Translate this page into:
Quality of Life as a Non-mortality Patient-centred Outcome in the Critically Ill: A Retrospective Analysis
*Corresponding author: Sonali Vadi, Intensive Care Unit, Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Mumbai, Maharashtra, India. sonalivadi@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vadi S, Gudka S, Deo P. Quality of Life as a Non-mortality Patient-centred Outcome in the Critically Ill: A Retrospective Analysis. Indian J Palliat Care. 2024;30:366-74. doi: 10.25259/IJPC_48_2024
Abstract
Objectives:
Mortality is a common gauged endpoint in critically ill patients. Reduced quality of life is an aligned repercussion of protracted critical illness. Baseline status, severity of illness and its trajectory influence the outcomes. Patient-oriented outcomes are those that matter the most to a patient. However, quite often, family approves of trade-offs with survivorship in the Indian context. We looked at non-mortality outcomes in patients on high-intensity life-sustaining interventions admitted to the intensive care unit (ICU) despite poor prognosis and died on full support or survived to be completely dependent.
Materials and Methods:
In this retrospective chart review study, we studied patients (1) who spent more than 1 month in the hospital enduring a myriad of distressing physical and psychological vicissitudes, (2) whose primary illness was fairly advanced (3) and either succumbed or survived to be impeded in their response to cognitive assessment and with severe functional impairment. Patient demographics, comorbidities, pre-morbid functional status, burden of critical illness, use of life-sustaining therapies, functional dependence in the last week of ICU stay, best neurological status in the last week pre-death or discharge, dying trajectories and economic analysis were noted.
Results:
Trends of clinical progress of 23 patients were deliberated. The mean age of males was 65 years and 61 years for females. Five patients had a Barthel index score of 10–20, indicating total dependency and two patients had a score of 21–60, indicating severe dependency. Two patients were cognitively impaired at baseline. The worst neurological status in the week before death or discharge was eye1, motor1, and verbaltracheostomised. Thirteen patients succumbed during ongoing treatment.
Conclusion:
Daily discussions on the dynamics of illness progression need to take place with family on a regular basis for patients managed in ICU. Realistic perceptions and grounded expectations from the families and caregivers are necessary for patient-centred outcomes.
Keywords
Patient-centred outcomes
Critical illness
Pre-intensive care unit health trajectory
Quality of life
Dignity
INTRODUCTION
‘The success of intensive care is not, therefore, to be measured only by the statistics of survival as if each death is a medical failure; it is to be measured by the quality of life preserved or restored, by the quality of dying of those in whose interest it is to die and by the quality of human relationships involved’ (ethicist Dunstan).[1] Healthcare providers face the issue of negotiating proper goals of treatment alongside relatives when faced with the question, ‘When is an extension of life too burdensome’? [2]
Dynamic shaping outcomes from a disease include the age of the patient, baseline health status, type of disease, its severity and therapy available for the same.[3] The degree of ability to reach baseline functional, cognitive, psychological and social performance is essential following a critical illness that is not put into perspective when analysing this group of patients. The manner in which patients and their families un-riddle the illness and its residuum influence crucial decision-making. Families and caregivers do opt for continuing life-maintaining treatment despite the poor prognosis. Such patient-centred outcomes are difficult to quantify and illustrate with traditional data and analysis. Contrary to this, mortality, an important patient outcome, is an easily measured binary variable and less susceptible to biases in determination. The following study looks at the morbidity burden of critical illness, a tangible aspect for the clinicians managing these complex patients that may not be crucial or difficult to accept for the families and caregivers.
Education and counselling of relatives about their patient’s overall health condition are a critical facet of communication during end-of-life care. Concepts of quality of life at endof-life, quality of end-of-life care and quality of dying and death[4] need to be understood. Priorities in terms of medical care change as per the trajectory they are on. We studied the quality of dying, a significant aspect of end-of-life care in the intensive care unit (ICU) but not easily measured. Specifically, we looked at patients who were on high-intensity life-sustaining intervention and cared for in the ICU despite poor prognosis and died with full support or survived to be completely dependent for illnesses having certainty in their trajectory from the available evidence.
METHODOLOGY
Study design
This retrospective chart-review study was conducted from March 2019 to January 2023 at a tertiary care centre in Mumbai, India. The study was approved by the institutional ethics committee [IEC- A Code: 002/2023].
Selection of patients
We included patients (1) who spent more than 1 month in the hospital enduring a myriad of distressing physical and psychological vicissitudes, (2) whose primary illness was fairly advanced, (3) and either succumbed or survived to be impeded in their response to cognitive assessment and with severe functional impairment. Interdisciplinary family meetings discussing goals of care and documentation of prognostic communication were customary and adhered to in these patients.
Data collected
Patient demographics (age and gender); Acute Physiology And Chronic Health Evaluation (APACHE) II score,[5] Charlson comorbidity index; comorbidities; diagnoses on admission; pre-morbid status (functional status by Barthel index,[6] frailty, cognitive status); burden of critical illness: use of life-sustaining therapies – invasive mechanical ventilation, renal replacement therapy, vasoactive medications, need for transfusion, need for cardiopulmonary resuscitation; code status and changes in code status; difficulties encountered – weaning from ventilator, vascular access issues; functional dependence in last week; total duration of ICU stay; cognitive function: best neurological status (Glasgow coma scale) in first 4 weeks and last week pre-death or discharge; any pressure sores and extremity contractures and self-pay versus insurance/third party.
Outcomes
We examined the association between life-sustaining interventions and quality of life.
Definitions
Barthel index score[6] – scores of 0–20 indicate total dependency, scores of 21–60 indicate severe dependency, scores of 61–90 indicate moderate dependency and scores of 91–99 indicate slight dependency.
Frailty[7] – Decline of normal stores with age and illness placing body systems at vulnerability.
Dying trajectories[8,9] – (1) trajectory with steady progression and usually a clear terminal phase (e.g.) malignancy, (2) trajectory with gradual decline, punctuated by episodes of acute deterioration and some recovery, with more sudden seemingly unexpected death (e.g.) respiratory failure or heart failure, (3) trajectory with prolonged gradual decline (e.g.) frail elderly, dementia and (4) trajectory of steady decline with rate of decline dependent on underlying pathology and other patient-related factors, i.e. comorbidities
Meaningful outcomes[10] – physical function, cognitive function, health-related quality of life, ability to return to work. Acute Physiology And Chronic Health Evaluation (APACHE) II score[5] - Interpretation of score - 0–10: Low risk - patients in this range have a relatively low risk of mortality; 11–20: Moderate risk - patients in this range have a moderate risk of mortality; 21–30: High risk - patients in this range have a high risk of mortality and ≥31: Very high risk - patients in this range have a very high risk of mortality.
Statistical analysis
Actual data are presented for all 23 patients. Scatter plot is used to present the expenditure incurred by the patient.
RESULTS
A total of 23 patients (14 males and 9 females) were studied [Table 1].
| Scenario | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age | 82 | 44 | 49 | 60 | 25 | 69 | 44 | 85 |
| Gender | Male | Female | Female | Male | Male | Male | Male | Male |
| Comorbidities | End-stage renal disease | Scleroderma | Hypothyroidism | Old stroke, progressive supranuclear palsy | None | Progressive supranuclear palsy, hypothyroid | Hypothyroid, high grade anaplastic ependymoma of spinal cord | Interstitial lung disease |
| Charlson comorbidity index (severity of comorbid diseases) | 7 (severe) | 2 (mild) | 1 (mild) | 7 (severe) | 0 | 3 (moderate) | 7 (severe) | 4 (moderate) |
| Status of primary illness | On maintenance haemodialysis | Advanced disease | --- | Residual hemiplegia | --- | End-stage progressive supranuclear palsy | Residual quadriplegia | Advanced interstitial lung disease |
| Number of medications at home | 8 | 4 | 1 | 7 | 0 | 12 | 7 | 4 |
| Baseline functional status (Barthel index) | 90 | 90 | 100 | 45 | 100 | 60 | 10 | 80 |
| Baseline cognitive status | Normal | Normal | Normal | Normal | Normal | Cognitively impaired | Normal | Normal |
| Diagnosis at current admission | Sepsis with multiorgan dysfunction Acute coronary syndrome with heart block Malignancy of urinary bladder |
Diffuse scleroderma | Post-COVID-19 infection, acute respiratory distress syndrome, multiorgan dysfunction | Acute ischaemic stroke-bilateral with raised intracranial pressure, internal carotid artery thrombosis, status epilepticus, central diabetes insipidus | Leptospirosis with multiorgan dysfunction, intracranial bleed following disseminated intravascular coagulation | Septic shock | Acute pneumonia with septic shock | Clostridium difficile colitis |
| Scenario | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Age | 70 | 66 | 31 | 75 | 67 | 66 | 59 | 88 |
| Gender | Male | Male | Female | Male | Male | Female | Female | Male |
| Comorbidities | Multiple myeloma | Hypertension, bronchial asthma | Tuberculosis, hypothyroid | None | Deep venous thrombosis, atrial fibrillation, hypertension | Morbid obesity, hypertension, diffuse astrocytoma of the brain | Diabetes mellitus ii, obesity | Hypothyroid |
| Scenario | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Charlson comorbidity index (severity of comorbid diseases) | 10 (severe) | 2 (mild) | 3 (moderate) | 9 (severe) | 10 (severe) | 9 (severe) | 9 (severe) | 4 (moderate) |
| Status of primary illness | Advanced multiple myeloma with compression fracture of multiple vertebrae | stable | --- | Post- chemotherapy and surgery |
--- | Post- chemotherapy |
Post- chemotherapy |
--- |
| Number of medications at home | 9 | 6 | 5 | 8 | 17 | 6 | 8 | 4 |
| Baseline functional status (Barthel index) | 10 | 80 | 80 | 100 | 80 | 20 | 20 | 80 |
| Baseline cognitive status | Cognitively impaired | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| Diagnosis at the current admission | Disseminated intravascular coagulation, retroperitoneal haematoma, multiorgan dysfunction syndrome | Urosepsis with septic shock | Rhomboencephalitis | Advanced metastatic carcinoma colon | Advanced carcinoma oesophagus with lung metastases and aspiration pneumonia | Septic shock | Metastatic carcinoma of the breast | Aspiration pneumonia |
| Scenario | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| Age | 67 | 50 | 63 | 55 | 68 | 89 | 79 | |
| Gender | Female | Male | Female | Male | Male | Female | Female | |
| Comorbidities | Diabetes mellitus ii, hypertension, chronic obstructive airway disease | End-stage renal disease, hypertension | Diabetes mellitus, hypertension, ischaemic heart disease | Carcinoma buccal mucosa | Hypertension | Diabetes mellitus, hypertension, coronary artery disease (s/p PTCA to LAD) | Diabetes mellitus, hypertension, chronic kidney disease, chronic liver disease, coronary artery disease (s/p CABG) | |
| Charlson comorbidity index (severity of comorbid diseases) | 6 (severe) | 3 (moderate) | 5 (severe) | 7 (severe) | 2 (mild) | 10 (severe) | 10 (severe) | |
| Scenario | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| Status of primary illness | --- | On maintenance haemodialysis | Stable | Carcinoma buccal mucosa with distant metastases (status post-chemotherapy | Post-operative | Stable | Stable | |
| Number of medications at home | 9 | 7 | 5 | 7 | 3 | 7 | 11 | |
| Baseline functional status (Barthel index) | 80 | 90 | 100 | 90 | 100 | 20 | 80 | |
| Baseline cognitive status | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| Diagnosis at current admission | Exacerbation of obstructive airway disease | Status post-evacuation of subdural haematoma, recurrent hospital-acquired infections, muscle wasting | Traumatic brain injury | Aspiration pneumonia | Vestibular schwannoma | Subdural haematoma, femur and radial fracture | Community-acquired pneumonia, uraemic encephalopathy, GI bleed, urosepsis, fluid overload, bilateral parieto-occipital infarct | |
PTCA: Percutaneous transluminal coronary angioplasty, LAD: Left anterior descending artery, CABG: Coronary artery bypass grafting, GI: Gastrointestinal
The mean age of males was 65 years and females was 61 years. Five patients had Barthel index scores of 10 to 20, indicating total dependency. Two patients had Barthel index scores of 21 to 60, indicating severe dependency. Two patients were cognitively impaired at baseline. Three patients (scenario 3 [age 49 years], scenario 5 [age 25 years] and scenario 11 [age 31 years]) had acute onset of infective illness. Other patients had advanced or end-stage primary disease. Nine patients were post-CPR [Table 2]. Eleven patients required re-transfer to the ICU following clinical instability. All of these patients had vascular access issues. All patients required antibiotics at some point during their hospital stay. The number of medications required per day in the week before death or discharge was, on average, eighteen. Twenty-one out of twenty-three patients were ventilator dependent.
| Scenario | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| APACHE II (predicted mortality %) | 27 (55%) | 4 (4%) | 9 (8%) | 20 (40%) | 36 (85%) | 29 (55%) | 25 (55%) | 24 (40%) |
| Post-cardiopulmonary resuscitation | Yes | No | Yes | Yes | No | No | Yes | No |
| Need for vasoactive medicines | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Need for transfusion | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ventilator dependent | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Need for renal replacement therapy | Yes | Yes | No | Yes | Yes | No | No | No |
| Vascular access issue | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Re-transfer to ICU | Yes | No | No | No | No | No | No | Yes |
| Recurrent hospitalisation | No | No | No | Yes | No | Yes | Yes | No |
| Antibiotics | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Antifungals | Yes | Yes | Yes | Yes | No | No | Yes | No |
| Number of medications per day (week prior discharge/death) | 20 | 16 | 13 | 24 | 17 | 13 | 21 | 23 |
| Pressure sores | Yes | No | Yes | Yes | No | Yes | No | Yes |
| Extremity contractures | Yes | No | No | Yes | No | Yes | Yes | No |
| Scenario | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| APACHE II (predicted mortality %) | 30 (73%) | 28 (55%) | 20 (40%) | 24 (40%) | 18 (24%) | 11 (15%) | 10 (15%) | 10 (15%) |
| Post-cardiopulmonary resuscitation | Yes | Yes | No | No | No | No | No | No |
| Need for vasoactive medicines | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Need for transfusion | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Ventilator dependent | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Need for renal replacement therapy | Yes | Yes | No | Yes | No | No | No | No |
| Vascular access issue | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Re-transfer to ICU | Yes | Yes | No | No | No | Yes | Yes | Yes |
| Recurrent hospitalisation | No | Yes | No | Yes | Yes | Yes | Yes | No |
| Antibiotics | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Antifungals | Yes | Yes | No | No | No | No | No | No |
| Number of medications per day (week prior discharge/death) | 25 | 28 | 16 | 12 | 16 | 18 | 14 | 40 |
| Pressure sores | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Extremity contractures | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Scenario | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| APACHE II (predicted mortality %) | 38 (85%) | 21 (40%) | 23 (40%) | 25 (55%) | 22 (40%) | 18 (24%) | 29 (55%) | |
| Post-cardiopulmonary resuscitation | Yes | No | No | No | Yes | Yes | No | |
| Need for vasoactive medicines | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Need for transfusion | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Ventilator dependent | Yes | No | Yes | Yes | Yes | Yes | Yes | |
| Need for renal replacement therapy | No | Yes | No | No | No | No | No | |
| Vascular access issue | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Re-transfer to ICU | No | Yes | No | No | Yes | Yes | Yes | |
| Recurrent hospitalisation | Yes | No | No | No | No | Yes | Yes | |
| Antibiotics | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Antifungals | No | Yes | No | Yes | No | Yes | Yes | |
| Number of medications per day (week prior discharge/death) | 16 | 16 | 11 | 12 | 10 | 15 | 12 | |
| Pressure sores | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| Extremity contractures | Yes | Yes | Yes | Yes | No | Yes | Yes |
ICU: Intensive care unit, APACHE: Acute Physiology and Chronic Health Evaluation
Best neurological status in the week before death or discharge was e4m3vt and worst e1m1vt [Table 3]. Three patients were clinically brain dead. As relatives or family were not ready to accept the actual situation of their patient, a formal declaration of their status could not be done.
| Scenario | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Best neurological status week 1 | e2m5v3 | e3m5v4 | e4m5v4 | e2m4vt | e4m6v5 | e3m4v2 | e4m1v5 | e1m1vt |
| Best neurological status week 2 | e2m5vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e3m1v3 | e1m1vt |
| Best neurological status week 3 | e2m5vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e2m1vt |
| Best neurological status week before discharge/death | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e2m1vt |
| Brain dead but still continuing treatment | No | No | No | Yes | Yes | No | No | No |
| Scenario | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Best neurological status week 1 | e3m4v2 | e3m3v2 | e3m5v2 | e4m3v5 | e3m4v1 | e3m1vt | e3m5v1 | e4m6v5 |
| Best neurological status week 2 | E2m3vt | e2m4v2 | e2m4v2 | e1m1vt | e1m1vt | e3m1vt | e3m5v1 | e4m5v3 |
| Best neurological status week 3 | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e2m1vt | e3m5v1 | e4m5vt |
| Best neurological status week before discharge/death | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt | e1m1vt |
| Brain dead but still continuing treatment | No | Yes | No | No | No | No | No | No |
| Scenario | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| Best neurological status week 1 | e1m1v1 | e4m6v5 | e1m1vt | e4m6v5 | e4m6v5 | e4m4vt | e4m5v4 | |
| Best neurological status week 2 | e1m1vt | e4m6v5 | e1m1vt | e4m6vt | e3m1vt | e4m4vt | e4m5v4 | |
| Best neurological status week 3 | e2m1vt | e3m4v3 | e4m1vt | e1m1vt | e3m1vt | e4m4vt | e2m2vt | |
| Best neurological status week before discharge/death | e1m1vt | e4m2vt | e4m4vt | e1m1vt | e1m1vt | e4m3vt | e3m3vt | |
| Brain dead but still continuing treatment | No | No | No | No | No | No | No |
e: Eye, m: Motor, v: Verbal [Glasgow coma scale]. ICU: Intensive care unit
The average duration of ICU stay was 121 days [Table 4]. Thirteen patients succumbed during ongoing treatment in the ICU. One patient was discharged against medical advice. Figure 1 depicts the economic impact of treatment.
| Scenario | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Dying trajectory | 4 | 4 | 4 | 3 | 4 | 3 | 3 | 4 |
| Duration of ICU stay | 135 | 62 | 34 | 95 | 87 | 30 | 108 | 109 |
| Outcome | Deceased | Deceased | Deceased | Deceased | Deceased | Deceased | Deceased | Transfer to other hospital |
| Scenario | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Dying trajectory | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 4 |
| Duration of ICU stay | 182 | 420 | 75 | 31 | 65 | 126 | 240 | 210 |
| Outcome | Deceased | Deceased | Discharged | Deceased | Deceased | Discharged | Deceased | Deceased |
| Scenario | 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| Dying trajectory | 1 | 4 | 1 | 4 | 4 | 4 | 4 | |
| Duration of ICU stay | 175 | 140 | 111 | 50 | 153 | 117 | 93 | |
| Outcome | Discharged | Discharged | Discharged | Discharged | Discharged | Discharged | Discharged | |
ICU: Intensive care unit
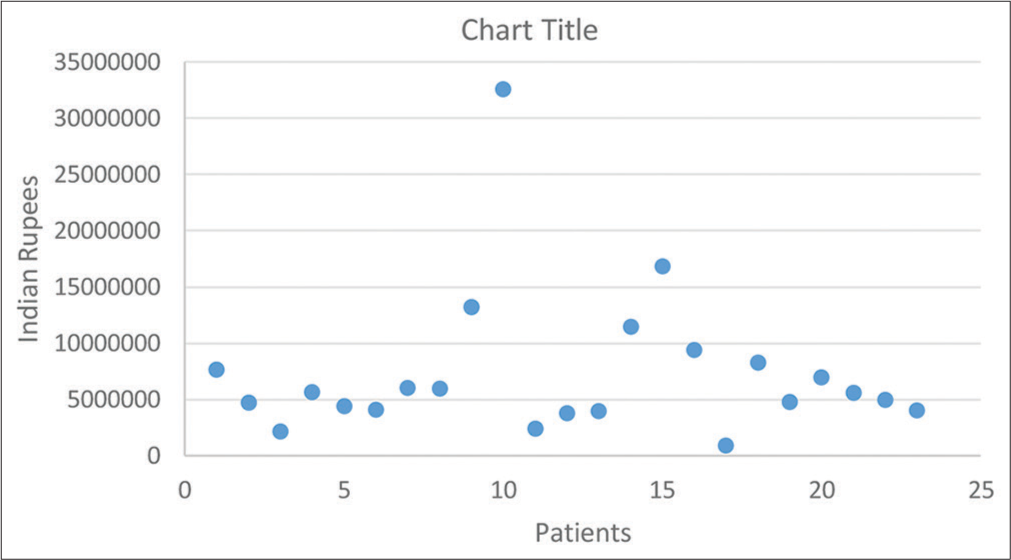
- Economic burden.
DISCUSSION
Death may be inevitable, but suffering and loss of dignity at the end of life need not be.[11] When medications and disease-oriented therapies do not help the critically ill, the focus of care shifts from prolonging life to promoting quality of life and quality of death. End-of-life involves multiple stakeholders, leading to disagreements about patient treatment or continuity of treatment. Overuse of medical services may be associated with wasteful utilisation. Patient preferences about end-of-life may not be conveyed or imprecisely communicated by family or next-of-kin to the treating team of doctors during the course of a prolonged illness. The lack of a longer relationship between acute care doctors and families augments discord. Emotions intensify conflicts during end-of-life discussions. Social aspects may also influence decision-making amongst the varied stakeholders involved. The quality of dying may also differ across varying religions and socioeconomic status, as well as healthcare providers and the systems they work in.[12]
Our findings
We analysed a spectrum of proximal endpoints, such as the use of life-sustaining therapies, the need for cardiopulmonary resuscitation, the need for disease-specific therapies, difficulties encountered, functional dependence, cognitive function, the best neurological status during an ICU stay and in the week before discharge or death and economic analysis.
Disease-centred outcomes are commonly researched in critical care. Our patient-centred outcomes observed that illness severity scores (APACHE II) did not correlate with a reduction in physical functioning. It is noteworthy that a low score does not equate to a good prognosis, and a high score does not equate to weak outcomes, as reflected in the cohort. Frailty, comorbidities and code status are not included in these scoring systems. An interplay between these parameters and variables measured in scoring systems influences outcomes. In an observational, multicentre study,[13] these variables did contribute to predictive performance for patients aged >65 years of age. This consideration also holds true irrespective of the age group, as in our cohort. Organ-supporting therapies may aid in reducing organ failure but do not affect the quality of life or survival. Survival does not necessarily equate to quality of life for a patient, an important facet of a patient-centred outcomes puzzle. The presence of co-morbidities negatively influenced the quality of life following critical illness. Compromised functional status reflected diminished physiologic reserves. These patients succumbed to frailty and the inability of disease-oriented therapies to prevent their decline.
Our findings apprise the need for assessing relatives’ viewpoints for their patients who require ICU care in the last few weeks of life for chronically ill patients and study the association between intensity of ICU care and the quality of dying perception Long-term cognitive and functional impairment as noted in those who survived underscores the value of counselling including communication of patient status, benefits versus detriments of life-prolonging therapies for those with advanced complex illnesses, the chronically critically ill and/or cognitively impaired, decision-making based on these discussions by team participating in immediate care as well as the need of involving palliative care to guide informed decisions by families and caregivers.[14]
One of the likely reasons that life-maintaining treatments are continued despite discussions seems to be sadness, fear, anxiety and guilt. Appraisal of baseline cognitive, functional, psychological and social stand-in and following how these keep up following ICU exposure need to be put into perspective when managing critically ill patients. Illness severity scores do not correlate with a reduction in physical functioning following a critical illness.[15] Understanding illness trajectories may ease conversations on prognosis and end-of-life care with family.
What constitutes meaningful outcomes is a point of contention. From whose perspective (healthcare personnel vs. family and caregiver) the terms meaningful and pertinent are debatable. For what purpose is meaning being gauged? This is another moot point. Elderly, the presence of multiple comorbidities and recurrent hospitalisation slow the magnitude of recovery from critical illness. The intensity of treatment should correspond to the ability of the patient to benefit in ways other than survival. A core set of outcomes besides survival to appraise following critical illness includes cognition, mental health, physical functioning, return to prior activities and health-related quality of life.[16] Hence, discussions with family members and caregivers should be focused on perceiving outcomes relevant to the patient.[17] Patient-important outcomes (i.e., those that influence quality of life) rather than clinically relevant ones should be the reason to partake in interventions in this group of patients. Clarity on what is achievable versus expectations needs to be teased out during daily discussions.
Intensive care is costly and resource-intensive. The socioeconomic burden of critical illness impacts families to the point that many have had to sell their personal property to support their ailing ones financially. The need for transfusions and antimicrobials in these patients contributes to the ethical aspects of clinical management.
Strengths
This is the first study from India to focus on soft outcomes and an important proximal end-point, quality of life. Our study incorporated a heterogeneous population spread across different age groups with severe diseases of varying diagnoses. These patients were on the negative side of the health spectrum. We used clinically validated scales such as the Charlson comorbidity index to measure the baseline status of patients. Our findings highlight the importance of focusing on patient-centred outcomes in critical care, understanding caregivers’ perspectives on patient outcomes and raising the important ethical question whether postponement of mortality offsets a negative effect on quality of life.
Limitations
The Barthel index was gathered as per various baseline patient parameters noted in the charts. We had to piece together the dying trajectories from patient information available in charts with practicable heuristics.
CONCLUSION
A number of findings from this study show a route for clinical care. Research is needed from a relative or next-ofkin perspective about an important patient-centred outcome, quality of life, for those patients who require ICU care with organ support in the last few weeks of life. Understanding the impact of the severity of illness, clinical progression, and trajectory of illness on family acceptance and perceptions of end-of-life care will help improve outcomes for patients and their families. Relatives and next-of-kin hold a myriad of expectations about a patient’s illness and its treatment. The escalating cost of medical care for this group of patients is a sensitive issue that can stir up controversy. Co-morbidities, frailty, quality of life and life span need to be considered in guidelines for medical cost coverage. To help the patient, we will need a broader outlook from the involved stakeholders, aiming for the possibility of bringing about changes in the current system. The findings of this study should be used as a live document for discussion on patient-centred outcomes in critical care units in India.
Ethical approval
The research/study was approved by the Institutional Review Board at Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, number IEC - A Code:002/2023, dated 7 June 2023.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Hard Questions in Intensive Care. A Moralist Answers Questions Put to Him at a Meeting of the Intensive Care Society, Autumn, 1984. Anaesthesia. 1985;40:479-82.
- [CrossRef] [PubMed] [Google Scholar]
- Research Agenda for Developing Measures to Examine Quality of Care and Quality of Life of Patients Diagnosed with Life-Limiting Illness. J Pain Symptom Manage. 1999;17:75-82.
- [CrossRef] [PubMed] [Google Scholar]
- The Value of Measuring Severity of Disease in Clinical Research on Acutely Ill Patients. J Chronic Dis. 1984;37:455-63.
- [CrossRef] [PubMed] [Google Scholar]
- The Concept of Quality of Life of Dying Persons in the Context of Health Care. J Pain Symptom Manage. 1999;17:93-108.
- [CrossRef] [PubMed] [Google Scholar]
- APACHE II: A Severity of Disease Classification System. Crit Care Med. 1985;13:818-29.
- [CrossRef] [PubMed] [Google Scholar]
- Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:56-61.
- [CrossRef] [Google Scholar]
- Living Well at the End of Life: Adapting Health Care to Serious Chronic Illness in Old Age. White Paper. RAND Health 2003 Available from: https://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf [Last accessed on 2024 Feb 21]
- [CrossRef] [Google Scholar]
- Patterns of Dying: Palliative Care for Non-Malignant Disease. Clin Med (Lond). 2004;4:39-44.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of Critical Illness: What is Meaningful? Curr Opin Crit Care. 2018;24:394-400.
- [CrossRef] [PubMed] [Google Scholar]
- Beyond Pain: Nurses' Assessment of Patient Suffering, Dignity, and Dying in the Intensive Care Unit. J Pain Symptom Manage. 2018;55:1591-8.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluating the Quality of Dying and Death. J Pain Symptom Manage. 2001;22:717-26.
- [CrossRef] [PubMed] [Google Scholar]
- Illness Severity Assessment of Older Adults in Critical Illness using Machine Learning (ELDER-ICU): An International Multicentre Study with Subgroup Bias Evaluation. Lancet Digit Health. 2023;5:e657-67.
- [CrossRef] [PubMed] [Google Scholar]
- Brain Dysfunction: Another Burden for the Chronically Critically Ill. Arch Intern Med. 2006;166:1993-9.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment and Predictors of Physical Functioning Post-Hospital Discharge in Survivors of Critical Illness. Ann Intensive Care. 2016;6:92.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding Patient-Important Outcomes After Critical Illness: A Synthesis of Recent Qualitative, Empirical, and Consensus-Related Studies. Curr Opin Crit Care. 2018;24:401-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effects on Health-Related Quality Oflife of Interventions Affecting Survival in Critically Ill Patients: A Systematic Review. Crit Care. 2022;26:126.
- [CrossRef] [PubMed] [Google Scholar]







