference was found in the decrease in pain intensity between 1 and 12 weeks after the intervention, comparing the experimental group with the control (P > 0.05). The adverse effects related to neurolysis were not serious and transitory, mentioning the most frequent adverse effects and reporting a percentage between 21% and 67% (with 17% for echoendoscopic neurolysis and 49% for percutaneous neurolysis).
Conclusion:
Celiac plexus neurolysis for the treatment of severe chronic pain secondary to oncological pathology in the upper hemiabdomen produces similar pain relief as conventional pharmacological analgesic treatment. It is a safe analgesic technique since the complications are mild and transitory.
Keywords
Abdominal neoplasms
Pancreatic neoplasms
Treatment
Chronic pain
Celiac plexus neurolysis
INTRODUCTION
The pain that cancer patients experience (cancer pain) is known as total pain[1] and can be acute, chronic, acute chronic, nociceptive, neuropathic, etc., or a combination of all of them. It occurs in up to 70% of patients[2] and is usually moderate to severe in intensity in 50% of patients who are in advanced stages of the disease.[3] Of the abdominal neoplasms, pain is more prevalent in patients with malignant tumours of the stomach and pancreas.[3,4]
The treatment of chronic cancer pain consists of the use of a multimodal therapy, based on pharmacology, taking into account the intensity of pain called the therapeutic ladder of the World Health Organization.[5] In this therapeutic algorithm, powerful opioid drugs play a fundamental role and interventional analgesia techniques are considered as the last step of treatment for chronic refractory pain.
In advanced neoplastic diseases, conventional therapies (pharmacological and non-pharmacological) often do not meet the objective of controlling it or have unacceptable side effects.[6] The continuous increase in the doses and the frequency of administration of opioids increases the risk of the appearance of side effects such as nausea, vomiting, gastrointestinal problems, and itching, among others, causing a decrease in the quality of life of the patient.[7] In these cases, interventional analgesia techniques are required.
A viable option, for the treatment of chronic refractory pain in the upper half of the abdomen within the interventional field, is the inhibition of the sympathetic nervous system. There are different techniques such as celiac plexus neurolysis (CPN) (percutaneous, endoscopic, or surgical) or the neurolysis of the splanchnic nerves (NSN) (percutaneous or surgical). NSN is used mainly as an alternative to CPN when anatomical changes, adhesions, lymphadenopathy, tumour infiltration, etc., may reduce the effectiveness of the latter or when it fails.[8] The celiac or solar plexus is located in the retroperitoneum, on the anterolateral wall of the aorta at the level of the body of the first lumbar vertebra (L1). It provides sympathetic, parasympathetic and sensory innervation to multiple intra-abdominal structures: pancreas, liver, gallbladder and bile duct, spleen, adrenal glands, kidneys, stomach, the lower third of the oesophagus, duodenum, and part of the transverse colon.[9,10]
Percutaneous neurolysis of the celiac plexus consists of a minimally invasive procedure that can be performed using different imaging techniques (fluoroscopy, ultrasound, computed tomography, or magnetic resonance imaging). It consists of the injection of a neurolytic agent (ethanol or phenol), which generates a Wallerian degeneration of the plexus,[9,10] interrupting the sensory pathway to the central nervous system to relieve pain for a long time.[11] In CPN, the approach can be posterior (patient in the prone position) or anterior (patient in the supine position). In the posterior approach, a unilateral or bilateral transcrural technique (identifying the L1 vertebral body), transaortic (accessing the left side of the L1 vertebral body through the aorta) or transdiscal (crossing the T12–L1 intervertebral disc and reaching splanchnic nerves) can be used. The anterior, ultrasound-guided, or tomography-guided approach usually uses a single puncture. The volume of the neurolytic substance is variable: between 10 mL and 30 mL depending on whether phenol or alcohol is used and it is generally preceded by the administration of contrast (except in the ultrasound technique) and a local anaesthetic drug.[8]
On the other hand, endoscopic neurolysis has the advantage of allowing direct access to the celiac plexus from the antecrural plane through 180° ultrasound cuts, allowing the puncture to be performed in real-time; the patient must be in the left lateral decubitus and low conscious sedation controlled by an anaesthesiologist. In the case of bilateral puncture, 10 mL of 0.25% bupivacaine plus and 10 mL of 98% alcohol are administered to each side of the aorta. Since alcohol makes it difficult to recognise structures by ultrasound, it is preferable to perform a single puncture in the anterior part of the aorta in the endoscopic technique.[12] CPN is a well-tolerated technique that is performed under local anaesthesia. The most frequent side effects are mild and transitory and depend on the type of technique used, known as, pain in the puncture site, diarrhoea, and orthostatic hypotension.[7,8] The main benefit of CPN is the reduction in a variable degree of the intensity of pain, although the evidence in this regard is limited concerning its safety and efficacy.[13-15] However, there are, indeed, up-to-date primary studies on CPN, where CPN is generally analysed by comparing alcohol volumes[16] or comparing the use of a single or double needle.[17]
Since CPN is the analgesic technique of choice for the treatment of refractory chronic cancer pain due to advanced neoplasms of the upper hemiabdomen, it is relevant for these patients to analyse the level of evidence on the efficacy and safety of the CPN.[11,18,19]
MATERIAL AND METHODS
A systematic review was carried out in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA),[20] following the CRD42021241713 protocol registered in PROSPERO (International prospective register of systematic reviews), available at https://www.crd.york.ac.uk/prospero/display_record. php?RecordID=241713.
We formulate the research question using the PICO methodology.[21] PATIENTS: Upper abdominal cancer with refractory chronic pain. INTERVENTION: CPN and COMPARATOR: Systemic pharmacological analgesic treatment. OUTCOME: Efficacy and safety.
Search strategy
A bibliographic search was carried out in five electronic databases (MEDLINE/PubMed, Cochrane, Scopus, Web of Science, and Google Scholar) between 1 January 2000 and 12 April 2021, including the following terms: Population: Cancer, neoplasms or abdominal pain and intervention: Neurolysis, nerve block or celiac plexus in English, Spanish and Portuguese. In addition, the list of bibliographic references was reviewed (snowball strategy) and a search was carried out in the grey literature [Annex 1].
Study selection
One author searched all five databases by title and abstracts; then, duplicates were identified and removed using the Mendeley 1.19.4 reference manager. Three authors independently reviewed the selected full-text articles following the following criteria:
Inclusion criteria
Randomised and controlled clinical trials evaluating the efficacy and safety of CPN (percutaneous or endoscopic ultrasound) in patients over 18 years of age with chronic pain due to upper abdominal cancer were included in the study.
Exclusion criteria
Systematic reviews and meta-analyses, studies compared with other procedures, surgical techniques, and radiofrequency neurolysis, studies that were not in full text, or those in which the methodology was not clearly specified were excluded from the study.
The doubts were resolved by consensus among the reviewers.
Data extraction
From the studies finally included in the review, the data were extracted by two of the authors independently, using a pre-designed data extraction sheet to collect information on the author and year of publication, country of origin, type of study, patients, comparator, characteristics, measurement, technique, and conclusions.
Quality assessment
The senior author assessed the quality of the data and the risk of bias in all the included studies, applying the Jadad scale modified by Oremus et al.,[22] which assigns a score from 0 to 5, considering the form of randomisation of patients, blinding and loss of individuals and excluding low-quality studies (score <3).
Synthesis of results
In studies with adequate quality and similar characteristics, global estimates were made in the pooled analysis using Review Manager 5.3 (RevMan 5.3);[23] the weighted difference of means (with 95% confidence interval) of the selected studies based on a random effects model, a variant of the inverse of the variance method that incorporates intra- and inter-study variability. The heterogeneity of the estimates was assessed using the I2 statistic, which describes the percentage of variation, not the sampling error between studies. An I2 value >75% indicates high heterogeneity.
RESULTS
Selected studies
A total of 744 publications were identified according to the search criteria, 13 of them were selected for qualitative synthesis, three of which met criteria for quantitative synthesis and efficacy of the procedure. Figure 1 shows the sequence that the selection of articles follows. The researchers in charge of the selection of articles had a good inter-rater agreement (Cohen’s Kappa Index 0.61). Seven studies compared the efficacy and safety of CPN with conventional pharmacological treatment except for Gao et al.’s,[24] who did not evaluate the safety of the intervention [Table 1]. Six studies compared the efficacy and safety of technical variations of the same procedure (access, imaging technique used, number of punctures, or substance used) except the case of Ugur et al.,[25] who did not assess safety [Table 2]. Thus, of the 13 studies selected, only 10 met the criteria for the analysis of the safety of the technique [Table 3].

- Flowchart of the selection of articles.
| AUTHOR | COMPARE | PATIENTS | MESUREMENT | TECHNIQUE | CONCLUSION |
|---|---|---|---|---|---|
| Wong et al. 2004 (EEUU) |
PER-CPB vs only pharmacological treatment | Pain due to PCa. Age 63 years +/- 11. Male 53% | Pain, quality of life, opioid use, opioid adverse effects | Posterior access, F-guide, 22G needle, Bu 0.5% 10 ml, Iopamidol 1-5 ml, OH 100% 10 ml | PER-CPB improves pain relief, without affecting quality of life, opioid adverse effects, or survival. |
| Jain et al. 2005 (India) |
PER-CPB vs only pharmacological treatment | Abdominal or back pain using morphine, PCa, and gallbladder. Age 48.6 vs 50.9 years. Male 50 vs 60% | Pain (VAS), quality of life, opioid use, Karnosfki | Posterior access, F-guide, 22G needle, lidocaine 1%, meglumine, OH 50% 20 ml | PER-CPB significantly reduced pain intensity, opioid requirement, and opioid-related adverse effects. Also improving quality of life and functionality. |
| Zhang et al. 2007 (China) |
PER-CPB vs only pharmacological treatment | Intractable pain due to PCa. Age 38-75 years. Male 62.5% | Pain (VAS), quality of life (appetite, sleep, communication), opioid use, complications | L1 posterior access, T-guide, 23G needle, 5 ml 1% lidocaine, 3 ml contrast, 20 ml 100% OH | PER-CPB is an effective and safe modality |
| Wyse et al. 2011 (Canada) |
Early EUS-CPB vs only pharmacological treatment | Inoperable PCa pain. Age 66.5 years +/- 9. Male 49% | Pain (Likert scale), morphine consumption, quality of life (DDQ-15), survival | 19G needle, F-guide, bilateral injection of Bu 0.5% 10 ml and OH 100% 20 ml | Early EUS-CPB reduces pain and can moderate morphine intake. It can be considered at the time of diagnosis. |
| Amr et al. 2013 (Egypt) |
Early PER-CPB vs initial pharmacological control | Severe pain due to inoperable PCa. Age 50 years +/- 11. Male 65% | Pain (VAS), duration of relief, quality of life (QLQ-C30), analgesic requirement, adverse effects | Transaortic access, T12-L1, F-guide, 22G needle, 1% lidocaine, 70% OH. Drugs: gabapentin, tramadol, acetaminophen, morphine sulphate, fentanyl patches | Pharmacologically controlling pain and then performing PER-CPB was shown to be more effective in relieving long-term pain, opioid use, and quality of life. |
| Gao et al. 2014 (China) |
PER-CPB vs only pharmacological treatment | Terminal pain due to PCa. Age 65 years +/- 10 | Pain (VAS), duration of pain, consumption of analgesics, quality of life (QLQ) | Celiac neurolysis, F-guide, Bu and ethanol | PER-CPB is an effective method that significantly reduces pain, for longer, less use of medications and improves quality of life. |
| Kanno et al. 2020 (Japan) |
EUS-CPB vs oxycodone and / or fentanyl | Pain due to PCa. Age 69 years +/- 10. Male 50% | Pain (VAS), quality of life, opioid use | Needle 22 or 25G, US-guide, Bu 0.25% 2-3 ml, solution 15-30 ml (iopaminol 5% + OH 99% 95%) | EUS-CPB did not show a significant difference in pain relief, quality of life or opioid use. |
PER-CPB: Percutaneous celiac plexus block, EUS-CPB: Endoscopic-ultrasound Celiac plexus block, PCa: Pancreas cancer, F: Fluoroscopic, T: Tomographic, US: Ultrasonographic, Bu: Bupivacaine; OH: Alcohol.
| AUTHOR | COMPARE | PATIENTS | MESUREMENT | TECHNIQUE | CONCLUSION |
|---|---|---|---|---|---|
| Ugur et al. 2007 (Turkey) | PER-CPB with two needles vs one needle with 2 stylets | Pain due to Pca | Number of punctures and fluoroscopy injection time | L1 Posterior access, F-guide, 21G needle, Bu 0.5% 15 ml, OH 50% 10 ml | The use of a single needle may be a more effective and appropriate method for beginners or practitioners of other specialties. |
| LeBlanc et al. 2011 (EEUU) |
EUS-CPB with one vs two needles | Pain due to PCa. Age 63 years. Male 48% | Pain, duration of remission, complications | US-Guide, injection at the level of the celiac trunk of 20 ml of Bu 0.75% + 10 ml of OH 98% | No difference was found in pain relief or safety of the procedure |
| LeBlanc et al. 2013 (EEUU) |
EUS-CPB using 10 vs 20 ml of OH | Inoperable PCa pain. Age 66 years +/- 14. Male 55% | Pain and quality of life and security. | US-guide, access to the posterior wall of the stomach, needle 22G, Bu 0.75% 20 ml and then OH 98% | Both alternatives are safe with similar results, but more research is needed. |
| Dolly et al. 2016 (India) |
PER-CPB using 20, 30 and 40 ml of OH | Gallbladder cancer pain. Age 25-70 years. Male 20% | Pain (VAS), quality of life, morphine consumption, adverse effects | Lateral access intervertebral disc (T12), F-guide, 25G needle, 2% lidocaine, 2 ml contrast, 70% OH | The use of 40 ml was more effective in celiac plexus neurolysis |
| Abdelghaffar et al. 2019 (Egypt) |
PER-CPB with one vs two needles | Abdominal cancer pain | Blockage failure, procedure time, pain (VAS), rescues, and complications | Antecrural posterior access, T-guide, phenol 10% 25 ml in total | The use of a single needle with change of position had a lower failure rate and shorter procedure time, with no difference in complications or pain relief |
| Saeed et al. 2019 (Pakistan) | PER-CPB using OH 50 and 100% | Inoperable PCa pain. Age 57 years +/- 12. Male 54% | Pain (VAS, PainScale), complications | Posterior access to body level L1, F-guide, lidocaine 2%, OH 15 ml | Complications (back pain, diarrhoea or hypotension) were more frequent with 100% alcohol. Pain control was similar. |
PER-CPB: Percutaneous Celiac plexus block, EUS-CPB: Endoscopic-ultrasound Celiac plexus block, PCa: Pancreas cancer, F: Fluoroscopic, T: Tomographic, US: Ultrasonographic, Bu: Bupivacaine, OH: Alcohol.
| AUTHOR | Procedure | Technique | n | Patients | % adverse effects | Transient hypotension | Local pain | Transient diarrhea | Lightheadedness or dizziness | Nausea or vomiting | Others adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jain et al. 2005 |
PER-CPB | Posterior access, F-guide, 22G needle, lidocaine 1%, meglumine, OH 50% 20 ml | 48 | Age 48.6 vs 50.9 years. Male 50 vs 60%. PCa and gallbladder, pain with opioid use | 20.8 | 10 cases | No SC | ||||
| Zhang et al. 2007 |
PER-CPB | L1 posterior access, T-guide, 23G needle, 5 ml 1% lidocaine, 3 ml contrast, 20 ml 100% OH | 29 | Age 38-75 years. Male 62.5%. Chronic pain due to Pca | 34.5 | 10 cases (two persist until day 7 and one until day 14) | 2 cases (persist until day 7) | 5 cases (one persists until day 7) |
2 cases (only one day) |
No SC | |
| Amr et al. 2013 |
PER-CPB | Transaortic access, T12-L1, F-guide, 22G needle, 1% lidocaine, 70% OH. | 60 | Age 50 years +/- 11. Male 65%. Severe pain due to PCa with use of tramadol | 53.3 | 8 cases (13%) resolved with parenteral hydration | 32 cases (53.3%), resolved in 48 hours without treatment | No SC | |||
| Dolly et al. 2016 |
PER-CPB | Lateral access intervertebral disc (T12), F-guide, 25G needle, 2% lidocaine, 2 ml contrast, 70% OH (20, 30, and 40 ml) | 30 (10 per group) |
Age 25-70 years. Male 20%. Gallbladder Cancer Pain | 53.3 | 12 in the injection site and 8 mild back pain | 4 cases | No SC. No difference between groups | |||
| Abdelghaffar et al. 2019 | PER-CPB | Antecrural posterior access, T-guide, phenol 10% 25 ml in total. One and two needles | 30 (G1:17 G2:13) | Abdominal cancer pain | 40 | 8 cases | one case | One case of hemorrhage and 3 of local infection | |||
| Saeed et al. 2019 |
PER-CPB | Posterior access to body level L1, F-guide, lidocaine 2%, OH 15 ml (50 and 100%) | 100 (50 per group) | Age 57 years +/- 12. Male 54%. Inoperable PCa pain | 67 | 64 vs 70% according to study group | 64 vs 70% according to study group: mild back pain | 64 vs 10% according to study group | No SC. Greater adverse effects with OH 100% | ||
| Wyse et al. 2011 |
EUS-CPB | 19G needle, F-guide, bilateral injection of Bu 0.5% 10 ml and OH 100% 20 ml | 96 (48 per group) |
Age 66.5 years +/- 9. Male 49%. Pain due to Pca | 0 | Only reports complications that prolong hospitalization | No SC | ||||
| LeBlanc et al. 2011 |
EUS-CPB | US-Guide, injection at the level of the celiac trunk of 20 ml of Bu 0.75% + 10 ml of OH 98% (1 or 2 needles) | 50 (G1:29 G2:21) | Age 63 years. Male 48%. Pain due to Pca | 36 | One case (2%) that resolved with intravenous fluids | 18 cases (36%) post- procedure pain |
No SC | |||
| LeBlanc et al. 2013 |
EUS-CPB | US-guide, access to the posterior wall of the stomach, needle 22G, Bu 0.75% 20 ml and then OH 98% (10 or 20 ml) | 20 (10 per group) |
Age 66 years +/- 14. Male 55%. Inoperable PCa pain | 30 | 2 cases (temporary) | one case | 3 cases | No SC. No difference between groups | ||
| Kanno et al. 2020 |
EUS-CPB | Needle 22 or 25G, US-guide, Bu 0.25% 2-3 ml, solution 15-30 ml (iopaminol 5% + OH 99% 95%) | 24 | Age 69 years +/- 10. Male 50%. Severe cancer pain | 33.3 | 3 cases | 3 cases | 2 cases | No SC |
PER -CPB: Percutaneous Celiac plexus block, EUS -CPB: Endoscopic -ultrasound Celiac plexus block, PCa: Pancreas cancer, F: Fluoroscopic, T: Tomographic, US: Ultrasonographic, Bu: Bupivacaine; OH: Alcohol, SC: Severe complications
Reduction of pain intensity comparing CPN with conventional pharmacological treatment
Four studies concluded that the decrease in pain was significantly greater in the group that received celiac neurolysis compared to the group that only used conventional pharmacological treatment.[24,26-30] The other three studies report that they found no significant difference in relation to this variable.[31-33]
Only three trials[24,30,33] fulfilled the conditions for the estimation of meta-analysis in relation to pain intensity with celiac neurolysis versus conventional pharmacological therapy [Table 4]. The difference in pain intensity means was −0.55, −0.39, 0.16, and −0.11 in weeks 1, 4, 8, and 12 after the procedure, but no confidence interval excluded zero [Figure 2]; heterogeneity was high (I2 78%) in week 1 comparisons, but 0% in all other comparisons. Of these three, only one shows a significant difference in the 1st week.
| Trial | Describe | Is adequate | Punctuation | |||
|---|---|---|---|---|---|---|
| Randomized | Double blind | Dropouts exclusions | Randomization | Blinding | ||
| Wong et al. 2004 | 1 | 1 | 1 | 1 | 1 | 5 |
| Jain et al. 2005 | 0 | 0 | 1 | -1 | -1 | -1 |
| Zhang et al. 2007 | 1 | 1 | 1 | 1 | -1 | 3 |
| Wyse et al. 2011 | 1 | 1 | 1 | -1 | -1 | 2 |
| Amr et al. 2013 | 1 | 0 | 0 | 1 | -1 | 1 |
| Gao et al. 2014 | 1 | 1 | 0 | 1 | -1 | 2 |
| Kanno et al. 2020 | 1 | 1 | 1 | 1 | -1 | 3 |
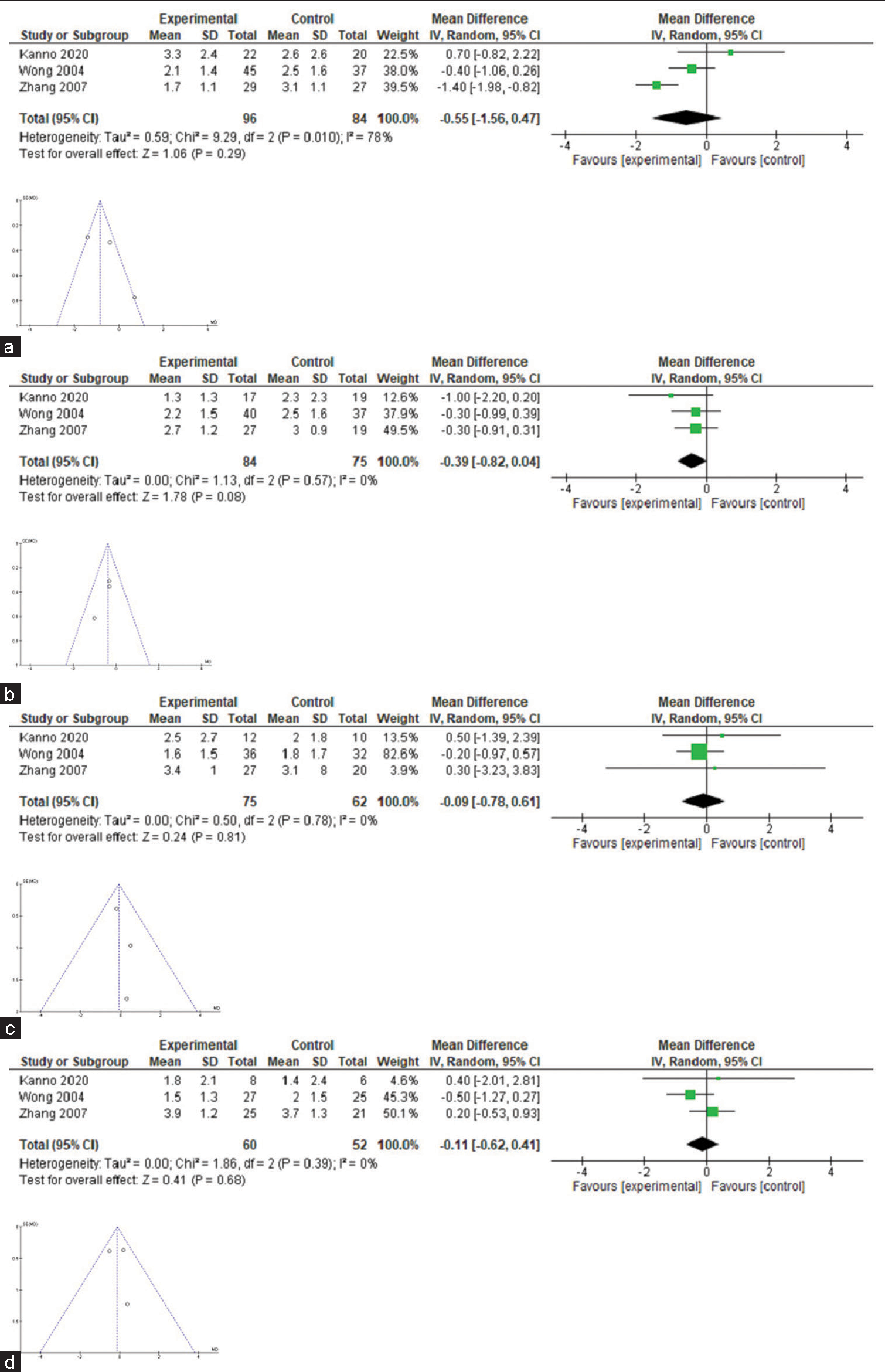
- Forest-plot comparing pain intensity in patients with advanced cancer (celiac plexus block versus conventional pharmacological treatment). (a) Pain relief at 1 week. (b) Pain relief at 4 week. (c) Pain relief at 8 week. (d) Pain relief at 12 week.
Reduction of opioid consumption
Three trials reported a significant decrease in opioid consumption in the group that received neurolysis compared to the control group[26,28,29,34] and one trial concluded that there was no difference in opioid consumption in the groups evaluated.[30] It was not possible to perform a quantitative synthesis of opioid use due to a lack of uniformity in the communication of the results.
Reduction of pain intensity comparing technical variations of the same procedure
Three studies compared the use of one versus two needles during CPN, different approaches, and imaging techniques. One of them concluded that using a needle had a lower failure rate and shorter procedure time compared to a double needle, but its safety was similar since there were no significant differences in the use of rescue analgesia and the presence of complications.[17] Furthermore, the other two did not find significant differences in pain relief or safety of the procedure,[23,25] mentioning it could be more appropriate for poorly trained personnel.
The use of 40 mL of 70% alcohol was more effective than 20 or 30 mL in percutaneous neurolysis in gallbladder cancer.[35] The third study concluded that using 15 mL of 50% alcohol had similar pain control as 100% alcohol in percutaneous neurolysis due to pancreatic cancer, but the latter presented greater complications.[17]
Technique-related adverse events in CPN.
In ten of the identified studies, transient (hours or a few days) and mild adverse effects related to CPN were reported (between 21% and 67%), mentioning orthostatic hypotension, dizziness/light-headedness, diarrhoea and pain in the puncture site; identifying that in the studies where neurolysis was applied percutaneously, they reported a greater number of adverse events compared to the endoscopic route: 49% versus 17%, respectively. In the study by Saeed et al.[26] for the percutaneous technique, 67% of complications were found, while the one of LeBlanc et al.,[35] 36% were observed for the endoscopic technique [Figure 3].
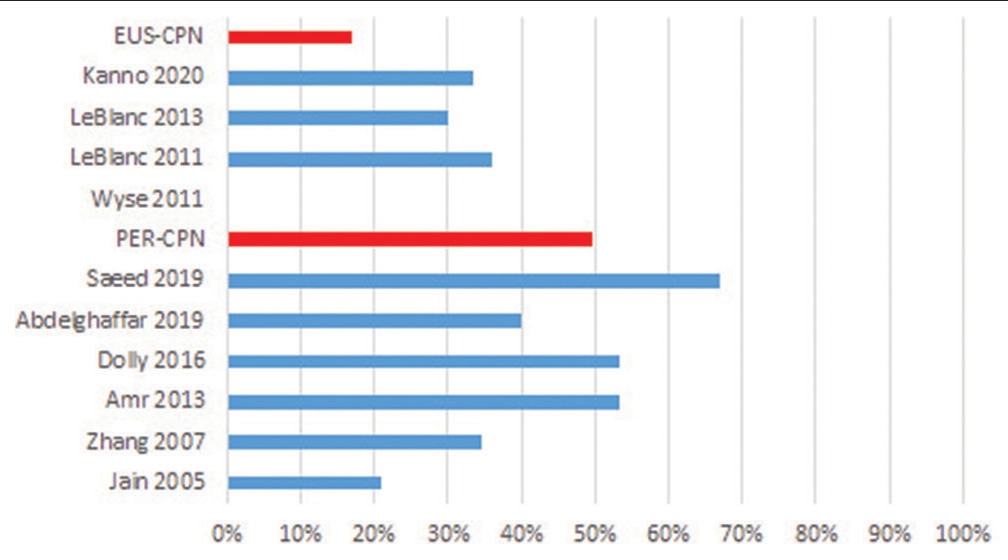
- Technique-related adverse effects (%). PER-CPN: Percutaneous celiac neurolysis EUS-CPN: Endoscopic-ultrasound Celiac neurolysis.
Quality of life
Six out of seven clinical trials that evaluate the efficacy and safety of CPN, compared to conventional analgesic treatment, analyse the quality of life and/or functionality of patients with the treatment. Three trials reported better quality of life or functional status of patients in the group that received neurolysis;[8,29,34] however, another three concluded that there were no significant differences between the groups[24,27,33] and one of them did not analyse this variable.[30] Numerical estimates could not be made because the studies used different ways of estimating the quality of life variable and in other cases due to the significant loss of patients to follow-up.
DISCUSSION
The goal of this study is to carry out an evaluation of the effectiveness and safety of CPN for the treatment of persistent chronic cancer pain caused by upper abdominal neoplasms in comparison with pharmacological analgesic treatment through a systematic review of the literature.
A total of 13 controlled clinical trials have been identified, a greater number than in the previous reviews,[11,18,19,36] comparing both technical variations of the procedure and conventional analgesic treatment in a highly variable number of patients, with heterogeneity being the rule. Only three clinical trials met the criteria for quantitative analysis,[24,30,33] because they analysed patients with pancreatic cancer, comparing various neurolysis techniques (endoscopy-guided[33] or computed tomography[30]) with analgesic treatment and monitoring the patients weekly to identify the intensity of pain and the need for opioid consumption, additionally, they analysed the variation in the quality of life after neurolysis. The most studied pathology of all upper hemiabdomen neoplasms among the selected articles was pancreatic cancer (eight studies). An increase in the number of studies with procedures guided by endoscopic ultrasound versus the percutaneous technique has been observed in recent years.
This review shows that CPN is performed using a wide variety of techniques, with the same objective of controlling pain in cancer patients, which, when compared with conventional treatment, provides a reduction in pain intensity higher in the first weeks of treatment with a decrease in opioid consumption and being satisfactory for the patient because it indirectly improves their quality of life with the presence of adverse effects that are, in general, transitory and manageable. If we focus exclusively to the three studies that met the criteria for quantitative synthesis,[24,30,33] we can say that with this level of evidence, there is no significant decrease in the intensity of pain due to upper hemiabdomen cancer in weeks 1, 4, 8 or 12 with the application of celiac neurolysis compared to conventional drug treatment.
However, when evaluating the included controlled clinical trials, we can observe that the intensity of pain in the follow-up of patients is significantly reduced at the beginning in both therapies, but less in subsequent weeks. In a single study, it was possible to identify continuity in the degree of decrease in pain intensity for those who received neurolysis, turning out to be significant,[24] while in the other studies, there were no significant differences with respect to patients receiving conventional pharmacological analgesic treatment.[33] In this sense, regarding the variation in pain intensity in upper abdominal cancer, several clinical trials report a significant decrease in pain in the group that received neurolysis[26,27,29-31] while others showed no variation,[31,33] but few studies met the criteria for quantitative synthesis that did not show a significant difference.
This differs from that reported by Nagels et al.,[18] who of 66 selected studies included five studies for quantitative synthesis and found a decrease in pain in the intervention group during the first 2 weeks. At the same time, in the review by Yan and Myers,[19] when analysing five randomised clinical trials, they found a significant decrease in the second, 4th, and 8th weeks after the procedure. Instead, different results were found by Nobre et al.,[37] who found no significant differences in pain relief in the 1st week after the procedure in the study group, while at 12 weeks, they were mainly observable in patients who received endoscopic ultrasound-guided CPN.
This discrepancy in the results obtained by the different studies and the absence of significant difference in the efficacy of pain control in CPN compared to conventional pharmacological treatment may be due to the rotation or combination of different drugs in the context of a strategy flexible multimodality to adapt to the changing needs of the patient,[38] but we could also attribute variable criteria for the selection of patients that could make these not representative of the type of advanced cancer patients with refractory pain to whom the technique is offered in the context of palliative care.[8] Last but not least, significant pain relief is more feasible in patients with pain intensity >7 according to the visual analogue scale than in patients with moderate pain intensity, frequently finding both types of pain. Both types of patients were included in the same study.[8]
Collaterally, since it is not a primary objective of the review, we present four studies that found a reduction in opioid consumption in patients with upper abdominal cancer pain who received celiac neurolysis, compared to conventional analgesic therapy,[26,28,29,34] being like that reported by Nagels et al.[18] and Yan and Myers.[19] These results could not be quantitatively evaluated due to methodological differences between them, such as the presence of patients with and without refractory pain, or the measurement of the variable, either qualitatively (presence and/or absence of consumption of these drugs), or quantitative (amount of your consumption). Although these results are not verifiable, they offer an indirect measure of the efficacy of celiac plexus block (CPB) compared to conventional analgesic therapy as an opioid-sparing strategy in patients with advanced refractory chronic cancer pain.
In the section on adverse effects related to the neurolytic technique, its incidence is variable in the ten selected studies, between 21% and 67%. The main adverse effects were orthostatic hypotension, diarrhoea, and pain at the puncture site in patients who received interventional treatment; and nausea, vomiting, and dizziness in those who received pharmacological treatment. Although in the aforementioned clinical trials, the population was homogeneous, the number of patients is small and the method of detecting these effects in each of the studies was not uniform, so it is not possible to do a quantitative analysis that allows us to offer conclusive results. It is interesting to note, for example, that the study by Wyse et al.[8] only collects the adverse effects and complications that prolong hospitalisation but does not register any event itself. However, if we compare the overall incidence of adverse effects separately in the studies that use both techniques, the difference in the appearance of adverse effects is evident, being higher when a percutaneous technique is performed, as shown in [Figure 3].
Analysing each study, it has been possible to identify that most of them use alcohol in different concentrations as the main neurolytic agent. When stratifying them according to the technique used, it is observed that, when percutaneous neurolysis is performed, using alcohol concentrations at 50%[29] has a lower frequency of adverse events than concentrations between 70% and 100%.[11,17,26,31,36] In contrast, with endoscopy-guided neurolysis, the use of 100% alcohol generates complications that prolong hospitalisation time,[28] while concentrations between 98% and 99% present transitory and rapidly resolving events.[16,17,35]
Adverse effects are non-serious and transitory, except in the study by Abdelghaffar et al.,[11] which uses 25 mL of 10% phenol in a single puncture to compare with 12.5 mL of 10% phenol on each side in a double puncture through the posterior route. This reports one case of haemorrhage and three cases of local infection, where most adverse events, including hypotension and diarrhoea, occurred more frequently in the double puncture group. However, the study with the highest incidence of adverse effects is the one of Saeed et al.,[26] which performs a posterior approach with a single puncture with 15 mL of 50% and 100% alcohol, which is surprising compared to transaortic,[36] transdiscal[17] or double puncture techniques that use even higher volumes of alcohol. In the aforementioned study, those patients that used 100% concentrations had a higher frequency of adverse events compared to those in whom 50% alcohol was used (hypotension and local pain that recovered in a few hours), while this is reversed when evaluating the presence of transient diarrhoea (<72 h duration) since this event was mainly presented by those in whom 50% alcohol was administered.
The adverse effects attributable to CPB that are revealed in the ten identified randomised clinical trials are mild and transitory and can be optimally managed until their disappearance. These findings are like those found in the literature previously.[11,18,19,36] The appearance of these effects is generally due to the anatomical characteristics of the patient or due to the technique used, whether it is open, endoscopic, unilateral, or bilateral; a reason for this is the presence of neurotoxicity due to the high consumption of opioids or can be due to the neurolytic agent, among others. Specifically, the occurrence of some of the effects (hypotension, diarrhoea, and abdominal pain) is attributable to neurolysis of sensory nerve fibres within the celiac plexus and its impact could be related to a proportional analgesic effect, but, depending on the type and technique performed, it may also be caused by the reduction of lumbar sympathetic tone, without an added analgesic benefit.[39]
One aspect evaluated in six of the seven selected clinical trials that are focused on evaluating the efficacy of CPN versus pharmacological analgesic treatment is the quality of life. We found three studies that show significant improvement in patients in favour of CPN[28,29,34] and another three studies where there are no differences with respect to conventional treatment.[24,27,33] The concept of health-related quality of life refers to the subjective perception, influenced by the current disease, of the ability of the patient to carry out the activities that he considers important in his life;[40] therefore, it can be related to the improvement of symptoms as significant as pain or with the reduction of opioid consumption. However, none of the studies has a methodological design to specifically evaluate this variable and whether it evolves in a dependent manner on different aspects of the disease. The loss of patients to follow-up and the methodological differences of the studies did not allow a quantitative synthesis.
Although quality of life is not an objective of this review, we can say that our findings coincide with those of the Yan and Myers review,[19] in which they report better functional scores at 4 weeks in patients who received CPN, turning out to be significant, while, in later weeks, they identified a functional deterioration in both groups. Similar to Nagels et al.,[18] in some studies incorporated into the meta-analysis, they identified that patients who received percutaneous CPN and those who received drug therapy improved their quality of life initially with a subsequent progressive decrease and without statistically significant difference between both therapies. Patients with an oncological disease see their quality of life altered due to the damages that the pathology itself generates in a very diverse way and it is difficult to assess, especially in advanced stages and short survival. CPB could be a complementary therapeutic alternative to improve it, even though its effect is maintained for short periods of time (a few weeks).[8]
Therefore, CPB constitutes a therapeutic alternative that can contribute to pain relief, improve quality of life, and reduce adverse pharmacological effects in certain patients with neoplasms of the upper hemiabdomen and refractory pain. This procedure can be associated with other therapeutic measures (such as systemic opioids) with better results.[37] However, contraindications of the procedure such as haemodynamic compromise, short life expectancy and advanced local compromise must be taken into account.[18]
In this study, the research of the past 20 years on the efficacy and safety of CPN in the treatment of chronic refractory pain due to cancer of the upper abdomen was analysed, following the recommendations of the PRISMA declaration and selecting those studies with the highest quality methodological for its analysis using RevMan 5.3 and, thus, obtain results of high methodological value.
The main limitations of this review were the wide range of time studied, due to the little updated evidence on the subject, the variability of the techniques found, the short duration of patient follow-up, and that, despite the large number of clinical studies Regarding the CPN, many of them are of low methodological quality.
CONCLUSION
According to the results of the meta-analysis carried out, CPN is not superior to pharmacological analgesic therapy for reducing the intensity of chronic refractory pain in patients with cancer of the upper abdomen. Although a quantitative analysis for safety could not be performed, the clinical trials reviewed conclude that CPN has mild and transient adverse effects (a few hours or <3 days).
However, according to the authors’ view, CPN is a treatment that can help patients with severe chronic pain refractory due to oncological pathology of the upper abdomen as a complement to pharmacological analgesic therapy, as it reduces severe pain in the first weeks after the procedure with other collateral benefits such as a reduction in opioid consumption and a temporary (a few weeks) improvement in quality of life.
Despite the extensive literature currently available, studies of higher methodological quality are necessary.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cancer Pain: A Review of Epidemiology, Clinical Quality and Value Impact. Future Oncol. 2017;13:833-41.
- [CrossRef] [PubMed] [Google Scholar]
- Practice Review: Evidence-based and Effective Management of Pain in Patients with Advanced Cancer. Palliat Med. 2020;34:444-53.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of Pain, Acceptance of Illness, Adjustment to Life, and Strategies of Coping with Illness among Patients with Gastric Cancer. Cancer Educ. 2020;35:724-30.
- [CrossRef] [PubMed] [Google Scholar]
- Pain in Patients with Pancreatic Cancer: Prevalence, Mechanisms, Management and Future Developments. Dig Dis Sci. 2017;62:861-70.
- [CrossRef] [PubMed] [Google Scholar]
- Celiac Plexus Block and Neurolysis in the Management of Chronic Upper Abdominal Pain. Semin Intervent Radiol. 2017;34:376-86.
- [CrossRef] [PubMed] [Google Scholar]
- Code of good clinical practice for oncologic pain management. Rev Soc Esp Dolor. 2011;18:98-117.
- [CrossRef] [Google Scholar]
- Practice Guidelines for Endoscopic Ultrasound-Guided Celiac Plexus Neurolysis. Endosc Ultrasound. 2017;6:369-75.
- [CrossRef] [PubMed] [Google Scholar]
- Review of Neurolytic Interventional Procedures in Pain Associated with Pancreatic Cancer. Algorithm Proposal. Rev Soc Esp Dolor. 2019;26:342-58.
- [Google Scholar]
- Celiac Plexus Neurolysis: An Effective Alternative in the Treatment of Persistent Abdominal Pain. Imagenes. 2016;5:59-63.
- [Google Scholar]
- Single Needle Versus Double Needle Celiac Trunk Neurolysis in Abdominal Malignancy Pain Management: A Randomized Controlled Trial. Rev Bras Anestesiol. 2019;69:284-90.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Ultrasonography-Guided Celiac Plexus Neurolysis in Patients with Pancreatic Disease and Pain Refractory to Medical Treatment. Gastroenterol Hepatol. 2005;28:114-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Computed Tomography-Guided Percutaneous Celiac Plexus Neurolysis in Relieving Pain Caused by Abdominal Malignancy. Menoufa Med J. 2018;31:525-30.
- [Google Scholar]
- Computed Tomography-Guided Celiac Plexus Neurolysis for Intractable. Egypt J Radiol Nucl Med. 2017;48:627-37.
- [CrossRef] [Google Scholar]
- Endoscopic Ultrasound-Guided Celiac Plexus Neurolysis in Pancreatic Cancer: A Prospective Pilot Study of Safety Using 10mL versus 20mL Alcohol. Diagn Ther Endosc. 2013;2013:327036.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Evaluation of Different Volumes of 70% Alcohol in Celiac Plexus Block for Upper Abdominal Malignsancies. South Asian J Cancer. 2016;5:204-9.
- [CrossRef] [PubMed] [Google Scholar]
- Celiac Plexus Neurolysis for Abdominal Cancer Pain: A Systematic Review. Pain Med. 2013;14:1140-63.
- [CrossRef] [PubMed] [Google Scholar]
- Neurolytic Celiac Plexus Block for Pain Control in Unresectable Pancreatic Cancer. Am J Gastroenterol. 2007;102:430-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of Endoscopic Ultrasound-Guided Celiac Plexus Block and Celiac Plexus Neurolysis for Managing Abdominal Pain Associated with Chronic Pancreatitis and Pancreatic Cancer. J Clin Gastroenterol. 2010;44:127-34.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med. 2015;162:777-84.
- [CrossRef] [PubMed] [Google Scholar]
- Interrater Reliability of the Modified Jadad Quality Scale for Systematic Reviews of Alzheimer's Disease Drug Trials. Dement Geriatr Cogn Disord. 2001;12:232-6.
- [CrossRef] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre. 2021. Available from: https://research.regionh.dk/en/organisations/det-nordiske-cochrane-center(ffaba9ca-9378-4bd0-a433-b842340abb6d).html [Last accessed on 2021 Nov 24]
- [Google Scholar]
- A Randomized Clinical Trial of Nerve Block to Manage End-Stage Pancreatic Cancerous Pain. Tumour Biol. 2014;35:2297-301.
- [CrossRef] [PubMed] [Google Scholar]
- Celiac Plexus Block with the Long Stylet Needle Technique. Adv Ther. 2007;24:296-301.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Effectiveness and Complications with Two Strengths of Dilution of Alcohol in Celiac Plexus Block in the Management of Relieving Pain. Pak J Med Health Sci. 2019;13:1015-7.
- [Google Scholar]
- The Effects of Early or Late Neurolytic Sympathetic Plexus Block on the Management of Abdominal or Pelvic Cancer Pain. Pain. 2004;110:400-8.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, Double-Blind, Controlled Trial of Early Endoscopic Ultrasound-Guided Celiac Plexus Neurolysis to Prevent Pain Progression in Patients with Newly Diagnosed, Painful, Inoperable Pancreatic Cancer. J Clin Oncol. 2011;29:3541-6.
- [CrossRef] [PubMed] [Google Scholar]
- Neurolytic Celiac Plexus Block: A Better Alternative to Opioid Treatment in Upper Abdominal Malignancies: An Indian Experience. J Pain Palliat Care Pharmacother. 2005;19:15-20.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Neurolytic Celiac Plexus Block on Pain Relief, Quality of Life, and Survival in Patients with Unresectable Pancreatic Cancer: A Randomized Controlled Trial. JAMA. 2004;291:1092-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Neurolytic Celiac Plexus Block Guided by Computerized Tomography on Pancreatic Cancer Pain. Dig Dis Sci. 2008;53:856-60.
- [CrossRef] [PubMed] [Google Scholar]
- A Prospective, Randomized, Double-Blind, Placebo Controlled Trial on the Efficacy of Ethanol Celiac Plexus Neurolysis in Patients with Operable Pancreatic and Periampullary Adenocarcinoma. J Am Coll Surg. 2015;220:497-508.
- [CrossRef] [PubMed] [Google Scholar]
- An Open Randomized Comparison of Clinical Effectiveness of Protocol-driven Opioid Analgesia, Celiac Plexus Block or Thoracoscopic Splanchnicectomy for Pain Management in Patients with Pancreatic and Other Abdominal Malignancies. Pancreatology. 2009;9:755-63.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of EUS-Guided Celiac Plexus Neurolysis Compared with Medication Alone for Unresectable Pancreatic Cancer in the Oxycodone/ Fentanyl Era: A Prospective Randomized Control Study. Gastrointest Endosc. 2020;92:120-30.
- [CrossRef] [PubMed] [Google Scholar]
- A Prospective, Randomized Study of EUS-Guided Celiac Plexus Neurolysis for Pancreatic Cancer: One Injection or Two? Gastrointest Endosc. 2011;74:1300-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Study between 2 Protocols for Management of Severe Pain in Patients with Unresectable Pancreatic Cancer: One-Year Follow-Up. Clin J Pain. 2013;29:807-13.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic-Ultrasound Versus Percutaneous-Guided Celiac Plexus Block for Chronic Pancreatitis Pain. A Systematic Review and Meta-Analysis. Rev Gastroenterol Peru. 2015;35:333-41.
- [Google Scholar]
- Endoscopic Ultrasound-Guided Celiac Plexus Neurolysis (EUS-CPN) Technique and Analgesic Efficacy in Patients with Pancreatic Cancer: A Systematic Review and Meta-Analysis. Pancreatology. 2021;21:434-42.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacotherapy of Cancer Pain. Analgesics and Adjuvants. Farm Profesional. 2008;22:44-9.
- [Google Scholar]
- CT-Guided Celiac Plexus Neurolysis: A Review of Anatomy, Indications, Technique, and Tips for Successful Treatment. Radiographics. 2011;31:1599-621.
- [CrossRef] [PubMed] [Google Scholar]
Annex 1: Search strategy
Efficacy and Safety of Celiac Plexus Neurolysis for the Management of Abdominal Cancer Pain: Systematic review and meta-analysis (2000–2021).
PICO:
Population: Cancer, Neoplasms, Pancreatic cáncer.
Intervention: Neurolysis, Neurolyses, Nerve Block, Celiac plexus, Coeliac plexus, Plexus coeliacus, Solar plexus
Comparison: Pain management
Outcome:
Safety: Adverse effects, Safety, Patient safety
Efficacy: Pain reduction, Efficacy, Rescue analgesia, Effectiveness
Spanish: Eficacia, Seguridad, Seguridad del paciente, Evaluación de Eficacia-Efectividad de Intervenciones; Plexo Celíaco, Neurolisis, Manejo del Dolor; Neoplasias (cáncer); Dolor, Dolor Abdominal, Dolor en Cáncer
Portuguese: Eficácia, Segurança, Segurança do Paciente, Avaliação de Eficácia-Efetividade de Intervenções; Plexo Celíaco, Manejo da Dor; Neoplasias (Câncer); Dor, Dor Abdominal, Dor do Câncer
Search definition:
Pubmed: 12-04-2021 (424resultados)
Search: ((((((‘Pancreatic Neoplasms’[MeSH Terms]) OR (‘Pancreatic Neoplasms’[Text Word])) OR (‘“Pancreatic Neoplasms’[Title/Abstract])) OR (((neoplasm*[Text Word]) OR (neoplasm*[Title/Abstract])) OR (neoplasms[MeSH Terms]))) OR (cancer[Text Word])) OR (cancer[Title/ Abstract])) AND ((((((((((‘celiac plexus’ [Title/Abstract]) OR (‘celiac plexus’[Text Word])) OR (‘coeliac plexus’[Text Word])) OR (‘coeliac plexus’[Title/Abstract])) OR (‘Plexus Coeliacus’[Title/Abstract])) OR (‘Plexus Coeliacus’[Text Word])) OR (‘Solar Plexus’[Text Word])) OR (‘Solar Plexus’[Title/Abstract])) OR (‘Celiac Plexus’[MeSH Terms])) AND (((((((Neurolysis[Title/Abstract]) OR (Neurolysis[Text Word])) OR (Neurolyses[Text Word])) OR (Neurolyses[Title/ Abstract])) OR (‘Nerve Block’[Title/Abstract])) OR (‘Nerve Block’[Text Word])) OR (‘Nerve Block’[MeSH Terms]))) Sort by: Most Recent
Cochrane: 12-04-2021 (97 document results)
((‘Pancreatic Neoplasms’):ti,ab,kw OR (‘Pancreatic Neoplasms’) (Word variations have been searched) OR ((Neoplasm*) OR (Neoplasm*):ti,ab,kw (Word variations have been searched)) OR ((Cancer) (Word variations have been searched) AND ((‘Celiac Plexus’) OR (‘Coeliac Plexus’) OR (‘Coeliac Plexus Block’) OR (‘Plexus Coeliacus’) OR (‘Solar Plexus’)) AND ((Neurolysis) OR (‘Neurolyses’) OR (‘Nerve Block Anesthesia’) OR (‘Nerve Block’) OR (‘Block’)) Scopus: 12-04-2021 (685 document results)
(TITLE-ABS-KEY (‘Pancreatic Neoplasms’) OR TITLE-ABSKEY (Neoplasm*) OR TITLE-ABS-KEY (Cancer)) AND ((TITLE-ABS-KEY (‘Celiac Plexus’) OR TITLE-ABS-KEY (‘Coeliac Plexus’) OR TITLE-ABS-KEY (‘Plexus Coeliacus’) OR TITLE-ABS-KEY (‘Solar Plexus’)) AND (TITLE-ABSKEY (Neurolysis) OR TITLE-ABS-KEY (Neurolyses) OR TITLE-ABS-KEY (‘Nerve Block’)))
Web of Science: 12-04-2021 (406 document results)
Todas las bases de datos: Colección principal de Web of Science, KCI - Korean Journal Database, MEDLINE®, Russian Science Citation Index, SciELO Citation Index (TI=(‘Pancreatic Neoplasms’ OR Neoplasm* OR Cancer) OR AB=(‘Pancreatic Neoplasms’ OR Neoplasm* OR Cancer)) AND (TI=(‘Celiac Plexus’ OR ‘Coeliac Plexus’ OR ‘Plexus Coeliacus’ OR ‘Solar Plexus’) OR AB=(‘Celiac Plexus’ OR ‘Coeliac Plexus’ OR ‘Plexus Coeliacus’ OR ‘Solar Plexus’)) AND (TI=Neurolysis OR Neurolyses OR ‘Nerve Block’ OR Block) OR AB=Neurolysis OR Neurolyses OR ‘Nerve Block’ OR Block)
# 4 406 #3 AND #2 AND #1
# 3 1.272 TI=(‘Celiac Plexus’ OR ‘Coeliac Plexus’ OR ‘Plexus Coeliacus’ OR ‘Solar Plexus’) OR AB=(‘Celiac Plexus’ OR ‘Coeliac Plexus’ OR ‘Plexus Coeliacus’ OR ‘Solar Plexus’)
# 2 1.034.317 TI=(Neurolysis OR Neurolyses OR ‘Nerve Block’ OR Block) OR AB=(Neurolysis OR Neurolyses OR ‘Nerve Block’ OR Block)
# 1 2.628.127 TI=(Cancer OR Neoplasm* OR ‘Pancreatic Cancer’) OR AB=(Cancer OR Neoplasm* OR ‘Pancreatic Cancer’)
Google Scholar: the first 200 results (Neoplasia OR Cancer) AND (Neurolisis OR Bloqueo) AND (‘Plexoceliaco’ OR ‘Plexo Solar’ OR Celiaco OR Solar)







