Translate this page into:
Overall Survival and Survival Time by Palliative Performance Scale: A Retrospective Cohort Study in Thailand
*Corresponding author: Kritsanee Saramunee, Social Pharmacy Research Unit, Faculty of Pharmacy, Mahasarakham University, Maha Sarakham, Thailand. kritsanee.s@msu.ac.th
-
Received: ,
Accepted: ,
How to cite this article: Vankun P, Saramunee K, Chaiyasong S. Overall survival and survival time by palliative performance scale: A retrospective cohort study in Thailand. Indian J Palliat Care 2022;28:295-300.
Abstract
Objectives:
The palliative performance scale (PPS) is a useful tool for predicting the survival time of palliative patients and for multidisciplinary teams in designing an appropriate care plan for patients and their families. This study aimed to (1) assess the survival time of palliative patients, (2) examine the factors associated with survival time and (3) investigate the proportion of patients whose survival time matched the time proposed by existing literature, within the Thai population.
Materials and Methods:
A retrospective cohort study was conducted with data drawn from five hospitals in one of the north-east provinces in Thailand. The study population comprised patients with a palliative diagnosis (ICD10: Z51.5) who had registered in one of the five hospitals between 1 October 2015 and 30 September 2017. Kaplan–Meier survival analysis was used to assess overall survival time and an extended Cox regression model to identify predictors of survival.
Results:
Of the 2792 registered patients, 1163 were included in the analysis. Most patients were male (55.62%), with a mean age of 64.59 years (±15.38), and were covered by the universal coverage insurance (77.72%). Approximately half (56.23%) of the participants had cancer and about a quarter (27.13%) had an initial PPS result of 30. The overall median survival time was 14 days (mean = 64.08, 95% CI: 12–16). Three significant predictors for survival included sex, hospital and initial PPS score.
Conclusion:
The survival time of palliative patients was relatively short. Sex, hospital and initial PPS were significant predictors of survival. The median survival time by PPS was similar to the values reported by the previous study but proportion of correct prediction was low. Therefore, it might be necessary to investigate the survival time of palliative patients by country independently.
Keywords
Palliative care
Survival
Palliative performance scale
Thailand
Kaplan–Meier
INTRODUCTION
Palliative care is an approach that focuses on improving patients’ and their families’ quality of life when facing life-threatening illnesses. This has been prioritised for patients close to the end-of-life stage, where complicated treatments are considered unnecessary.[1] In general, palliative care is delivered by a multiprofessional specialist team that needs at least basic training. A well-designed referral system is also essential to be integrated into this service to refer severe cases to other levels of care.[2]
In 2021, Thailand become an ageing society; this means that the proportion of its older adults has exceeded 20% of the population; this has several effects for the country, including an increase in healthcare needs.[3] Palliative care has been identified as a high demand service for older people, and it is expected that palliative care will be available to 80% of endof-life patients in the country. In 2018, the Ministry of Public Health included palliative care as one of the key performance indicators for all public hospitals,[4] which generally involves the following steps: First, when no other curative treatment is applicable, a patient is diagnosed as requiring palliative care. Once a doctor has officially confirmed this diagnosis, the palliative patient is then registered at the palliative care centre of the hospital. Second, the patient’s functional status is assessed using the palliative performance scale (PPS). At the early palliative stage, patients may remain admitted to the hospital where they are receiving palliative care. Otherwise, the patients or their families may choose to go home and receive palliative care from a primary care team.
PPS was developed by Anderson et al. (1996). It assesses patients’ function from five dimensions: Ambulation, activity level and evidence of disease, self-care, oral intake and status of consciousness.[5] The PPS yields results, ranging from 0% (death) to 100% (fully ambulatory), in 10% increments. Moreover, this scale is also useful for predicting the survival time of palliative patients, and such data can assist multidisciplinary teams in designing an appropriate care plan for patients and their families. The previous studies have confirmed that PPS is a good predictor of survival time in palliative patients; however, the studies showed median survival times that were slightly different.[6-8] A meta-analysis conducted by Downing et al.[6] which pooled data from hospitalised patients in developed countries, demonstrated the combined survival time of PPS 10–70% from 2 to 78 days.[6] Meanwhile, a retrospective cohort study in Canada reported a survival time of PPS 10–70% from 1 to 63 days.[8] Another prospective Canadian study reported that the 5-month survival rate of cancer patients was higher among those with greater initial PPS result.[7]
Based on these results, since PPS can help predict survival time, we may hypothesise that the values it yields for survival time differ by country. Moreover, the available evidence on this variable comes predominantly from developed countries, especially Canada, where palliative care is well established. In Thailand, such evidence is more limited, leading healthcare professionals to frequently utilise PPS-predicted survival time based on the study of Downing et al.[6] Another study that compared PPS survival time between cancer and non-cancer patients at a hospital in Thailand showed no differences between the groups regarding overall survival time.[9] Therefore, this study aimed to examine survival time among Thai palliative care patients. The results would be valuable for multidisciplinary specialist teams in the country to estimate survival time and thus deliver better healthcare services.
Objective
The purposes of this study were (1) to assess the survival time of palliative patients, (2) examine factors associated with palliative survival time and (3) investigate the proportion of patients whose survival time matched that in the range of a previous study.[6]
MATERIALS AND METHODS
Design and setting
This was a retrospective cohort study. Data were drawn from an electronic database of five pioneering hospitals – one at the provincial and four at the district level – regarding palliative care services in one of the north-east provinces in Thailand. This study received ethical approval from all relevant institutes, including Mahasarakham University Research Ethics Committee, the participating provincial hospital and the local public health office, which covered all participating district hospitals.
Population and sample
The study population included diagnosed patients who required palliative care (ICD10: Z51.5) and had registered to one of the five hospitals from 1 October 2015 to 30 September 2017. These patients were observed from the first date of palliative care diagnosis (index date) until death. The last date of the cohort observation was 30 September 2018.
Study variables included sex, age, hospital name, health insurance, diagnosis, initial PPS result, first date of palliative care diagnosis, first date of palliative care treatment and death date. Those with missing initial PPS results or incomplete patient records were excluded from the study. If the hospital registration reported missing data on the patient’s death, the death date was tracked using the civil registry office. Patients who survived the last observation date were censored.
Data analysis
Overall survival
Statistical analysis was performed using STATA® version 15. We used descriptive statistics, including percentage, mean or median and standard deviation. Kaplan–Meier survival analysis was used to assess overall survival time and univariate Cox regression to screen for potential covariates of the survival model.
Evidence has proven that age, sex, hospital, cancer and initial PPS result are factors associated with survival time.[6-8] Moreover, we included the health insurance scheme as a covariate due to the slightly different benefits package between adults in Thailand.[10] Covariates with P < 0.20 were highly likely to be associated with survival time and therefore included in the initial Cox proportional hazard model. We used the backward elimination approach to refine and identify the most valid model. Schoenfeld residuals were employed to test the proportional hazards assumption. If the assumption was violated in its control for time-varying covariates, we performed an extended Cox regression model.
Survival time by initial PPS
Downing et al. proposed the widely used estimation of survival time by PPS.[6] In this study, we reported the median survival time of each PPS score with a 95% confidence interval (CI), computing the frequency and percentage of patients whose survival time fell within the range of a prior research.[6]
RESULTS
There were 2792 registered patients at the palliative care centre across the five hospitals. Of these, 1163 were excluded due to unavailability of PPS score (n = 154), loss of patient records (n = 940) and unknown current status (n = 69). Thus, 1629 participants were included in the analysis. Thirty-three patients were censored as they survived after the last observation date. The most of the sample was male (55.62%), the mean age was 64.59 (±15.38) and most held universal coverage (77.72%). Approximately half (56.23%) of the participants had cancer and about a quarter (27.13%) had an initial PPS result of 30. About one-third (36.53%) were first diagnosed as requiring palliative care by hospital A [Table 1].
| n | % | |
|---|---|---|
| Sex | ||
| Male | 906 | 55.62 |
| Female | 723 | 44.38 |
| Age at enrolment (years) | ||
| Mean (SD) | 64.59 (15.38) | |
| Median (min, max) | 66 (<1, 97) | |
| Insurance | ||
| Universal coverage | 1,266 | 77.72 |
| Civil servant scheme | 201 | 12.34 |
| Social security scheme | 55 | 3.38 |
| Out-of-pocket or uninsured | 107 | 6.56 |
| Disease | ||
| Cancer | 916 | 56.23 |
| Non-cancer | 713 | 43.77 |
| PPS (%) | ||
| 10 | 247 | 15.16 |
| 20 | 175 | 10.74 |
| 30 | 442 | 27.13 |
| 40 | 221 | 13.57 |
| 50 | 168 | 10.31 |
| 60 | 241 | 14.79 |
| ≥70 | 135 | 8.29 |
| Hospital | ||
| A (provincial 1000 beds) | 595 | 36.53 |
| B (district 264 beds) | 77 | 4.73 |
| C (district 172 beds) | 528 | 32.41 |
| D (district 69 beds) | 225 | 13.81 |
| E (district 60 beds) | 204 | 12.52 |
PPS: Palliative performance scale
Survival patterns
[Figure 1] shows the Kaplan–Meier survival estimates of palliative care patients and the number at risk. The median survival time was 14 days (mean = 64.08, 95% CI: 12–16); the total time at risk for all patients was 104,391 person-days, of which 1596 deaths were observed. The mortality rate after receiving palliative care was 1.53 times in 100 person-days (0.015%).
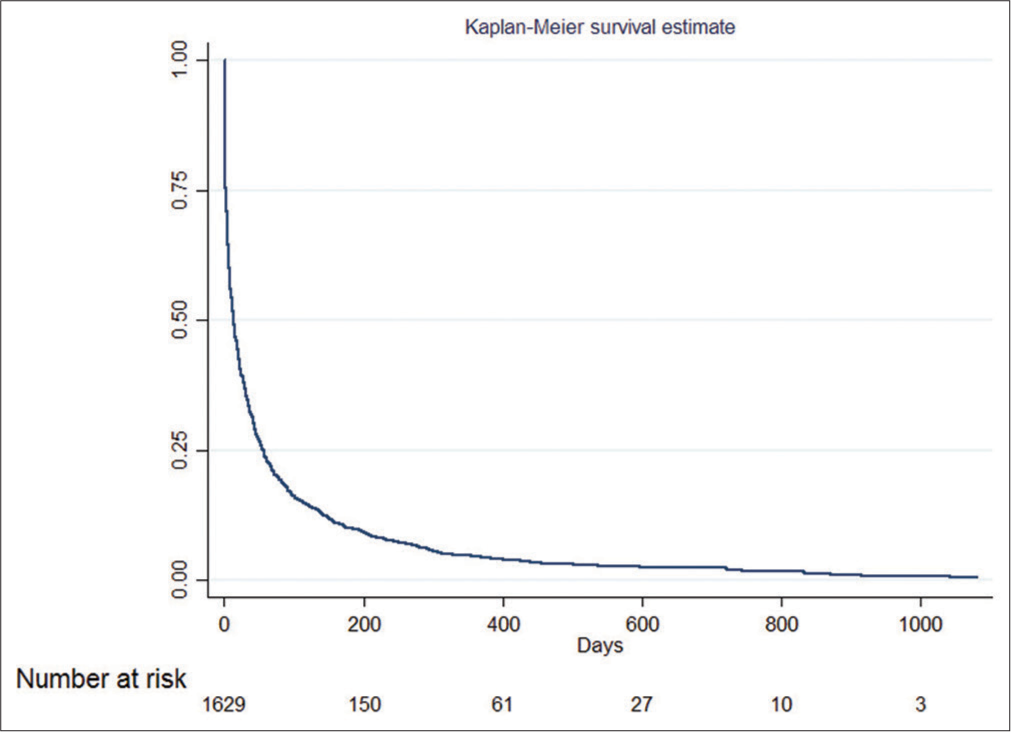
- Kaplan–Meier survival estimate of palliative care patients and the number at risk.
Univariate analysis
From the univariate Cox regression, five covariates were found as potential predictors of survival time in palliative patients: Age, sex, hospital, disease and initial PPS result [Table 2]. Although not associated with the univariate analysis, the health insurance scheme was also included in the multivariate analysis because of its impact on health outcomes.[10]
| Covariates | Median survival time, days (95% CI) | Crude HR (95% CI) | P-value |
|---|---|---|---|
| Agea | 0.99 (0.99–0.99) | 0.017 | |
| Sex | |||
| Male | 12 (10–14) | 1 | |
| Female | 16 (13–21) | 0.83 (0.76–0.93) | <0.001 |
| Hospital | |||
| A (provincial 1000 beds) | 6 (4–7) | 1 | |
| B (district 264 beds) | 12 (8–22) | 0.66 (0.52–0.84) | 0.001 |
| C (district 172 beds) | 35 (29–42) | 0.45 (0.39–0.51) | <0.001 |
| D (district 69 beds) | 20 (13–24) | 0.58 (0.49–0.68) | <0.001 |
| E (district 60 beds) | 9 (5–13) | 0.79 (0.67–0.93) | 0.004 |
| Insurance | |||
| Universal coverage | 14 (12–16) | 1 | |
| Civil servant scheme | 9 (6–16) | 1.04 (0.89–1.21) | 0.587 |
| Social security scheme | 16 (7–32) | 1.07 (0.81–1.39) | 0.647 |
| Out-of-pocket or uninsured | 15 (10–28) | 1.11 (0.91–1.35) | 0.311 |
| Disease | |||
| Non-cancer | 12 (9–14) | 1 | |
| Cancer | 16 (13–18) | 1.12 (1.01–1.24) | 0.028 |
| Initial PPS (%) | |||
| 10 | 1 (1–2) | 1 | 0.007 |
| 20 | 3 (2–4) | 0.77 (0.63–0.93) | <0.001 |
| 30 | 9 (7–11) | 0.58 (0.49–0.67) | <0.001 |
| 40 | 22 (15–30) | 0.39 (0.32–0.46) | <0.001 |
| 50 | 35 (24–55) | 0.34 (0.27–0.41) | <0.001 |
| 60 | 43 (37–52) | 0.29 (0.25–0.35) | <0.001 |
| ≥70 | 86 (62–117) | 0.21 (0.16–0.28) | <0.001 |
Multivariate analysis
The initial model included six covariates. Age, health insurance scheme and disease did not significantly contribute to survival time [Table 3]; thus, these factors were removed using the backward elimination approach. Then, three covariates (i.e., sex, hospital and initial PPS result) were found to be significantly associated with survival time. However, the proportional hazard assumption of the three-covariate model was violated because of the time-varying PPS variable. Therefore, we performed the extended Cox regression by including the three covariates, but while treating PPS as a time-varying variable.
| Covariates | HR (95% CI) | |||
|---|---|---|---|---|
| Initial model | P-value | Final model | P-value | |
| Age | 1.00 (0.99–1.00) | 0.879 | ||
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.84 (0.76–0.93) | 0.001 | 0.84 (0.76–0.93) | 0.001 |
| Hospital | ||||
| A (provincial 1000 beds) | 1.00 | 1.00 | ||
| B (district 264 beds) | 0.72 (0.56–0.92) | 0.009 | 0.70 (0.55–0.89) | 0.004 |
| C (district 172 beds) | 0.59 (0.51–0.69) | <0.001 | 0.59 (0.52–0.68) | <0.001 |
| D (district 69 beds) | 0.44 (0.38–0.53) | <0.001 | 0.54 (0.46–0.63) | <0.001 |
| E (district 60 beds) | 0.71 (0.51–0.69) | <0.001 | 0.70 (0.59–0.83) | <0.001 |
| Insurance | ||||
| Universal coverage | 1.00 | |||
| Civil servant scheme | 0.90 (0.77–1.05) | 0.189 | ||
| Social security scheme | 1.02 (0.77–1.34) | 0.911 | ||
| Out-of-pocket or uninsured | 0.91 (0.74–1.13) | 0.406 | ||
| Disease | ||||
| Non-cancer | 1.00 | |||
| Cancer | 1.03 (0.92–1.15) | 0.592 | ||
| PPS (%) | ||||
| 10 | 1.00 | 1.00 | ||
| 20 | 0.69 (0.57–0.86) | 0.001 | 0.63 (0.52–0.77) | <0.001 |
| 30 | 0.52 (0.44–0.61) | <0.001 | 0.38 (0.32–0.45) | <0.001 |
| 40 | 0.34 (0.28–0.41) | <0.001 | 0.18 (0.15–0.23) | <0.001 |
| 50 | 0.32 (0.26–0.39) | <0.001 | 0.11 (0.86–0.14) | <0.001 |
| 60 | 0.29 (0.23–0.36) | <0.001 | 0.06 (0.05–0.09) | <0.001 |
| ≥70 | 0.19 (0.15–0.24) | <0.001 | 0.02 (0.01–0.03) | <0.001 |
PPS: Palliative performance scale, HR: Hazard ratio
The results of the final model illustrate that sex, hospital and PPS were significantly associated with survival time [Table 3]. The hazard ratio (HR) of female palliative care patients was lower than that of male patients (HR = 0.84, 95% CI: 0.76–0.93), indicating that women have a longer survival time. Thereafter, we used the provincial hospital as a reference to compare the HR of the hospitals. It was found that the HR was lower among district hospitals, but not in order by hospital size. Moreover, the PPS variable was deemed as time varying, in that the higher the PPS, the lower the HR (using PPS of 10 as a reference); thus, patients with high PPS had longer survival times [Table 3].
Survival time by initial PPS
[Table 4] shows the median survival times (95% CI) in days by the initial PPS result based on the present study and the results of a previous meta-analysis.[6] We used the same order proposed by Downing et al. for analysing the median survival times by initial PPS result.[6] However, our values were slightly lower in comparison to the past research; using the survival time proposed by the previous meta-analysis, we were able to partially and correctly estimate the survival time in Thai palliative patients, which was of approximately 5–15% for the initial PPS of 10–50% and about 40% for the initial PPS of 60–70%.
| PPS (%) | Median survival time, days (95% CI) | n of patients | n(%) patients whose survival fell within the value in a previous meta-analysisa | |
|---|---|---|---|---|
| Previous meta-analysisa | Our study | |||
| 10 | 2 (2–2) | 1 (1–2) | 247 | 20 (8.10) |
| 20 | 4 (3–5) | 3 (2–4) | 175 | 27 (15.43) |
| 30 | 13 (12–14) | 9 (7–11) | 442 | 21 (4.75) |
| 40 | 24 (21–27) | 22 (15–30) | 221 | 14 (6.33) |
| 50 | 37 (32–42) | 35 (24–55) | 168 | 8 (4.76) |
| 60 | 48 (17–79) | 43 (37–52) | 241 | 101 (41.91) |
| 70 | 78 (25–131) | 86 (62–117) | 74 | 31 (41.89) |
DISCUSSION
This study examined the overall survival time and factors related to survival time among Thai palliative patients. It was found that the overall median survival time was 14 days (95% CI: 12.01–15.99), which was in the middle range (9–28 days) based on the previous meta-analysis.[6]
However, our median survival time was longer than that in a Canadian study, which was of 8 days.[8] Thus, we hypothesise that these variations in overall survival time among different studies might owe to factors such as race, the healthcare system or details regarding the palliative care service in the countries.
Three factors were found to be significant predictors of survival time in palliative patients: Sex, hospital and initial PPS result. These predictors find consistency in the previous reports.[6-8,11] Regarding sex, the present study iteratively confirmed that female survival time (16 days) was longer than that of males (12 days), and that the HR of the female group was 0.84 (95% CI: 0.76–0.93), indicating that the female mortality risk was about 14% less than that of males. This is consistent with the previous studies.[6,7]
Regarding hospital, we defined this predictor differently from the prior research, which used the term ‘location’ instead of ‘hospital.’[8] In Thailand, palliative care is currently operated by palliative care centres at public hospitals. Tertiary care and home-based care are inclusive but there is no hospice centre. This denotes that the services provided by tertiary care and home-based care in Thailand are not like those in developed countries. Hence, in Thailand, we compared all palliative care services delivered by each hospital, and our results showed that HR was lower among district hospitals, but not in order by hospital size [Table 3]. We conducted this examination not to critique the service by hospital, but to demonstrate that survival time differences might be related to the care processes used by each hospital. These results suggest that palliative care teams from different hospitals should share and learn how they can improve their palliative care service delivery. Moreover, we observed that the provincial hospital was likely to have a higher mortality risk and shorter survival time, a reality that may have been related to the nature of tertiary care, denoting that these hospitals receive patients in severer conditions.
In our study, the results for survival time by initial PPS concur with those in the previous studies; they showed that the higher the PPS, the longer the survival time.[6-8,11] However, we also discovered that the survival estimates proposed by the meta-analysis might not be applicable to Thai patients.[6] The survival time proposed by Downing et al. could only correctly predict 5–15% for a 10–50% PPS or 40% for a 60–70% PPS [Table 4].[6] However, on the other side, the median survival time (95% CI) by PPS of both studies (Downing et al.[6] vs. our study) were similar or overlapped. These contradict findings may suggest that further research is needed to compare survival time between different countries. Baik et al. previously commented that additional research is needed from countries such as South America, Africa, Europe and Asia to obtain more reliable survival measures from various ethnicities.[11] Nevertheless, the use of PPS by multidisciplinary palliative care teams can allow them to grasp an idea of how long a patient may survive; this may lead to more appropriate care plan development and assessment. Therefore, our results reiterate the need for survival time assessments with improved accuracy in the Thai population.
Strengths and limitations
To the best of our knowledge, this was the first large-scale study in Thailand to estimate the survival time of palliative patients using PPS. Still, it has some limitations. Of importance, the Thai version of the PPS we used was proven to have high specificity (>70%) and low sensitivity (<60%);[9] still, the same study recommended this tool for use only to predict survival time, denoting that it would not be appropriate to be used in screening endeavours.[9] A huge number of patient records were lost and not compiled in this data set, selection bias might exist as it could affect the survival time.
Moreover, we were not able to identify who assessed the patients for their PPS because data on the matter were unavailable; still, we remark the importance of ascertaining the professional performed the PPS assessment. If the person is not well trained in the process, this would surely question the accuracy of the predicted survival time based on the PPS results. Thus, future studies should explore differences by different assessors.
Finally, this study might be affected by the ‘tail effect,’ which means patients with long survival time, because there were a small number of patients with low initial PPS who had longer survival times.[7]
Implications to practice
Respect, autonomy, empowerment and communication are the core concepts of palliative care, which are believed to elevate patient dignity, particularly in the end-of-life stage.[12] Moreover, the world is continually moving toward what is beginning to be known as the era of ageing societies, denoting that palliative care will be of great importance for our future society. In the developing countries like Thailand, where the older adult population is rapidly increasing, we see the need for palliative care to be established in every hospitals, as this will widen the accessibility of such care for those in need. Our results corroborate this notion.
This research demonstrated that the hospital was a key predictor of survival time in palliative patients. Therefore, care delivery across hospitals at different levels should be harmonised, and the quality of their services should be simultaneously enhanced.
Moreover, we observed that the survival time based on the PPS was specific to Thai patients, and these data should, thus, be disseminated to the multidisciplinary palliative care teams. Nonetheless, as aforementioned, PPS assessment results might vary by assessors. Thus, the Ministry of Public Health and other relevant stakeholders should endeavour to provide these professionals with support for their assessment skills through promoting regular training programmes.
CONCLUSION
The survival time of palliative patients in Thailand was shown to be relatively short, and the variables of sex, hospital and initial PPS result were shown to be significant predictors of survival time in these patients. The median survival time by PPS was similar to the values reported by the previous study but proportion of correct prediction was low. Hence, the future research is warranted to investigate survival time in palliative patients by country.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Faculty of Pharmacy, Mahasarakham University.
Conflicts of interest
There are no conflicts of interest.
References
- WHO Definition of Palliative Care. Geneva: World Health Organization; Available from: https://www.who.int/cancer/palliative/definition/en [Last accessed on 2021 Sep 15]
- [Google Scholar]
- Global Consensus Based Palliative Care Definition. 2018. [Last accessed on 2021 Sep 15]https://www.hospicecare.com/what-we-do/projects/consensus-based-definition-of-palliative-care/definition
- [Google Scholar]
- Report on the 2017 Survey of the Older Persons in Thailand Bangkok, Thailand: Statistical Forecasting Division, National Statistical Office; 2018.
- [Google Scholar]
- Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5-11.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245-54.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of palliative performance scale and survival in patients with cancer receiving home-based palliative care. J Palliat Care. 2018;33:95-9.
- [CrossRef] [PubMed] [Google Scholar]
- Using the palliative performance scale to provide meaningful survival estimates. J Pain Symptom Manage. 2009;38:134-44.
- [CrossRef] [PubMed] [Google Scholar]
- Using palliative performance scale (PPS) to predict survival lengths of palliative care patients with and without cancer. Udonthani Hosp Med J. 2019;27:294-302.
- [Google Scholar]
- Thailand's Universal Coverage Scheme: Achievements and Challenges-an Independent Assessment of the First 10 Years (2001-2010) Nonthaburi, Thailand: Health Insurance System Research Office; 2012.
- [Google Scholar]
- Using the palliative performance scale to estimate survival for patients at the end of life: A systematic review of the literature. J Palliat Med. 2018;21:1651-61.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of patient dignity in care at the end of life. Ulster Med J. 2016;85:45-8.
- [Google Scholar]







