Translate this page into:
Perception of Social Support and Prevalence of Self-Reported Depressive Symptoms among Patients with Head-and-Neck Squamous Cell Carcinoma Treated at a Tertiary Cancer Centre in North India
*Corresponding author: Ahitagni Biswas, Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi, India. dr_ahitagni@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Rani B, Sinha AP, Sharma KK, Prasad BV, Srinivasan M, Biswas A. Perception of Social Support and Prevalence of Self-Reported Depressive Symptoms among Patients with Head-and-Neck Squamous Cell Carcinoma Treated at a Tertiary Cancer Centre in North India. Indian J Palliat Care. 2024;30:336-41. doi: 10.25259/IJPC_56_2023
Abstract
Objectives:
This study was conducted to determine and correlate the perception of social support and the prevalence of self-reported depressive symptoms among patients with head-and-neck squamous cell carcinoma (HNSCC).
Materials and Methods:
This cross-sectional study included 100 patients with HNSCC receiving treatment at a tertiary cancer centre in north India. They were enrolled by a convenient sampling technique. Subsequently, data regarding sociodemographic profile, clinical profile, perception of social support and prevalence of self-reported depressive symptoms were collected through face-to-face interviews using a subject datasheet, Multidimensional Scale of Perceived Social Support (MSPSS) and Patient Health Questionnaire-9.
Results:
Most of the HNSCC patients, 37%, were in the 42–54 years age category. A male gender predilection (85%) was noted. The two most common subsites involved were the oral cavity (61%) followed by the oropharynx (26%). A majority, 60% of the patients had high social support. Among the subscales of the MSPSS, high social support was obtained majorly from the family (98%), followed by significant others (66%) and friends (52%). The prevalence of self-reported moderate-to-severe depressive symptoms was noted in 36% of patients. The perception of social support and the prevalence of self-reported depressive symptoms showed a weak negative correlation (r = −0.262, P = 0.008).
Conclusion:
Despite receiving high social support, there was a high prevalence of self-reported moderate-to-severe depressive symptoms in patients with HNSCC. Therefore, it is pertinent to monitor the mental health of patients afflicted with HNSCC and provide mental health rehabilitation as per their needs.
Keywords
Head-and-neck cancer
Perception
Prevalence
Social support
Depressive symptoms
INTRODUCTION
Head-and-neck cancer is a group of cancers that arise from the oral cavity, oropharynx, larynx, hypopharynx and nasopharynx. They account for a significant global cancer burden, with an annual incidence of more than 750,000 cases and a mortality of around 350,000 per year.[1] Due to the complexity of the anatomy and the function of the sites affected by head-and-neck squamous cell carcinoma (HNSCC), these patients experience significant physical, psychological and social problems as a consequence of the disease and its treatment.[2] Patients with HNSCC are particularly prone to psychosocial problems due to the adverse impact of the tumour and its subsequent treatment on their communication and emotional expression.[3] In a hospital-based cross-sectional study on head-and-neck cancer patients, Yadav et al. reported that 49% of the patients had major depressive disorder (MDD), 13% of the patients had MDD with melancholic features, and 10% had dysthymia. The authors concluded that depressive disorders are highly prevalent among head-and-neck cancer patients, and the healthcare team has to be sensitive to this issue.[4]
In addition, the importance of social support cannot be overemphasised as it positively impacts people who have cancer.[5] Social support may be vital in the head-and-neck cancer patient population because this disease may disrupt daily activities due to altered speech, eating and facial aesthetics. Patients with HNSCC report less social support at 12 months post-treatment than they do at the time of diagnosis, and social support-seeking behaviours are the most prevalent strategies for coping among such patients.[6] Some studies have shown that adequate social support benefits patients with head-and-neck cancer in coping with cancer-related symptoms and decreasing anxiety and depression. Social support measures prevent social isolation and ensure that the relationship between the individual and the society is maintained.[2]
Only a limited number of studies from the Indian subcontinent have evaluated the perceived social support and depression among head-and-neck cancer patients.[4,7,8] The aim of the present study was to assess and correlate the perception of social support and the prevalence of self-reported depressive symptoms among patients with HNSCC being treated at a major tertiary cancer centre in north India.
MATERIALS AND METHODS
Study design and sample size calculation
This cross-sectional study was conducted on 100 patients with HNSCC from October 2020 to July 2021 after the obtainment of ethical clearance from the Institute Ethics Committee. In the context of a pilot study, the sample size was computed using the statistical formula N= 4pq/d2, where p stands for prevalence, d for precision and q is 1-p. With the anticipated prevalence of each of the two outcomes, that is, social support and depression (p) being 50%, the absolute precision (d) being 10%, and the confidence level being 95%, the calculated sample size (4×50× [100–50]/102) was100.
Data collection
Patients with primary HNSCC, aged between 18 and 65 years and able to understand and communicate in Hindi/English language, were enrolled in this study by convenient sampling method. Patients with known psychiatric illnesses were excluded from this study. A total of 105 patients with HNSCC were screened for inclusion in the study. Out of them, five were excluded (two had known psychiatric illnesses, and three did not meet the age inclusion criteria). All patients were explained about the purpose of the study and confidentiality, and informed consent was taken. Subsequently, the clinico-demographic data were collected by a self-structured tool developed by the researchers. Perception of social support was determined by the Multidimensional Scale of Perceived Social Support (MSPSS). This is a 12-item standardised, brief, psychometrically sound measure of the subjective assessment of the adequacy of the received emotional and social support from the three sources, that is, family, friends and significant others, developed by Zimet et al. in 1988. Response choices are in the form of a 7-point Likert-type scale, that is, 1- very strongly disagree to 7- very strongly agree. The minimum score of MSPSS is 12, and the maximum possible score is 84. A mean score ranging from 1 to 2.9 is considered low support; a score of 3–5 is considered moderate support, and 5.1–7 is considered high support. The internal consistency of the MSPSS, through Cronbach’s coefficient alpha for the total scale, was 0.87.[9] Depressive symptoms (in the preceding 2 weeks) were assessed using patient health questionnaire-9 (PHQ-9), a 9-item questionnaire. The criteria for response choices are 0 (not at all) to 3 (nearly every day). The total score of PHQ-9 ranges from 0 to 27. The severity of depressive symptoms is graded as none or minimal (0–4), mild (5–9), moderate (10–14), moderately severe (15–19) and severe (20–27). PHQ-9 items showed good internal (Cronbach’s alpha = 0.85) and test-retest reliability (interclass correlation coefficient = 0.92).[10] The approximate time taken to respond to the questionnaires ranged from 25 to 30 min.
Statistical analysis
The data were analysed using the Statistical Package for the Social Sciences version 25.0 using descriptive and inferential statistics. The association between the perception of social support and the clinico-demographic variables was assessed using the Kruskal–Wallis test for three or more groups and the Mann–Whitney test for two groups. The association between the prevalence of depressive symptoms and the clinico-demographic variables was assessed using the Fisher exact test. Karl Pearson’s coefficient of correlation was used to assess the correlation of perception of social support with the prevalence of self-reported depressive symptoms. P ≤ 0.05 was considered statistically significant.
RESULTS
Most of the HNSCC patients, 37%, were in the 42–54 years age category. A majority of patients, 85%, were male. The two most common subsites involved were the oral cavity (61%) followed by the oropharynx (26%). A significant number of patients, 85%, were diagnosed at an advanced stage (stage III-IV). A family history of cancer was noted in 12% of the patients. More than half, 56%, of the patients were receiving concurrent chemoradiotherapy as the treatment modality at the time of analysis [Table 1].
| Variables | f (%) |
|---|---|
| Demographic profile | |
| Age | |
| 18–30 years | 6 (6) |
| 30–42 years | 21 (21) |
| 42–54 years | 37 (37) |
| 54–65 years | 36 (36) |
| Gender | |
| Male | 85 (85) |
| Female | 15 (15) |
| Marital status | |
| Married | 93 (93) |
| Unmarried | 5 (5) |
| Others | 2 (2) |
| Educational status | |
| Illiterate | 24 (24) |
| High school certificate | 46 (46) |
| Secondary school | 15 (15) |
| Graduate or above | 15 (15) |
| Occupation | |
| Unemployed | 74 (74) |
| Employed | 12 (12) |
| Others | 14 (14) |
| Family income in rupees | |
| ≤10,001/month | 60 (60) |
| 10,002–29,972/month | 26 (26) |
| More than 29,973 | 14 (14) |
| Place of residence | |
| Urban | 65 (65) |
| Rural | 35 (35) |
| Clinical profile | |
| Tumour site | |
| Oral cavity | 61 (61) |
| Oropharynx | 26 (26) |
| Others | 13 (13) |
| Time since diagnosis | |
| ‘less than 6 months | 41 (41) |
| More than 6 months | 59 (59) |
| Tumour stage | |
| Early-stage (I, II) | 15 (15) |
| Advanced stage (III, IV) | 85 (85) |
| Comorbid illness | |
| Yes | 26 (26) |
| No | 74 (74) |
| Family history of mental illness | |
| Yes | 2 (2) |
| No | 98 (98) |
| Family history of cancer | |
| Yes | 12 (12) |
| No | 88 (88) |
| Treatment modality being received at the time of analysis | |
| Palliative chemotherapy | 1 (1) |
| Neoadjuvant chemotherapy | 3 (3) |
| Radical radiotherapy | 5 (5) |
| Palliative radiotherapy | 8 (8) |
| Post-op radiotherapy | 15 (15) |
| Surgery | 12 (12) |
| Concurrent chemoradiotherapy | 56 (56) |
| ECOG PS | |
| Good (0–1) | 58 (58) |
| Poor (2–4) | 42 (42) |
f (%) = frequency (percentage). HNSCC: Head and neck squamous cell carcinoma, ECOG: Eastern cooperative oncology group, PS: Performance status
Only 2% of the patients had low social support, while 38% of the patients had moderate and 60% of the patients had high social support. Among the subscales of the MSPSS, high social support was obtained majorly from the family (98%), followed by significant others (66%) and friends (52%) [Table 2]. There was no statistically significant association of the clinico-demographic variables with the perception of social support, except for the eastern cooperative oncology group (ECOG) performance status (PS) (P = 0.001) [Table 3]. Out of 100 patients, 18% had moderate depressive symptoms, 12% had moderately severe, and 6% had severe depressive symptoms [Figure 1]. A weak negative correlation was found between the perception of social support and the prevalence of self-reported depressive symptoms (r = −0.262, P = 0.008). Five categories (none to minimal, mild, moderate, moderately severe and severe) of the PHQ-9 tool were merged into two (minimal to mild and moderate to severe). The association of depressive symptoms with the tumour stage (P = 0.045) and the ECOG PS (P = 0.006) were statistically significant. The other clinico-demographic variables did not show any association with depressive symptoms [Table 4]. Patients with moderate-to-severe depressive symptoms were referred to the psychiatry outpatient department for further evaluation.
| Subscales MSPSS | f (%) |
|---|---|
| Family support | |
| Low support | 1 (1) |
| Moderate support | 1 (1) |
| High support | 98 (98) |
| Support from significant others | |
| Low support | 11 (11) |
| Moderate support | 23 (23) |
| High support | 66 (66) |
| Support from friends | |
| Low support | 26 (26) |
| Moderate support | 22 (22) |
| High support | 52 (52) |
MSPSS: Multidimensional scale of perceived social support, HNSCC: Head and neck squamous cell carcinoma
| Variables | Perception of social support | ||
|---|---|---|---|
| f (%) | Median (IQR) | P-value | |
| Age | |||
| 18–30 years | 6 (6) | 66 (46.5–84) | 0.208** |
| 30–42 years | 21 (21) | 61 (50–84) | |
| 42–54 years | 37 (37) | 71 (59.5–84) | |
| 54–65 years | 36 (36) | 84 (60–84) | |
| Gender | |||
| Male | 85 (85) | 70 (60–84) | 0.963* |
| Female | 15 (15) | 72 (57–84) | |
| Marital status | |||
| Married | 93 (93) | 78 (60–84) | 0.125** |
| Unmarried | 5 (5) | 60 (43–76) | |
| Widowed | 2 (2) | 36.5 (12-) | |
| Educational status | |||
| Illiterate | 24 (24) | 64.5 (53–84) | 0.680** |
| High school certificate | 46 (46) | 81 (60–84) | |
| Secondary school | 15 (15) | 84 (60–84) | |
| Graduate or above | 15 (15) | 63 (57–84) | |
| Occupation | |||
| Unemployed | 74 (74) | 84 (60–84) | 0.614** |
| Employed | 12 (12) | 62 (58.5–84) | |
| Others | 14 (14) | 71.5 (54.75–84) | |
| Family income in rupees | |||
| ≤10,001/month | 60 (60) | 67 (53–84) | 0.449** |
| 10,002–29,972/month | 26 (26) | 84 (59.75–84) | |
| More than 29,973/month | 14 (14) | 71 (60.75–84) | |
| Place of residence | |||
| Urban | 65 (65) | 70 (59.5–84) | 0.716* |
| Rural | 35 (35) | 78 (60–84) | |
| Site of tumour | |||
| Oral cavity | 61 (61) | 63 (57.5–84) | 0.138** |
| Oropharynx | 26 (26) | 84 (60–84) | |
| Others | 13 (13) | 72 (56–84) | |
| Time since diagnosis | |||
| less than 6 months | 41 (41) | 84 (60–84) | 0.090* |
| More than 6 months | 59 (59) | 66 (57–84) | |
| Tumour stage | |||
| Early-stage (stage I-II) | 15 (15) | 61 (53–84) | 0.453* |
| Advanced stage (stage III-IV) | 85 (85) | 72 (60–84) | |
| Comorbid illness | |||
| Yes | 26 (26) | 64 (55.75–84) | 0.427* |
| No | 74 (74) | 72 (60–84) | |
| Family history of cancer | |||
| Yes | 12 (12) | 84 (63-84) | 0.115* |
| No | 88 (88) | 68 (57.5-84) | |
| Treatment modality | |||
| Chemotherapy | 4 (4) | 78 (66.75–84) | 0.249** |
| Radiotherapy | 28 (28) | 62 (52–84) | |
| Surgery | 12 (12) | 65 (58.5–84) | |
| Concurrent chemoradiotherapy | 56 (56) | 84 (60–84) | |
| ECOG PS | |||
| Good (0–1) | 58 (58) | 84 (60–84) | 0.001* |
| Poor (2–4) | 42 (42) | 60 (49.75–84) | |
Kruskal–Wallis H test**, Mann–Whitney U-test*. HNSCC: Head-and-neck squamous cell carcinoma, ECOG: Eastern cooperative oncology group, PS: Performance status, f: frequency; IQR: interquartile range
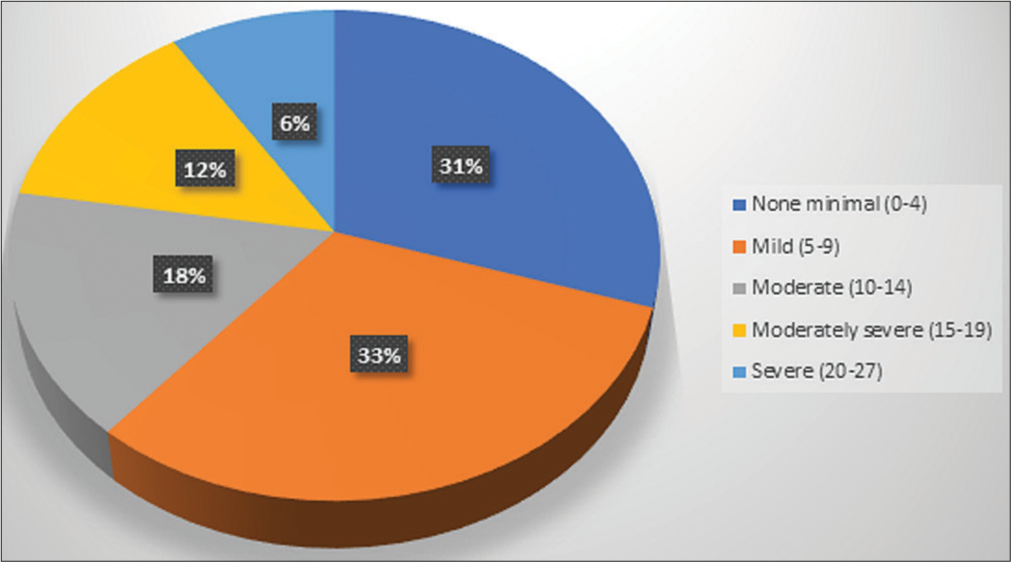
- Pie chart showing the prevalence of self-reported depressive symptoms in patients with head-and-neck squamous cell carcinoma (n = 100).
| Variables | Self-reported depressive symptoms | ||
|---|---|---|---|
| Minimal to mild (%) | Moderate to severe (%) | P-value# | |
| Age | |||
| 18–30 years | 3 (50) | 3 (50) | 0.668 |
| 30–42 years | 12 (57.1) | 9 (42.9) | |
| 42–54 years | 24 (64.9) | 13 (35.1) | |
| 54–65 years | 25 (69.4) | 11 (30.6) | |
| Gender | |||
| Male | 56 (65.9) | 29 (34.1) | 0.390 |
| Female | 8 (53.3) | 7 (46.7) | |
| Marital status | |||
| Married | 59 (63.4) | 34 (36.6) | 0.838 |
| Unmarried | 4 (80) | 1 (20) | |
| Widowed | 1 (50) | 1 (50) | |
| Educational status | |||
| Illiterate | 16 (66.7) | 8 (33.3) | 0.770 |
| High school certificate | 27 (58.7) | 19 (41.3) | |
| Secondary school | 10 (66.7) | 5 (33.3) | |
| Graduate or above | 11 (73.3) | 4 (26.7) | |
| Occupation | |||
| Unemployed | 46 (62.2) | 28 (37.8) | 0.062 |
| Employed | 11 (91.7) | 1 (8.3) | |
| Others | 7 (50) | 7 (50) | |
| Family income in rupees | |||
| ≤10,001/month | 36 (60) | 24 (40) | 0.464 |
| 10,002–29,972/month | 17 (65.4) | 9 (34.6) | |
| More than 29,973/month | 11 (78.6) | 3 (21.4) | |
| Place of residence | |||
| Urban | 45 (69.2) | 20 (30.8) | 0.190 |
| Rural | 19 (54.3) | 16 (45.7) | |
| Tumour site | |||
| Oral cavity | 37 (60.7) | 24 (39.3) | 0.570 |
| Oropharynx | 17 (65.4) | 9 (34.6) | |
| Others | 10 (76.9) | 3 (23.1) | |
| Time since diagnosis | |||
| less than 6 months | 28 (68.3) | 13 (31.7) | 0.528 |
| More than 6 months | 36 (61) | 23 (39) | |
| Tumour stage | |||
| Early stage (stage I-II) | 6 (40) | 9 (60) | 0.045 |
| Advanced stage (stage III-IV) | 58 (68.2) | 27 (31.8) | |
| Comorbid illness | |||
| Yes | 14 (53.8) | 12 (46.2) | 0.240 |
| No | 50 (67.6) | 24 (32.4) | |
| Family history of cancer | |||
| Yes | 9 (75) | 3 (25) | 0.529 |
| No | 55 (62.5) | 33 (37.5) | |
| Treatment modality | |||
| Chemotherapy | 3 (75) | 1 (25) | 0.626 |
| Radiotherapy | 15 (53.6) | 13 (46.4) | |
| Surgery | 8 (66.7) | 4 (33.3) | |
| Concurrent chemoradiotherapy | 38 (67.9) | 18 (32.1) | |
| ECOG PS | |||
| Good (0–1) | 44 (75.9) | 14 (24.1) | 0.006 |
| Poor (2–4) | 20 (47.6) | 22 (52.4) | |
#Fisher’s exact test. HNSCC: Head-and-neck squamous cell carcinoma, ECOG: Eastern cooperative oncology group, PS: Performance status
DISCUSSION
In this study of 100 patients with HNSCC, a male preponderance (85%) was noted, typical for an HNSCC cohort.[11,12] Most of them were diagnosed with oral cavity cancer (61%), as reported in other studies.[13-15] The majority of the patients (85%) presented at an advanced stage due to delayed health-seeking behaviour in the Indian subcontinent, reflecting the results of some other studies.[12]
Social support plays a crucial role among patients with cancer, especially of the head and neck, because the site of the tumour itself is distressing, and treatment-related sequelae are also unique to these patients, often leading to impaired communication and emotional expression. In the present study, most of the patients (60%) had overall high social support. These findings are consistent with the results of the studies by Eadie et al. and Ng et al.[13,14] In the social support subscales, the patients received social support mostly from their families, mirroring similar findings from the study by Somasundaram and Devamani.[7] This could be due to the sociocultural structure of the Indian families, where people take more responsibility for looking after their family members. In the present study, the patients with good PS (ECOG PS 0–1) had significantly higher perceptions of social support, reflecting similar findings from the study by Yilmaz et al.[15]
The prevalence of depression (clinical diagnosis or symptoms of depression) among head-and-neck cancer patients is high and depends on the type of measurement tools and the assessment time.[16] In a systematic review, Haisfield-Wolfe et al. reported that over the period, prevalence rates of depression vary from 13 to 40% at diagnosis, 25–52% during treatment and 11–45% in the first 6 months after treatment.[17] In the present cross-sectional study, due to the convenient sampling technique and the ongoing coronavirus disease 2019 (COVID-19) pandemic, patients with HNSCC were enrolled irrespective of any specific time point in their illness trajectory, and serial temporal assessments were not done at predefined time points. The prevalence of self-reported moderate-to-severe depressive symptoms (PHQ-9 score of 10–27) was 36% in our study.
It is notable that despite receiving high social support (60% of patients), there was still a high prevalence of self-reported moderate to severe depressive symptoms (36% of patients) in the current study. A weak negative correlation (P = 0.008) was found between the perception of social support and the prevalence of self-reported depressive symptoms. This suggests that social support alone may not be sufficient to alleviate depressive symptoms in this population, and additional interventions may be needed to address mental health concerns. A plethora of studies have demonstrated that higher social support is significantly associated with less depressive symptoms and a higher general mental health score.[3,6,18-20] However, in the study by Katz et al. on multiple regression analysis, social support was not related to depressive symptoms in surgically treated patients with HNSCC.[21]
A higher locoregional disease burden in patients with stage III-IVB HNSCC may result in increased difficulty in eating, swallowing, pain, discomfort and sleeping issues reflected by a relatively higher prevalence of depression.[22] However, on subgroup analysis in the present study, the prevalence of moderate-to-severe depressive symptoms was 60% versus 31.8% in patients with early and advanced stages, respectively. This paradoxical result may be attributed to the relatively low number of patients with early-stage cancer (15%) in this study. In addition, the patients with poor PS (ECOG PS 2–4) were significantly more likely to have moderate-to-severe depressive symptoms in this study. This is in concordance with the results of studies by Yadav et al. and Hammerlid et al.[4,23] Further, this may be explained by the fact that patients with HNSCC, who have a considerable limitation of day-to-day activity, are more likely to be diagnosed with psychological distress, MDD and melancholic features.
Relative heterogeneity pertaining to the sites of HNSCC, the different treatment modalities, the time points of assessment of perception of social support and the prevalence of self-reported depressive symptoms, and the lack of temporal assessment of these parameters at predefined time points (e.g. before, during and after treatment) are some of the limitations of this cross-sectional study, which was conducted in challenging circumstances amidst the resource limitations of a tertiary cancer centre in a low-middle income country during an ongoing COVID-19 pandemic. The convenience sampling method used in this study might have introduced selection bias and limited the generalisability of the findings. We relied on self-reported measures of depressive symptoms, which may not accurately reflect the true prevalence of depression in the study population. Objective assessment of depression by clinical interviews or diagnostic assessments, alongside self-reported data, may provide a more accurate estimate of the prevalence of depression. Finally, the cross-sectional design of our study limits the ability to establish causal relationships between social support and depressive symptoms.
Based on the study results, we recommend the implementation of routine screening for depression in patients with HNSCC with high symptom burden using validated assessment tools, followed by personalised intervention plans. These interventions may include cognitive behavioural therapy, pharmacological treatments and psychoeducation to help patients manage their symptoms effectively. Future directions for improving mental health support in patients with HNSCC could involve (a) enhanced screening protocols: regular and systematic screening for mental health issues at various stages of cancer treatment to ensure timely intervention; (b) multidisciplinary approaches: collaboration between oncologists, psychiatrists, psychologists and social workers to create a holistic treatment plan that addresses both physical and mental health needs; (c) patient education: providing resources and information to patients and their families about the psychological impact of cancer and available support services; (d) telehealth services: expanding access to mental health support through telehealth platforms, especially for patients in remote areas or those with mobility challenges and (e) research and training: conducting further research on the mental health needs of such patients and training healthcare providers to recognise and address these needs effectively.
CONCLUSION
Despite receiving high social support, there was a high prevalence of self-reported moderate to severe depressive symptoms in patients with HNSCC in north India. These findings emphasise the need for targeted mental health monitoring and rehabilitation and underscore the importance of a multidisciplinary approach to cancer care that addresses the psychological and social needs of patients, particularly those with high symptom burden.
Acknowledgments
We express gratitude to all the study participants for their co-operation and devotion of time during the data collection period, without which it might not have been possible to complete this project.
Ethical approval
The research/study was approved by the Institutional Review Board at Institute Ethics Committee for Postgraduate Research AIIMS New Delhi, number IECPG-294/22 July 2020, dated 22 July 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [Google Scholar]
- Assessing Functional Status and Social Support in the Head and Neck Cancer Patients. Eur J Oncol Nurs. 2014;18:S49.
- [CrossRef] [Google Scholar]
- Negative and Positive Influences of Social Support on Depression in Patients with Head and Neck Cancer: A Prospective Study. Psychooncology. 2000;9:20-8.
- [CrossRef] [Google Scholar]
- Prevalence of Depressive DISORDers among Head-and-neck Cancer Patients: A Hospital-based, Cross-Sectional Study. Indian J Psychiatry. 2019;61:409-14.
- [CrossRef] [Google Scholar]
- Perceived Social Support and Its Impact on Depression and Health-related Quality of Life: A Comparison between Cancer Patients and General Population. Jpn J Clin Oncol. 2017;47:728-34.
- [CrossRef] [Google Scholar]
- Influence of Social Support on Health-related Quality of Life Outcomes in Head and Neck Cancer. Head Neck. 2007;29:143-6.
- [CrossRef] [Google Scholar]
- A Comparative Study on Resilience, Perceived Social Support and Hopelessness among Cancer Patients Treated with Curative and Palliative Care. Indian J Palliat Care. 2016;22:135-40.
- [CrossRef] [Google Scholar]
- Assessment of Fatigability,Depression, and Self-esteem among Head-and-neck Carcinoma Patients in a Tertiary Care Hospital in South India. J Cancer Res Ther. 2019;15:645-52.
- [CrossRef] [Google Scholar]
- The Multidimensional Scale of Perceived Social Support. J Pers Assess. 1988;52:30-41.
- [CrossRef] [Google Scholar]
- The PHQ-9: Validity of a Brief Depression Severity Measure. J Gen Intern Med. 2001;16:606-13.
- [CrossRef] [Google Scholar]
- Depression, Anxiety, Fatigue, and Quality of Life in a Large Sample of Patients Suffering from Head and Neck Cancer in Comparison with the General Population. BMC Cancer. 2021;21:94.
- [CrossRef] [Google Scholar]
- Clinical Predictors of Quality of Life in Patients with Head and Neck Cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401-8.
- [CrossRef] [Google Scholar]
- Role of Psychosocial Factors on Communicative Participation among Survivors of Head and Neck Cancer. Otolaryngol Head Neck Surg. 2018;159:266-73.
- [CrossRef] [Google Scholar]
- Anxiety, Depression, Perceived Social Support and Quality of Life in Malaysian Breast Cancer Patients: A 1-year Prospective Study. Health Qual Life Outcomes. 2015;13:205.
- [CrossRef] [Google Scholar]
- Social Support and Quality of Life in a Group of Cancer Patients (Ankara, Turkey) Turk J Med Sci. 2017;47:732-7.
- [CrossRef] [Google Scholar]
- Prevalence of Depression in Cancer Patients: A Meta-analysis of Diagnostic Interviews and Self-report Instruments. Psychooncology. 2014;23:121-30.
- [CrossRef] [Google Scholar]
- Prevalence and Correlates of Depression among Patients with Head and Neck Cancer: A Systematic Review of Implications for Research. Oncol Nurs Forum. 2009;36:E107-25.
- [CrossRef] [Google Scholar]
- Correlates of Depressed Mood in Ambulatory Head and Neck Cancer Patients. Psychooncology. 1999;8:494-9.
- [CrossRef] [Google Scholar]
- Caring for Head and Neck Oncology Patients. Does Social Support Lead to Better Quality Of Life? Can Fam Physician. 1996;42:1712-20.
- [Google Scholar]
- Sociodemographic Risk Indicators for Depressive Symptoms among Persons with Oral Cancer or Oral Epithelial Dysplasia. J Oral Maxillofac Surg. 2005;63:513-20.
- [CrossRef] [Google Scholar]
- Psychosocial Adjustment in Head and Neck Cancer: The Impact of Disfigurement, Gender and Social Support. Head Neck. 2003;25:103-12.
- [CrossRef] [Google Scholar]
- Depression and Survival in Head and Neck Cancer Patients. Oral Oncol. 2017;65:76-82.
- [CrossRef] [Google Scholar]
- A Prospective Multicentre Study in Sweden and Norway of Mental Distress and Psychiatric Morbidity in Head and Neck Cancer Patients. Br J Cancer. 1999;80:766-74.
- [CrossRef] [Google Scholar]







