Translate this page into:
A Prospective Longitudinal Study to Demonstrate the Utility of the Palliative Prognostic Index in Forecasting the Short-term Survival of Patients with Advanced Cancer in India
*Corresponding author: Avinash Tiwari, Department of Palliative Medicine and Supportive Care, Sanjeevani CBCC USA Cancer Hospital, Raipur, Chhattisgarh, India. doctoravinashtiwari@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tiwari A, Ghoshal A, Deodhar JK, Muckaden MA. A Prospective Longitudinal Study to Demonstrate the Utility of the Palliative Prognostic Index in Forecasting the Short-term Survival of Patients with Advanced Cancer in India. Indian J Palliat Care. 2024;30:353-7. doi: 10.25259/IJPC_104_2024
Abstract
Objectives:
In this study, our primary objectives were to validate the palliative prognostic index (PPI) tool in the context of palliative care for patients with advanced cancer. Specifically, we aimed to assess the accuracy of the PPI in predicting actual survival in these patients through prospective validation.
Materials and Methods:
To achieve our objectives, we enrolled a cohort of 227 advanced cancer patients receiving palliative care. The study population comprised 132 (58.1%) men and 95 (41.9%) women, with a median age of 52 years (Range: 20–81). Among them, 56 (24.7%) underwent chemotherapy, and 26 (11.5%) underwent palliative radiotherapy. We utilised the PPI score to categorise patients into three prognostic groups: (a) PPI score <4 indicating likely survival of more than 6 weeks; (b) PPI score 4–6 indicating likely survival shorter than 6 weeks; and (c) PPI score >6 indicating likely survival <3 weeks.
Results:
Through our analysis, we found that the PPI demonstrated limited predictive capabilities, particularly for short-term survival (<3 weeks). The PPI’s performance metrics included a positive predictive value of 45.24%, a negative predictive value of 100%, a sensitivity of 100.00% and a specificity of 88.94%.
Conclusion:
In conclusion, our study establishes the limited reliability of the PPI in predicting short-term survival (<3 weeks) among patients in palliative care with advanced cancer. These findings underscore the PPI’s potential as a valuable tool for healthcare professionals, aiding in the development of treatment plans and facilitating discussions on end-of-life care options with patients and their families. In addition, the PPI may assist healthcare professionals in identifying individuals who could benefit from more aggressive interventions or those approaching the end of life, thereby guiding the provision of additional support and care.
Keywords
Advanced cancer
Prognostic factors
Prognostic tool
Palliative care
INTRODUCTION
Patients with advanced cancer, their families and the healthcare staff looking after them often want to know about their prognosis.[1-4] It has a central role in decision-making regarding treatment options, preparation for death and timely resolution of end-of-life issues. However, it has been noted quite often that clinician predictions are inaccurate and over-optimistic and were the subject of a recent review.[5] In 2005, a Working Group of the Research Network of the European Association for Palliative Care identified evidence-based recommendations regarding prognostication in advanced cancer (stage III or stage IV, relapsed, refractory cancer).[6] Again, in June 2018, a panel of prognostic researchers and clinicians convened an international prognostication workshop at the Multinational Association for Supportive Care in Cancer annual meeting in Vienna, Austria.[7] Apart from these, evidence-based prognostication in patients with advanced cancer has been examined through various models incorporating performance status, symptoms such as anorexia–cachexia, dyspnoea and delirium and laboratory parameters such as leucocytosis, lymphopenia and high C-reactive protein.[7,8] Most of these models have found their predictions of survival to be associated with actual survival but limited by the strength of the association, with correlation coefficients varying from 0.2 to 0.65.[6] One of the six key recommendations of the working group of the European Association for Palliative Care was the systematic use by health workers of prognostic scores designed to divide patients into groups with significantly different survival times.[6] They also recommended that a clinical prediction of survival be used in partnership with attention to prognostic factors when assessing prognosis. The two prognostic scores or tools specifically considered in the report of the working group were the Palliative Prognostic (PaP) score and the Palliative Prognostic Index (PPI).
The PaP score is based on Karnofsky’s performance status, the presence or absence of dyspnoea and anorexia, white blood cell counts and the clinician’s prediction of survival (given high weightage). It has been validated successfully, both in Italy and Australia, in hospitals and hospices for patients with cancer.[9-11] Potential limitations of the PaP are the omission of delirium, the dependence on laboratory testing and the higher weightage given to the clinician’s prediction of survival.
The PPI was developed and successfully validated in hospice in patients with advanced cancer in Japan.[12] They subsequently conducted a study which demonstrated improved accuracy of physicians’ survival predictions with the use of the PPI.[13] The PPI relies on the assessment of performance status using the palliative performance scale (PPS),[14] oral intake and the presence or absence of dyspnoea, oedema and delirium but does not require blood tests or incorporate a clinical prediction of survival. The resulting score puts the patient into one of three groups, predicting survival of shorter than 3 weeks (PPI score >6), shorter than 6 weeks (PPI score >4), or more than 6 weeks (PPI score ≤4). Our study represents the first attempt to validate the PPI in a geographically and culturally different population in India on patients with advanced cancer attending a palliative care clinic.
MATERIALS AND METHODS
This study was carried out in a specialist palliative care clinic in a tertiary cancer care centre in India. Approval for the study was granted by the Institutional Ethics Committee at the Tata Memorial Hospital, project number 3128, and registered with the Clinical Trials Registry – India (CTRI)/2019/03/018141. No formal sample size was calculated for this exploratory study. All adult patients with advanced cancer (any type of stage III or stage IV, relapsed, refractory cancer) referred to the service over a period (April 2019 to June 2021 to June 2021) were included in the study. Families that could not provide reliable means of communication to verify the living status of study participants were excluded from the study. Patients were assessed at the time of first contact with the service. Demographics and information required to determine the PPI were recorded by the registered nurses or doctors who first assessed the patient. Experience in palliative care of the nurses and doctors ranged from months to several years.
The five variables used to determine the PPI are as follows: oral intake, the presence or absence of oedema, dyspnoea at rest, delirium and performance status, as measured by the PPS. The PPS measures physical performance and is measured in 10% decrements from fully ambulatory and healthy (100%) to death (0%). The PPS score, oral intake and presence of dyspnoea at rest were recorded as reported by the patient. If this was not possible, they were determined by observation and by discussion with family or nursing staff. Patients receiving total parenteral nutrition or feeding through enterostomies were recorded as having a ‘normal’ oral intake. Delirium was diagnosed according to the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.[15] Delirium was judged to be absent if considered to be caused by a single medication, as per the protocol of the original development and validation study of the PPI.[12] Information regarding the date of death was obtained from the family while they were contacted as a part of routine practice, and subsequently, the actual survival, in days, from enrolment was calculated.
Application of the cutoff points of PPI 4 and PPI 6 splits the sample into three groups based on the PPI score. Kaplan– Meier survival curves were constructed for each of the three groups, and a Cox proportional hazards regression was used to examine the relationship between survival and PPI as a continuous covariate. Positive predictive value (PPV) and negative predictive value (NPV) predictions of survival of <3 weeks and <6 weeks were calculated.[16] Statistical analysis was conducted using the Statistical Package for the Social Sciences Statistics for Windows, Version 24.0.[17]
RESULTS
We approached 242 patients referred to the service over a period (April 2019 to June 2021 to June 2021), but 227 (93.8%) consented to this study – 132 (58.1%) were men, and 95 (41.9%) were women. The median age of the included population was 52 (Range: 20–81) years; 56 (24.7%) were receiving chemotherapy, while 26 (11.5%) were on palliative radiotherapy. Table 1 shows all demographic and clinical characteristics of the patient.
| Items | n(%) |
|---|---|
| Total number of patients | 227 |
| Male | 132 (58.1) |
| Female | 95 (41.9) |
| Mean age | 52 (Range: 20–81) |
| Receiving chemotherapy | 56 (24.7) |
| Receiving palliative radiotherapy | 26 (11.5) |
| No cancer-modifying treatment | 145 (63.9) |
| Sites of cancer | |
| Bone and soft tissue | 6 (2.6) |
| Breast | 17 (7.5) |
| Gastrointestinal | 61 (26.9) |
| Genitourinary | 13 (5.7) |
| Gynaecological | 35 (15.4) |
| Haematolymphoid | 14 (6.2) |
| Head and neck | 40 (17.6) |
| Neurological (brain and spinal cord) | 2 (0.9) |
| Thoracic | 39 (17.2) |
PPI components
Oral intake was severely reduced (< mouthfuls) in 33 (14.5%), moderately reduced (> mouthfuls) in 134 (59.1%) and normal in 60 (26.4%) patients,
Oedema was present in 82 (36.1%) patients,
Dyspnoea at rest was present in40 (17.6%) of patients,
Delirium was present in 14 (6.2%) patients,
On the Performance Status, as measured by the PPS, 136 (59.9%) patients had PPS between 30 and 50, 84 (37.0%) had PPS more or equal to 60% and 7 (3.1%) scored 10–20% [Table 2].
| Palliative performance scale (%) | |
| 10–20 | 7 (3.1) |
| 30–50 | 136 (59.9) |
| ≥60 | 84 (37.0) |
| Oral intake (%) | |
| Severally reduce | 33 (14.5) |
| Moderately reduce | 134 (59.1) |
| Normal | 60 (26.4) |
| Oedema (%) | |
| Present | 82 (36.1) |
| Absent | 145 (63.9) |
| Dyspnoea at rest (%) | |
| Present | 40 (17.6) |
| Absent | 187 (82.4) |
| Delirium (%) | |
| Present | 14 (6.2) |
| Absent | 213 (93.8) |
The PPI was used to split patients into three subgroups as follows: Group 1 corresponds to patients with PPI ≤4, Group 2 corresponds to those with PPI >4 and ≤6 and Group 3 corresponds to patients with PPI >6.
Survival was calculated from the time of enrolment to the time of death (if it occurred during the study period) or to the time of censoring of data. At the time of analysis, actual survival data were available for 171 (75.3%) patients, with a survival range of 37–62 days. A Kaplan–Meier curve was constructed for each of the groups. The median survival for Groups 1, 2 and 3 were 78, 24 and 26 days, respectively. The 95% confidence intervals (CI) for these are summarised in Table 3 and demonstrate the substantial statistically significant difference in the median survival times (P < 0.001). The actual Kaplan–Meier survival curves for the three groups are shown in Figure 1. A Cox proportional hazards regression was used to examine the relationship between survival and PPI as a continuous covariate; the Hazard Ratio associated with a one-unit increase in PPI score is 1.297 (95% CI 1.163, 1.391), P < 0.001.
| Palliative prognostic index | Total number of patients | Events | Meana | Median | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | 95% confidence interval | Estimate | Std. Error | 95% confidence interval | |||||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||||
| ≤4 | 42 | 36 | 19.649 | 6.143 | 7.608 | 31.690 | 4.000 | 0.883 | 2.270 | 5.730 |
| 4–6 | 55 | 49 | 12.800 | 3.848 | 5.258 | 20.342 | 5.000 | 0.915 | 3.208 | 6.792 |
| >6 | 130 | 86 | 42.870 | 4.588 | 33.877 | 51.862 | 16.000 | 2.820 | 10.472 | 21.528 |
| Overall | 227 | 171 | 31.696 | 3.198 | 25.427 | 37.964 | 9.000 | 1.159 | 6.729 | 11.271 |
| Overall comparisons. | ||||||||||
| Chi-square | df | Sig. | ||||||||
| Log Rank (Mantel-Cox) | 40.786 | 2 | 0.000 | |||||||
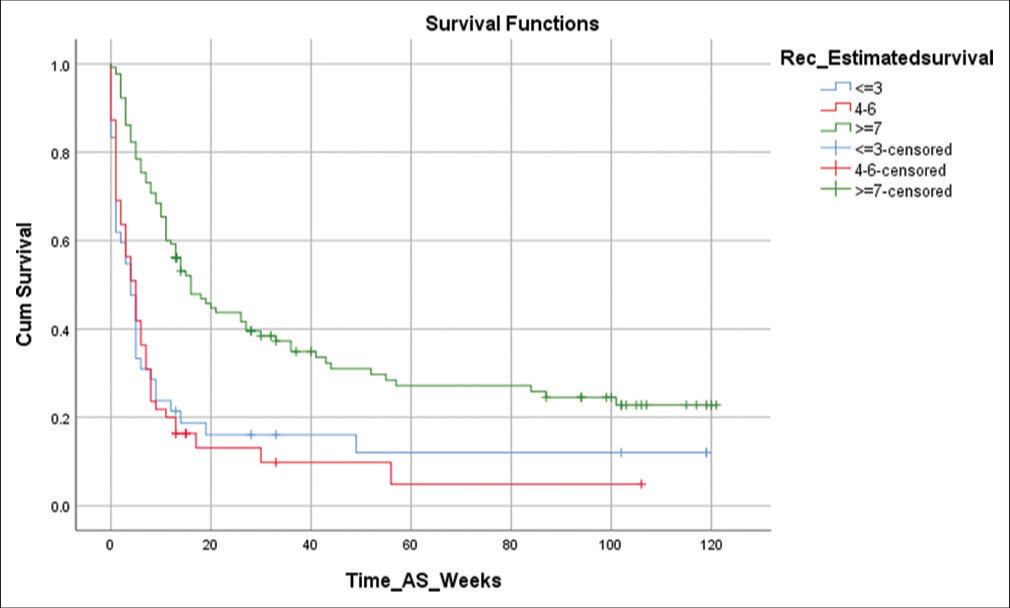
- Kaplan–Meier survival curves for each palliative prognostic index group.
Using the PPI, survival of <3 weeks (PPI >6) was predicted with a PPV of 45.24%, NPV of 100%, positive likelihood ratio of 9.04, sensitivity of 100.00% and specificity of 88.94%, while survival of <6 weeks (PPI 4–6) was predicted with a PPV of 20%, NPV of 86.05%, positive likelihood ratio of 1.37, sensitivity of 31.43% and specificity of 77.08% [Appendix 1].
DISCUSSION
In this study, we have tried to validate the PPI in an Indian population. It is one of the first such attempts to our knowledge, and we have followed the method described by previous work on PPI, dividing the population of patients with advanced cancer into three groups, each with a significantly different survival profile.[13,18] In addition, we have shown that the scope of validity of the PPI extends to patients attending a palliative care clinic beyond the characteristics of the inpatient hospice setting in which it was originally developed.[12]
A direct comparison of patient characteristics in this study with those in the study by Morita et al.[12] and Stone et al.[18] shows that there are many demographic variances between the patients included in this study and those patients in whom the tool was originally developed and validated [Appendix 1]. In this study, 100% were enrolled on the clinic, while in Stone et al., 73.7% of patients’ place of care was the hospital, 25.8% were home, and 0.5% were hospice at the time of enrolment in the study, whereas all patients included in the initial validation study by Morita et al. were hospice inpatients. Cancer of the upper gastrointestinal tract accounted for 22% in this study, while 5% of patients in Stone et al. and 23% of patients in Morita et al., reflecting recognised variances in cancer demographics between India, Ireland and Japan. The mean age in this study was 52 years, versus 70 years in Stone et al. and 67 years in Morita et al. The incidence of recorded symptoms was comparable, except for delirium (6.2 vs. 23% in Morita et al. vs. 9% in Stone et al.).[12,18] Research into the incidence of delirium in patients with advanced cancer has shown it to be in the estimates of 4–12% in the community, 9–57% across hospital palliative care consultative services and 6–74% in inpatient palliative care units.[19] The comparatively low incidence of delirium and a high proportion of people with good performance status in this study is likely to reflect the fact that many patients were referred to palliative care clinics earlier in their disease trajectory and that patients receiving antineoplastic therapy were included in the study.
In this study, performance measures for PPI to predict survival of fewer than 3 weeks (PPI >6) were highly specific and sensitive, comparable to the study by Morita et al.[12] Although the PPV was less than in the original study (NPV [%] for PPI >6 is more), the positive likelihood ratio is high, 9.04, implying better performance of this model than that of PPI 4–6 (survival shorter than 6 weeks). This suggests that the PPI has a prominent level of accuracy in those patients that it identifies as having a short prognosis, but it will not identify all patients with a short prognosis. There is some evidence that prior experience in oncology or palliative care is associated with increased prognostic accuracy.[5] The fact that the PPI predicts 3-week survival better than 6 weeks in this population suggests that it may be used by medical and nursing staff not experienced in oncology or palliative care to attain accuracy comparable, if not superior, to that of physicians with significant experience for predicting short-term survival. Elsewhere, the use of the PPI has been shown to improve the clinical predictions of survival by doctors experienced in palliative care.[13] This finding of increased accuracy using a combination of a prognostic tool and the clinician prediction of survival (CPS) has also been demonstrated using the support prognostic model,[20] intensive care unit scoring systems[21] and the hospitalised elderly longitudinal project survival model.[22]
A recent review has highlighted the importance of prognostic tools in combination with the CPS to estimate the survival of patients with advanced cancer.[7] The PPI is quick and easy to use and does not require invasive tests like blood sampling. It incorporates common clinical signs and symptoms recorded in routine care and does not require specialist knowledge to ensure accuracy. With proper training generalists, palliative care providers may use it to achieve prognostic accuracy comparable to specialists in palliative care. [23,24] We have shown that it is valid for use in patients with advanced cancer who are receiving antineoplastic therapies attending a palliative care clinic.
Limitation of the study
It is a single-centre study from a tertiary cancer centre in Mumbai, India. There is a possibility that these results might vary if done in other parts of the country. Another potential bias might be due to recruitment being limited only to patients attending the clinic and the inability to capture data from in-patients or those under hospice care. Due to the small sample size, we could not appreciate the effect of cancer-modifying treatment or complementary/alternative medicine in this study, limiting its results. Performance measures like PPV are not intrinsic to the test but depend on the prevalence, which could not be accounted for in this study.[25]
CONCLUSION
Short-term survival (<3 weeks) of patients with advanced cancer attending a palliative care clinic can be predicted by the PPI scoring system with limited accuracy. These scores could help researchers construct and compare future prognostic models for such patients. The method of scoring used for the PPI is easy, objective and reproducible; bereft of any invasive examinations such as blood sampling.
Ethical approval
The research/study was approved by the Institutional Review Board at the Institutional Ethics Committee at the Tata Memorial Hospital, number project number 3128, dated 08 th October 2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Arun Ghoshal and Dr. Jayita K Deodhar are on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary material available on:
Financial support and sponsorship
Nil.
References
- Discussions of Life Expectancy and Changes in Illness Understanding in Patients With Advanced Cancer. J Clin Oncol. 2016;34:2398-403.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced Cancer Patients' Understanding of Prognostic Information: Applying Insights from Psychological Research. Cancer Med. 2019;8:4081-8.
- [CrossRef] [PubMed] [Google Scholar]
- "I Want to Live, but …" the Desire to Live and Its Physical, Psychological, Spiritual, and Social Factors among Advanced Cancer Patients: Evidence from the APPROACH Study in India. BMC Palliat Care. 2022;21:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- To Tell or Not to Tell: Exploring the Preferences and Attitudes of Patients and Family Caregivers on Disclosure of a Cancer-Related Diagnosis and Prognosis. J Glob Oncol. 2019;5:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11:e0161407.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Factors in Advanced Cancer Patients: Evidence-based Clinical Recommendations--a Study By the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostication in Advanced Cancer: Update and Directions for Future Research. Support Care Cancer. 2019;27:1973-84.
- [CrossRef] [PubMed] [Google Scholar]
- Development of Prognostication Model of 60-Day Survival in Ambulatory Cancer Patients Receiving Palliative Care. Symptoms Surviv. 2022;40(16 Suppl):e24091.
- [CrossRef] [Google Scholar]
- Diagnostic Accuracy of the Palliative Prognostic Score in Hospitalized Patients with Advanced Cancer. J Clin Oncol. 2004;22:4823-8.
- [CrossRef] [PubMed] [Google Scholar]
- Independent Prospective Validation of the PaP Score in Terminally Ill Patients Referred to a Hospital-based Palliative Medicine Consultation Service. J Pain Symptom Manage. 2001;22:891-8.
- [CrossRef] [PubMed] [Google Scholar]
- A New Palliative Prognostic Score: A First Step for the Staging Of Terminally Ill Cancer Patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:231-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Palliative Prognostic Index: A Scoring System for Survival Prediction of Terminally Ill Cancer Patients. Support Care Cancer. 1999;7:128-33.
- [CrossRef] [PubMed] [Google Scholar]
- Improved Accuracy of Physicians' Survival Prediction for Terminally Ill Cancer Patients Using the Palliative Prognostic Index. Palliat Med. 2001;15:419-24.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative Performance Scale (PPS): A New Tool. Palliat Care. 1996;12:5-11.
- [CrossRef] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders United States: American Psychiatric Association; 2013.
- [CrossRef] [Google Scholar]
- Released 2016 In: IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016.
- [Google Scholar]
- Prospective Validation of the Palliative Prognostic Index in Patients with Cancer. J Pain Symptom Manage. 2008;35:617-22.
- [CrossRef] [PubMed] [Google Scholar]
- The Incidence and Prevalence of Delirium Across Palliative Care Settings: A Systematic Review. Palliat Med. 2019;33:865-77.
- [CrossRef] [PubMed] [Google Scholar]
- The SUPPORT Prognostic Model. Objective Estimates of Survival for Seriously Ill Hospitalized Adults. Ann Intern Med. 1995;122:191-203.
- [CrossRef] [PubMed] [Google Scholar]
- ICU Scoring Systems Allow Prediction of Patient Outcomes and Comparison of ICU Performance. Crit Care Clin. 1996;12:503-14.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of Survival For Older Hospitalized Patients: The HELP Survival Model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(S1):S16-24.
- [CrossRef] [Google Scholar]
- Generalist Versus Specialist Palliative Medicine. J Palliat Med. 2022;25:193-9.
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge and Use of Prognostic Scales by Oncologists and Palliative Care Physicians in Adult Patients with Advanced Cancer: A National Survey (ONCOPRONO Study) Cancer Med. 2022;11:826-37.
- [CrossRef] [PubMed] [Google Scholar]
- Quantifying How Tests Reduce Diagnostic Uncertainty. Arch Dis Child. 2007;92:404-8.
- [CrossRef] [PubMed] [Google Scholar]







