Translate this page into:
Psychometric Evaluation of Patient Health Questionnaire 9 Hindi for Use with Patients with Cancer in Community Palliative Care Settings
*Corresponding author: Tushti Bhardwaj, Department of Social Work, Dr. Bhim Rao Ambedkar College, University of Delhi, New Delhi, India. tushti.p@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhardwaj T, Arora N, Rajvanshi A. Psychometric Evaluation of Patient Health Questionnaire 9 Hindi for Use with Patients with Cancer in Community Palliative Care Settings. Indian J Palliat Care. doi: 10.25259/IJPC_250_2024
Abstract
Objectives
Patient Health Questionnaire 9 (PHQ-9) has previously undergone validation with patients with various types of cancer, but psychometric validation of PHQ-9 Hindi among patients with cancer receiving palliative care services in northern regions of India is required. This study aimed at psychometric validation of a culturally adapted version of PHQ-9 Hindi for early screening of depression among cancer patients receiving palliative care services in a community setting.
Materials and Methods
A sample of adult patients (n = 228) with cancer receiving palliative care services in a community setting participated in the research. A 50% subset of the sample was contacted for repeat assessment twice, each after a period of 3–4 weeks. For validation, a previously adapted version of PHQ-9 Hindi, along with a demographic sheet, was employed to collect data. In addition, we used the recently validated Integrated Palliative Care Outcome Scale to assess the validity of PHQ-9 within palliative care settings. Exploratory and confirmatory factor analyses were conducted, followed by test-retest reliability, interclass correlation, construct validity and divergent validity examination.
Results
The exploratory factor analysis revealed a two-factor solution consistent with the hypothesised model, yielding two sub-scales namely, physical and emotional concerns. The confirmatory factor indices within our sample were conclusive suggesting a relatively good fit between the hypothesised model and the observed data, thus confirming the cross-cultural validity of PHQ-9 Hindi. The physical sub-scale confirmed moderate internal consistency (α = 0.5) while the emotional sub-scale presented high internal consistency (α = 0.734). The associations between PHQ-9 Hindi with IPOS Hindi individual items were significant (P < 0.001).
Conclusion
A psychometrically validated version of PHQ-9 Hindi has been presented to screen depression among patients with cancer in northern parts of India. Further research is required to adapt and check the validity of PHQ-9 in other regional languages among different populations across the country.
Keywords
Adult
Cancer
Community setting
Palliative care
Patient health questionnaire-9
Psychometric validation
INTRODUCTION
Chronic and advanced illnesses, including cancer, pose considerable emotional concerns to the patients. Depression is one of the most prominent and prevalent psychological symptoms among patients with cancer. Depression affects almost 20–40%[1] of patients with cancer irrespective of the disease trajectory.[2-4] It carries a heightened risk of emotional and somatic repercussions that ultimately undermine the quality of life of the patients.[5] It is important to identify and address depressive symptoms early and provide psychotherapeutic and pharmacological support to add quality to the lives of patients.[6] Unfortunately, depression often goes underdiagnosed and undertreated among cancer patients as mainly physical symptoms overpower their health-related concerns.[6]
Patient Reported Outcome Measures (PROMs) are generally used to identify several concerns of the patients as these tools assess the concerns quickly and holistically. These assessments serve as valuable tools for gauging the severity of a particular issue affecting a person. A few such PROMs namely, the World Health Organisation Quality of Life Questionnaire-100 and 23, the European Organisation for Research and Treatment of Cancer Questionnaire, Karnofsky Performance Scale and Patient Health Questionnaire (PHQ) are widely used in Indian settings. PHQ in general and the PHQ-9 depression scale, in particular, are used in both research and practice in mental health settings.[7-9] PHQ-9 has been translated in Hindi, validated against DSM-IV[10] and previously used in psychiatric settings.
It has also been reported that many of the translations of PHQ variants have been conducted by MAPI using standard methodology and researchers have substantiated the validity and reliability of PHQ-9 across diverse contexts.[11-14] PHQ-9 has also been previously validated with patients with different types of cancers.[1] However, a review of available literature suggests that the psychometric validation of PHQ-9 Hindi among patients with cancer, especially in palliative care settings, has not been extensively explored. This necessitates the need for psychometric validation of PHQ-9 Hindi and examines its responsiveness for use among patients with cancer. We, thus, aim to undertake psychometric validation of previously adapted PHQ-9 Hindi[15] with patients with cancer receiving palliative care services in community settings in northern parts of India. Palliative care services are provided in various settings namely, hospitals, hospices and home care or community settings. This research focused on patients receiving palliative care services in community settings. Few of them were also receiving curative treatment from hospitals in addition to home care services for the management of symptoms.
MATERIALS AND METHODS
Setting
The research was implemented at one of the charity organisations providing palliative care services in North India.
Sample
Data were collected by a total of 21 palliative care teams to ensure representation of patients from all geographical locations of Delhi NCR. Each team had a target of completing data from 11 patients selected through a convenience sampling technique. Following the basic rule of thumb for validation studies, we targeted a minimum of 180 patients (p/M ratio of 20 patients per item or a minimum of 150–300 patients).[16,17] We could achieve a sample of 228 patients (n = 228). All adult patients (18+ years) with a confirmed diagnosis of cancer and receiving home-based palliative care services, having the capacity to consent, ability to speak and understand Hindi were approached by the team during assigned data collection week. A subset of 50% of the total sample who gave consent and were available for repeat assessment were followed up on two more occasions at intervals of 3–4 weeks.
Data collection and validation
To ensure rigour and maintain uniformity in data collection, all enumerators were trained in data collection by the principal investigator (TB) and co-investigator (NA) through two group training sessions. The training was focused on making comprehension of every PHQ-9 item clear to enumerators, imparting skills to interview patients for each item of PHQ-9 through role plays and recording participants’ responses on the four given response categories. Enumerators were trained for coding, data management and maintaining summary log sheets for repeat assessment. Their progress was monitored by telephonic discussions with TB and field visits by a researcher (PC). Data collection was conducted in participants’ homes after obtaining written informed consent. Data were recorded using the paper-pen method. All enumerators were required to submit the data sheets with the field data monitor (PC and AR) before commencing repeat assessments to avoid chances of replication and subjective biases. Repeat assessment was conducted by the same interviewers.
Materials
The adapted version of PHQ-9 Hindi and a demographic sheet were used to collect data. In addition, the recently validated Integrated Palliative Care Outcome Scale (IPOS) for use in palliative care settings[18] was used to examine the validity of PHQ-9 against IPOS. PHQ-9 Hindi version is a brief nine-item scale to screen depression among patients. Many variants of PHQ are available; however, PHQ-9 is more often used in practice. PHQ-9 assesses depression through nine items, each of which is scored between 0 and 3, providing a score ranging from 0 to 27. PHQ family of measures were developed by researchers with a grant supported by Pfizer Inc.[19] All measures are in the public domain. Although PHQ measures are free to use, we obtained permission for their validation in a palliative care setting. IPOS is a 17-item multidimensional scale for assessing physical, emotional, communication, spiritual and practical issues of the patients with advanced illness.[20] It is widely used with cancer and non-cancer patients.[21]
At baseline PHQ-9, IPOS and a demographic sheet were used. At repeat assessment, only PHQ-9 and a single item of Global Health Rating were used to understand the change in participants’ health status from the last assessment.
Analysis
Five student volunteers were trained by TB in data extraction from hard copy to data entry in the Statistical Package for the Social Sciences (SPSS) (IBM SPSS 26). The field data monitor (PC) supervised data entry. TB and PC together checked 30% of the randomly selected sheets entered by each student volunteer for data entry errors and corrections were made. A very negligible error rate (0.08%) was reported in data entry, hence terminating data checking further.
To assess the structural validity of the scale, we used exploratory factor analysis through principal component analysis and scree plots. For confirmatory factor analysis (CFA), we used structural equation modelling using IBM SPSS AMOS 27. We evaluated the model fit using Chi-square, confirmatory fit index (CFI) and root mean square error of approximation measures for small samples. To examine reliability, we used Cronbach alpha for internal consistency of the scale and sub-scales and item-total correlation for assessing the discriminate ability of the instrument. Test-retest reliability was calculated using a t-test and inter-rater reliability was calculated using inter-class correlation coefficient. We assessed construct validity by relating the score of the PHQ-9 baseline with IPOS-relevant items using Spearman’s rank order correlation. For divergent validity, we compared PHQ-9 scores with cancer staging as well as PHQ-9 scores of baselines and follow-up of those patients who reported improvement in their health using Cohen’s d coefficient. Analysis was conducted using the IBM SPSS 28 version.
RESULTS
The study involved participants (n = 228) with diverse demographic and clinical characteristics. Mean age of the participants was approximately 51.24 (12.5) years a range spanning 69 years (min. 20 years and max. 89 years).
The majority of participants were female (68%), and married (77.2%), with either no formal education (25.4%) or primary education (31.1%). Most of them were either poor (43.4%) or lower middle (50.4%). Income status showed that 24.1% were living comfortably, while 32.5% found it very difficult to manage daily expenses. Most participants belonged to nuclear families (64.5%), with household sizes of 2–4 people (46.5%) or 5–6 people (37.3%).
The cancer types varied, most participants were in stage 4 (30.7%) and stage 3 (17.5%). Patients with early-stage diagnosis (stages 1 and 2 together) were very few (9.2%). The cancer stage of a substantial proportion of participants was either unknown (10.5%) or not applicable (15.8%). The unknown category for cancer staging included those patients whose cancer stage was unknown due to the non-availability of papers or not being aware. Not applicable cancers refer to those where TNM staging is not applicable like haematological cancers. A total of 15.4% had a history of cancer in their family, and comorbidities were present [Table 1].
| Participants | ||
|---|---|---|
| n | % | |
| Sex | ||
| Female | 155 | 68 |
| Male | 73 | 32 |
| Marital status | ||
| Unmarried | 9 | 3.9 |
| Married/having partner | 176 | 77.2 |
| Window/widower | 40 | 17.5 |
| Divorced | 2 | 0.9 |
| Education | ||
| No formal education/illiterate | 58 | 25.4 |
| Primary (7 years of education) | 71 | 31.1 |
| Sr. secondary (14 years of education) | 64 | 28.1 |
| Graduate and above | 21 | 9.2 |
| Social economic | ||
| Poor | 99 | 43.4 |
| Lower middle | 115 | 50.4 |
| Upper middle | 4 | 1.8 |
| Family structure | ||
| Nuclear | 147 | 64.5 |
| J oint | 71 | 31.1 |
| Cancer type | ||
| Breast (excludes skin of breast) | 53 | 23.2 |
| Lip, oral cavity and pharynx | 36 | 15.8 |
| Gynaecological cancer | 18 | 7.9 |
| Digestive organs | 25 | 11 |
| Respiratory system and intrathoracic organs | 16 | 7 |
| Not available/others | 80 | 35.1 |
| Cancer stage | ||
| Stage 1 | 3 | 1.3 |
| Stage 2 | 18 | 7.9 |
| Stage 3 | 40 | 17.5 |
| Stage 4 | 70 | 30.7 |
| Unknown/Not available | 24 | 10.5 |
| Not applicable | 36 | 15.8 |
Exploratory factor analysis
We followed Kaiser’s rule to identify emerging factors from the scree plot. The plot suggested [Figure 1] that only two factors should be retained as the value dropped below 1 when more than two factors were considered.[22] Exploratory factor analysis suggested a two-factor approach: Physical concerns (4 items) and emotional concerns (5 items). Following Kaiser’s rule, an item with a value >0.5 was retained to constitute the given factor[23] [Table 2].
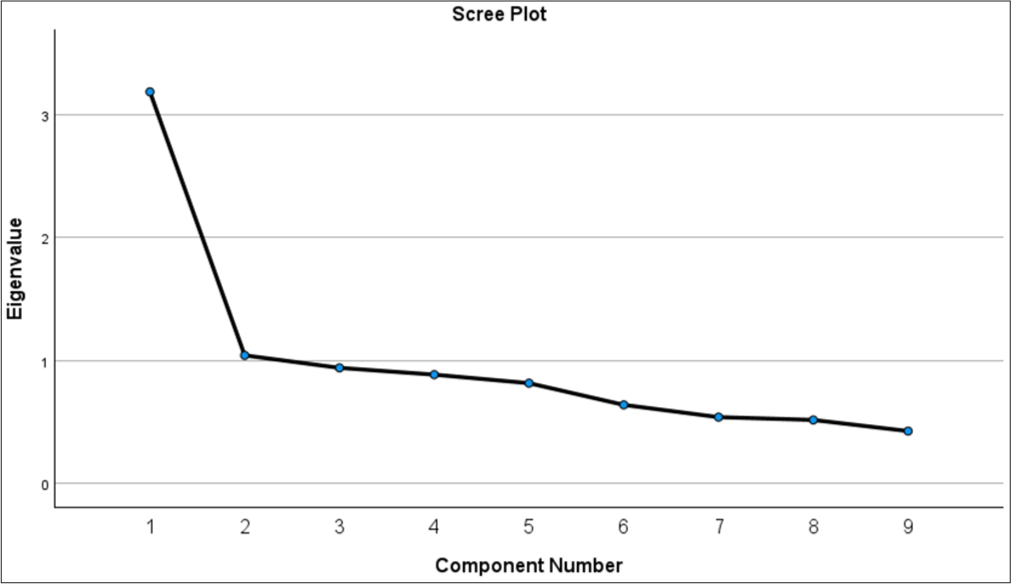
- Scree plot to identify number of factors.
| Components | ||
|---|---|---|
| Component 1 | Component 2 | |
| PHQ item 1 | 0.223 | 0.498 |
| PHQ item 2 | 0.533 | 0.292 |
| PHQ item 3 | 0.071 | 0.553 |
| PHQ item 4 | 0.278 | 0.629 |
| PHQ item 5 | 0.065 | 0.731 |
| PHQ item 6 | 0.733 | 0.207 |
| PHQ item 7 | 0.539 | 0.249 |
| PHQ item 8 | 0.551 | 0.537 |
| PHQ item 9 | 0.844 | −0.057 |
Extraction Method: Principal Component Analysis. Rotation Method: Varimax with Kaiser Normalisation. Rotation converged in 3 iterations. Component 1: Emotional Concerns, Component 2: Physical Concerns PHQ: Patient health questionnaire, Red colour scheme: Factor solution for emotional component, Yellow colour scheme: Factor solution for physical component
CFA
CFA fit indices were CFI = 0.980 and RMSEA = 0.031, TLI = 0.966 indicating medium fit of the model to the data (χ2 = 31.679, df = 26, χ2/df = 1.218, P = 0.204). Since CFI and RMSEA parameters approached the minimums, they were within the defined parameters recommended for small samples.[24]
The standardised parameter estimates of the modified model are shown in Figure 2. Physical concerns accounted for 62– 35% of the variance while emotional concerns presented a relatively large variance ranging from 76% to 9% of PHQ-9 items. Item no. 9 which centred on suicidal thoughts under emotional concerns had poor factor loading, that is 0.09 followed by factor loading of item no. 3 which dealt with sleep disturbances under physical concerns (factor loading 0.35). The correlation between physical and emotional concerns was very high (0.97) suggesting a strong fit of the factors in the model.
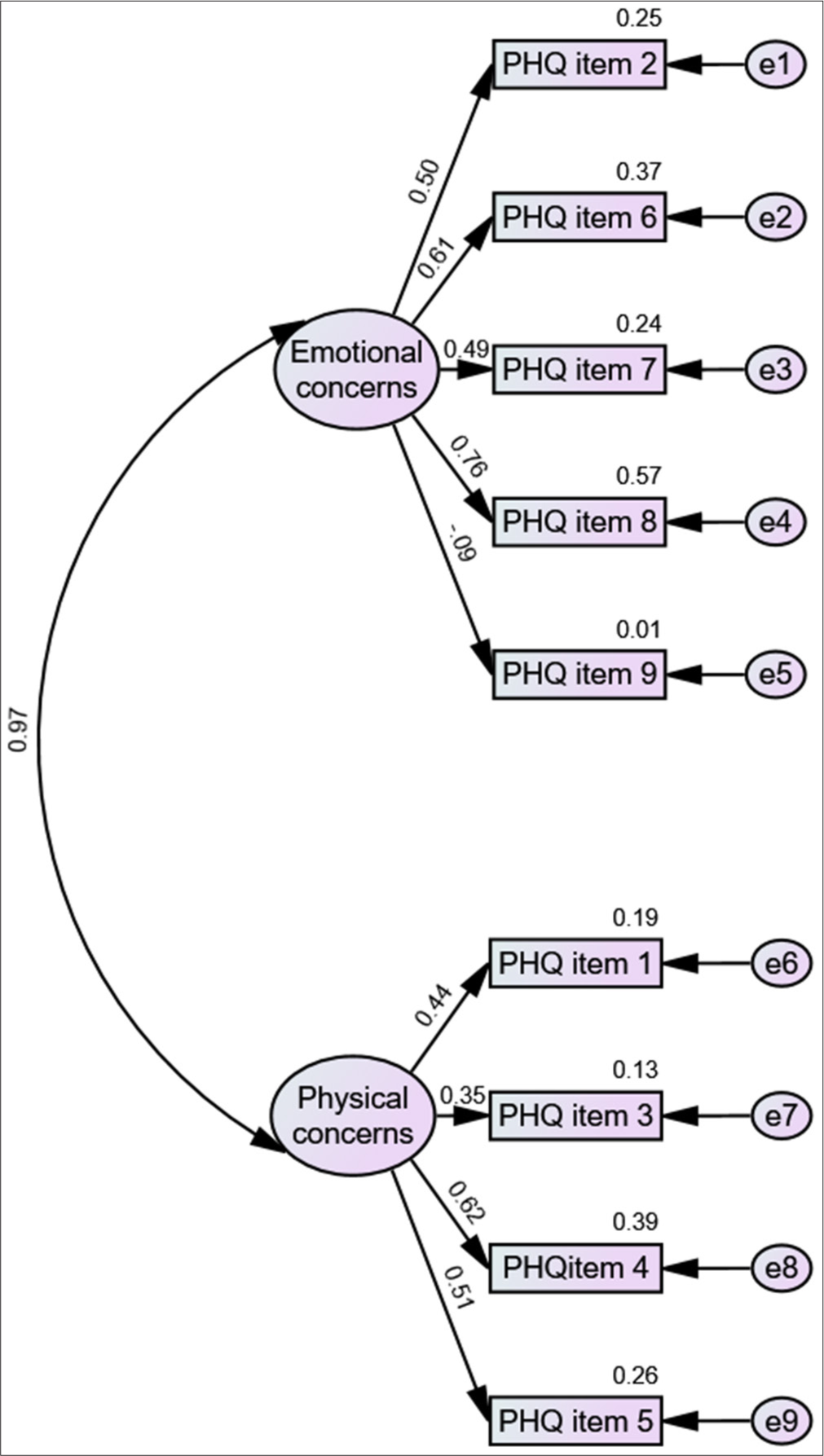
- Confirmatory factor analysis model of Patient Health Questionnaire-9 (PHQ) Hindi. e: measurement error.
The total scores and the sub-scale scores have been calculated by simple addition of relevant item scores. Cases with missing values were not included in the analysis. Data show completeness, missing values were negligible and within the acceptable parameters.
None of the items under the physical concerns domain showed floor or ceiling effects, responses were very well distributed among all the four given options. Two items under emotional concerns focussing on the difficulty in concentration (item no. 7) and suicidal tendency (item no.9) reflected floor effects (61% and 69%, respectively, on the lowest response category, [Table 3]. Missing value for both sub-scales, that is physical and emotional sub-scales was within acceptable limits, that is <10%.[25,26] [Table 4].
| Item | Not at all (0) | Few days (1) | More than half days (2) | Almost every day (3) | Mean (±SD) | Missing (%) |
|---|---|---|---|---|---|---|
| Physical concerns | 3.9 | − | − | − | 5.2 (2.8) | 6.1 |
| Lack of interest | 36.4 | 27.2 | 12.3 | 21.9 | 1.2 (±1.1) | 2.2 |
| Difficulty sleeping | 39.5 | 21.9 | 16.2 | 20.2 | 1.1 (±1.1) | 2.2 |
| Tired/lack of energy | 10.1 | 33.3 | 27.2 | 28.1 | 1.7 (±0.9) | 1.3 |
| Loss of appetite | 37.3 | 28.5 | 12.3 | 18.0 | 1.1 (±1.1) | 3.9 |
| Emotional concerns | 11.0 | − | − | − | 4.2 (±3.5) | 5.7 |
| Feeling sad | 24.6 | 41.2 | 16.7 | 16.2 | 1.2 (±1.0) | 1.3 |
| Feeling bad/unsuccessful | 43.6 | 28.9 | 11.8 | 13.2 | 0.9 (±1.0) | 2.2 |
| Difficulty concentration | 61.0 | 17.1 | 6.6 | 13.6 | 0.7 (±1.0) | 1.8 |
| Slow/figidity | 52.6 | 23.7 | 8.3 | 12.7 | 0.8 (±1.0) | 2.6 |
| Suicide | 69.3 | 16.7 | 4.8 | 6.1 | 0.4 (±0.8) | 3.1 |
PHQ: Patient health questionnaire, SD: Standard deviation
| n=228 | Number of items | Mean | SD | Floor effect (%) | Ceiling effect (%) | Missing (%) |
|---|---|---|---|---|---|---|
| Total | 09 | 9.5 | 5.5 | 1.3 | 0.4 | 10.1 |
| Physical | 04 | 5.2 | 2.8 | 3.9 | 1.8 | 6.1 |
| Emotional | 05 | 4.2 | 3.5 | 11.0 | 1.3 | 5.7 |
PHQ: Patient health questionnaire, SD: Standard deviation
Reliability
Item-total correlation
The physical sub-scale showed moderate internal consistency (α = 0.527), and the emotional sub-scale presented high internal consistency (α = 0.734). Internal consistency of the overall scale was also high (α = 0.759). Item-total correlation for each item of the scale was within the accepted limits [Table 5]. The intraclass correlation coefficient among physical and emotional sub-scales showed moderate agreement [Table 6].
| Sub-scale | Item | Item-total correlation |
|---|---|---|
| Physical | Lack of interest | 0.543** |
| Difficulty sleeping | 0.587** | |
| Tired/lack of energy | 0.486** | |
| Loss of appetite | 0.558** | |
| Emotional | Feeling sad | 0.606** |
| Feeling bad/unsuccessful | 0.654** | |
| Difficulty concentration | 0.577** | |
| Slow/figidity | 0.735** | |
| Suicide | 0.549** |
| ICC coefficient | |||||||
|---|---|---|---|---|---|---|---|
| ICC | 95% confidence interval | F test with true value 0 | |||||
| Lower Bound | Upper Bound | Value | df1 | df2 | Sig | ||
| Physical scale average measures | 0.552 | 0.325 | 0.702 | 2.230 | 93 | 93 | <0.001 |
| Emotional sale average measures | 0.646 | 0.449 | 0.770 | 3.073 | 96 | 96 | <0.001 |
ICC: Intraclass correlation, df: degrees of freedom, F: Analysis of Variance
Test-retest reliability
We hypothesised that there is no significant difference between Time 1–Time 2 and Time 1–Time 3 score of PHQ-9 Hindi scale. To test this, we compared the sub-scale scores of Time 1 and Time 2 for those whose repeat assessment was undertaken. We also compared Time 1 and Time 3 scores of the same population using paired one sample t-test. We compared the mean difference of PHQ-9 Hindi sub-scale scores among baseline and follow-up assessment sub-scale scores among patients who felt the same. Test-retest reliability analysis for both Time 1–Time 2 and Time 1–Time 3 failed to reject the null hypothesis (H0), indicating that there was no significant difference between baseline and follow-up.
In addition, we also tested interclass correlation and interrater reliability using two-way mixed effect model where people effects were random, and measure effects were fixed. Results indicated that difference between the mean scores was not significant between repeated test scores.
Inter-rater reliability was moderate for physical scale with 0.552 (0.32–0.07, 95% confidence interval [CI], n = 94) for average measures. Standard error of measurement was low (0.313). For emotional sub-scale, inter-rater reliability was high with 0.646 (0.44–0.77, 95% CI, n = 97). Standard error of measurement for emotional sub-scale was low (0.355).
Validity
As hypothesised, associations between PHQ-9 Hindi with IPOS Hindi individual items were all significant (P < 0.001) [Table 7]. Correlations between PHQ-9 Hindi sub-scales and IPOS individual selected items were in the predicted direction. The correlation was positive with IPOS items since higher scores on both scales indicate worse outcomes. For example, PHQ-9 Hindi physical sub-scale correlates moderately with shortness of breath, weakness/lack of energy, poor appetite and poor mobility. Interestingly, emotional items of PHQ-9 Hindi have much stronger correlation with IPOS items namely, feeling anxious/worried, feeling depressed and peace. The correlation of emotional sub-scale of PHQ-9 Hindi with physical concerns-based items of IPOS was relatively weaker while correlation with emotional concerns of IPOS was stronger and same was true for physical sub-scale of PHQ-9.
| PHQ Domain | Shortness of breath | Weakness or lack of energy | Poor appetite | Poor mobility | Feeling anxious or worried | Feeling depressed | Feeling at peace |
|---|---|---|---|---|---|---|---|
| PHQ physical | 0.256** | 0.442** | 0.406** | 0.361** | 0.311** | 0.421** | 0.323** |
| N | 212 | 208 | 210 | 213 | 212 | 213 | 211 |
| PHQ emotional | 0.285** | 0.394** | 0.359** | 0.411** | 0.484** | 0.508** | 0.506** |
| N | 213 | 208 | 212 | 214 | 213 | 214 | 213 |
| PHQ total | 0.313** | 0.469** | 0.422** | 0.444** | 0.445** | 0.535** | 0.457** |
| N | 203 | 199 | 202 | 204 | 203 | 204 | 203 |
PHQ: Patient health questionnaire, IOPS: Integrated palliative care outcome scale, ** significant at 0.01, N: Number of cases
PHQ-9 physical sub-scale has discriminative or known-groups validity while with advanced cancer have higher mean scores (mean [M] = 10.09, standard deviation [SD] = 5.82) compared to those with early cancer (M = 7.7, SD = 4.3). Similar trend was observed in physical and emotional sub-scales when compared with disease staging. Physical sub-scale scores for patients with early cancer was relatively lesser (M = 5.0, SD = 2.5) than patients with advanced stage cancer (M = 5.5, SD = 2.9). In case of emotional sub-scale, mean score for early-stage cancer patients was much lesser (M = 2.6, SD = 2.1) than advanced cancer patients (M = 4.5, SD = 3.7).
In order to estimate the effect size, we calculated Cohen’s d using paired sample t-test for first time administration of PHQ-9 Hindi and second time assessment. Results obtained were significant (t = 3.72, P < 0.001, df = 90) whereas the effect size was weak (Cohen’s d = 0.388).[27]
We assessed the ability of PHQ-9 to detect change in patient’s status and present evidence for detecting minimally clinical important differences.
We hypothesised that PHQ-9 score would change if there were improvement or deterioration in patient’s condition during subsequent assessments. Conversely, PHQ-9 score would remain the same if patient’s status does not change in subsequent assessment. We included those patients who reported themselves as ‘better’ compared to the previous assessment. Moderate to strong effect sizes were shown by both sub-scales and the scale as a whole, though the effect size of physical sub-scale was relatively smaller [Table 8].
| PHQ Domain Assessment Time | Paired Differences | t | df | One-Sided p | Cohen’ d value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | |||||||
| Lower | Upper | |||||||||
| Pair 1 | Total PHQ Time 1 - PHQ Time 2 | 4.075 | 5.404 | 0.854 | 2.347 | 5.803 | 4.769 | 39 | <0.001 | 0.754 |
| Pair 2 | PHQ Physical Time 1 - PHQ Physical Time 2 | 1.35714 | 3.17630 | 0.49011 | 0.36734 | 2.34695 | 2.769 | 41 | 0.004 | 0.427 |
| Pair 3 | PHQ emotional Time 1- PHQ emotional Time 2 | 2.65854 | 3.26810 | 0.51039 | 1.62700 | 3.69008 | 5.209 | 40 | <0.001 | 0.813 |
PHQ: Patient health questionnaire, MCID: Minimally clinical important differences, df: degrees of freedom, t: student’s t-test
DISCUSSION
We examined the psychometric validation of PHQ-9 Hindi for the early screening of depression among patients with cancer receiving palliative care services. The process of scale validation involved two phases. The first phase involved the cultural adaptation which was already undertaken for PHQ-9 and published elsewhere.[15] The second phase related to assessment of psychometric properties of the adapted scale, which is reported here. Findings of this research relate to a sample of either uneducated or less educated patients belonging to poor or lower middle class, living in a nuclear family structure.
In palliative care settings, generally most of the patients are seen at advanced stages which are also evident in present research from the cumulative percentage of patients at stage 3 and stage 4. Previous studies have also reported that depression is frequently observed among advanced stage or terminally ill cancer patients.[28,29] In India, previous research have also indicated that depressive and anxiety disorders show a crude prevalence of 3.3%. This has accounted for 19–33.8% of disease-adjusted life years attributed to mental disorders.[30,31] Such evidence indicating high prevalence of depression and other psychiatric morbidities among patients with cancer makes it even more significant that a culturally valid instrument to screen patients early for depressive symptoms be developed.
Exploratory factor analysis presented a two-factor solution in line with the hypothesised model with two sub-scales namely, physical and emotional concerns. Confirmatory factor analysis indices in our sample were conclusive, and we can infer that there is a relatively good fit between the hypothesised model and the observed data confirming cross-cultural validity of PHQ-9 Hindi. Confirmatory factor analysis in previous research has also supported a two-factor model of PHQ-9 being a better fit with heterogenous cancer population.[14,32] Notably, two items centred on suicidal thoughts and sleep disturbances exhibited low factor loadings. Previous research reported that factor loading of PHQ-9 was within the acceptable limits.[33] This suggests that PHQ-9 Hindi is a reliable measure to efficiently assess the concerns of patients with cancer.
PHQ-9 Hindi items were appropriately distributed. None of the items for this sample showed floor or ceiling effects for physical sub-scale; however, items on suicide and difficulty concentration under emotional sub-scale presented floor effect. It is important to note that the floor and ceiling effects of both the consolidated sub-scales were within the acceptable limit indicating suitability of PHQ-9 Hindi among cancer patients.[34-37]
Missing data analysis presented a very small percentage suggesting high value of data completeness. PHQ-9 has presented high data completeness in previous research[32] as well suggesting its suitability in clinical practice. This gives an advantage to use PHQ-9 in clinical practice for two reasons, one being a brief scale and second having the quality of yielding high data completeness.
The overall internal consistency of PHQ-9 Hindi was found to be significant in the present research which is in line with previous evidence. Internal consistency of PHQ-9 by previous research conducted in varied settings was also found to be high suggesting that the items of PHQ-9 Hindi are consistent with each other and effective to yield meaningful results.[7,38] Furthermore, the present research noted a strong correlation between physical and emotional concerns. This supported the two-factor model suitability for such heterogeneous population which has also been reported in previous research.[7] However, other researchers[38,39] reported single factor solution of PHQ-9 with patients with major depressive disorder and university students suggesting further scope of confirmatory factor analysis of PHQ-9 Hindi in varied populations and cultural contexts. Previous researchers[39-43] have also examined the internal consistency of PHQ-9. The present research showed a high test-retest reliability coefficient for the total scores which have also been reported by other researchers,[40-44] confirming that PHQ-9 has excellent internal reliability.
Convergent validity of PHQ-9 Hindi was confirmed against IPOS Hindi. Previous research reported that the total score of PHQ-9 exhibited a positive correlation with the total score of HAMD-17.[40] We examined if emotional sub-scales of PHQ-9 Hindi presented convergent validity with IPOS supporting the capability to assess depression and anxiety among patients receiving palliative care services. Findings suggested that PHQ-9 Hindi emotional sub-scales appropriately assess emotional concerns of the patients as the scores relate well with other scales both in previous and present research. PHQ-9 Hindi physical sub-scale confirmed discriminative or known-groups validity when compared with patients with advanced cancer who presented higher mean scores as compared to those with early cancer. Previous research have also confirmed that physical concerns of the patients with advanced stage cancer are more overwhelming than those at early stages of cancer.[45,46] This has implication for clinical practice necessitating early screening and intervention with patients who are at advanced stages of cancer to improve their quality of life. Alongside, PHQ-9 Hindi has presented moderate to strong effect size in both physical and emotional sub-scales. Very few research studies have reported the effect size in their study reflecting the need of in-depth psychometric analysis in future research.[8,47]
CONCLUSION
PHQ-9 Hindi is a valid and reliable tool to screen Hindi speaking patients with cancer who may have depressive symptoms. India is a multilingual and culturally diversified country, so it is important that PHQ-9 is tested in other parts of the country in other regional languages before use. This tool will help the practitioners in palliative care settings to identify patients with depressive symptoms early and provide appropriate timely interventions to them. PHQ-9 Hindi, being a brief scale, gives an edge to the practitioners to quickly make an assessment without any discomfort to the patients with cancer.
Acknowledgment
We thank Can Support home care team members who helped us in data collection. Our special thanks to Ms. Anu Paul and Ms. Pallika Chaudhary (PC) for providing training support and coordination during data collection. We also thank all participants for their valuable time. We are thankful to student volunteers Nashrah Ali Siddiqui, Praney Aggarwal, Prerita Bahri, Swastika Borgohain and Anisha for their support in data entry. We thank our language editor Dr. N.Victoria Chanu for her precious time to proof read and edit this manuscript.
Ethical approval
The research/study was approved by the Institutional Review Board at Institutional Ethics Committee-Human Research Cansupport, number IEC CanSupport 3/2022, dated 23rd May 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Patient-reported Anxiety and Depression Measures for Use in Indian Head and Neck Cancer Populations: A Psychometric Evaluation. J Patient Rep Outcomes. 2021;5:44.
- [CrossRef] [PubMed] [Google Scholar]
- Depression and Anxiety in Patients with Cancer. BMJ. 2018;361:k1415.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Depression and Anxiety among Cancer Patients. Caspian J Intern Med. 2014;5:167-70.
- [Google Scholar]
- Depression and the Cancer Patient. J Clin Psychiatry. 1990;51(Suppl):12-7. discussion 8-9
- [Google Scholar]
- Depression Screening in Cancer Patients: A Narrative. J Nurs Educ Pract. 2018;8:11-5.
- [CrossRef] [Google Scholar]
- Routine Depression Screenings for Advanced Cancer Patients: Reducing Disparities, Identifying Depression, and Improving Quality of Life. J Hosp Palliat Nurs. 2020;22:12-6.
- [CrossRef] [PubMed] [Google Scholar]
- The PHQ-9: Validity of a Brief Depression Severity Measure. J Gen Intern Med. 2001;16:606-13.
- [CrossRef] [PubMed] [Google Scholar]
- The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr Ann. 2002;32:509-15.
- [CrossRef] [Google Scholar]
- Monitoring Depression Treatment Outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42:1194-201.
- [CrossRef] [PubMed] [Google Scholar]
- Translation and Validation of Brief Patient Health Questionnaire against DSM IV as a Tool to Diagnose Major Depressive Disorder in Indian Patients. J Postgrad Med. 2007;53:102.
- [CrossRef] [PubMed] [Google Scholar]
- Psychometric Comparison of PHQ-9 and HADS for Measuring Depression Severity in Primary Care. Br J Gen Pract. 2008;58:32-6.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring Depression Outcome with a Brief Self-report Instrument: Sensitivity to Change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the General Population. Gen Hosp Psychiatr. 2006;28:71-7.
- [CrossRef] [PubMed] [Google Scholar]
- Psychometric Comparison of the PHQ-9 and BDI-II for Measuring Response During Treatment of Depression. Cogn Behav Ther. 2011;40:126-36.
- [CrossRef] [PubMed] [Google Scholar]
- Cultural Adaptation of Patient Health Questionnaire-9 in Hindi for Use with Patients with Cancer in Community Palliative Care Settings. Indian J Palliat Care. 2023;29:292.
- [CrossRef] [PubMed] [Google Scholar]
- Sample Size Used to Validate a Scale: A Review of Publications on Newly-developed Patient Reported Outcomes Measures. Health Qual Life Outcomes. 2014;12:2.
- [CrossRef] [PubMed] [Google Scholar]
- Sample Size Requirements for the Internal Validation of Psychiatric Scales. Int J Methods Psychiatr Res. 2011;20:235-49.
- [CrossRef] [PubMed] [Google Scholar]
- Translation and Cross-cultural Adaptation of the Integrated Palliative Care Outcome Scale in Hindi: Toward Capturing Palliative Needs and Concerns in Hindi Speaking Patients. Palliat Med. 2023;37:391-401.
- [CrossRef] [PubMed] [Google Scholar]
- Validation and Utility of a Self-report Version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282:1737-44.
- [CrossRef] [PubMed] [Google Scholar]
- A Brief, Patient-and Proxy-reported Outcome Measure in Advanced Illness: Validity, Reliability and Responsiveness of the Integrated Palliative care Outcome Scale (IPOS) Palliat Med. 2019;33:1045-57.
- [CrossRef] [PubMed] [Google Scholar]
- Starting from Scratch: Implementing Outcome Measurement in Clinical Practice. Ann Palliat Med. 2018;7(Suppl 3):S253-61.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluating Model Fit In: Structural Equation Modeling: Concepts, Issues, and Applications. Thousand Oaks, CA, US: Sage Publications. Inc; 1995. p. :76-99.
- [Google Scholar]
- How Can I Deal with Missing Data in My Study? Aust N Z J Public Health. 2001;25:464-9.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple Imputation: A Primer. Stat Methods Med Res. 1999;8:3-15.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing Effect Sizes in Follow-up Studies: ROC Area, Cohen's d, and r. Law Hum Behav. 2005;29:615-20.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological Distress Following First Recurrence of Disease in Patients with Breast Cancer: Prevalence and Risk Factors. Breast Cancer Res Treat. 2000;61:131-7.
- [CrossRef] [PubMed] [Google Scholar]
- Psychotherapy for Depression among Patients with Advanced Cancer. Jpn J Clin Oncol. 2012;42:1113-9.
- [CrossRef] [Google Scholar]
- Are the PHQ-9 and GAD-7 Suitable for Use in India? A Psychometric Analysis. Front Psychol. 2021;12:676398.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of Mental Disorders across the States of India: The Global Burden of Disease Study 1990-2017. Lancet Psychiatry. 2020;7:148-61.
- [CrossRef] [PubMed] [Google Scholar]
- Enabling Cross-cultural Data Pooling in Trials: Linguistic Validation of Head and Neck Cancer Measures for Indian Patients. Qual Life Res. 2021;30:2649-61.
- [CrossRef] [PubMed] [Google Scholar]
- Translation, Cultural Adaptation, and Validation of the PHQ-9 and GAD-7 in Kinyarwanda for Primary Care in the United States. PLos One. 2024;19:e0302953.
- [CrossRef] [PubMed] [Google Scholar]
- Floor and Ceiling Effects, Time to Completion, and Question Burden of PROMIS CAT Domains among Shoulder and Knee Patients Undergoing Nonoperative and Operative Treatment. JBJS Open Access. 2019;4:e0015.
- [CrossRef] [PubMed] [Google Scholar]
- Quality Criteria were Proposed for Measurement Properties of Health Status Questionnaires. J Clin Epidemiol. 2007;60:34-42.
- [CrossRef] [PubMed] [Google Scholar]
- Psychometrics of the Patient-reported Outcomes Measurement Information System Physical Function Instrument Administered by Computerized Adaptive Testing and the Disabilities of Arm, Shoulder and Hand in the Orthopedic Elbow Patient Population. J Shoulder Elbow Surg. 2018;27:515-22.
- [CrossRef] [PubMed] [Google Scholar]
- Psychometric Properties of the PROMIS Physical Function Item Bank in Patients with Spinal Disorders. Spine. 2014;39:158-63.
- [CrossRef] [PubMed] [Google Scholar]
- The Reliability and Validity of PHQ-9 in Patients with Major Depressive Disorder in Psychiatric Hospital. BMC Psychiatry. 2020;20:474.
- [CrossRef] [PubMed] [Google Scholar]
- Validity and Reliability of the Patient Health Questionnaire Scale (PHQ-9) among University Students of Bangladesh. PLoS One. 2022;17:e0269634.
- [CrossRef] [PubMed] [Google Scholar]
- The Validity and Reliability of the PHQ-9 on Screening of Depression in Neurology: A Cross Sectional Study. BMC Psychiatry. 2022;22:98.
- [CrossRef] [PubMed] [Google Scholar]
- The Patient Health Questionnaire-9 vs. the Hamilton Rating Scale for Depression in Assessing Major Depressive Disorder. Front Psychiatry. 2021;12:747139.
- [CrossRef] [PubMed] [Google Scholar]
- The Patient Health Questionnaire-9 for Detection of Major Depressive Disorder in Primary Care: Consequences of Current Thresholds in a Crosssectional Study. BMC Fam Pract. 2010;11:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- The Patient Health Questionnaire (PHQ-9) as a Tool to Screen for Depression in People with Multiple Sclerosis: A Cross-sectional Validation Study. BMC Psychol. 2022;10:281.
- [CrossRef] [PubMed] [Google Scholar]
- Patient Health Questionnaire-9 (PHQ-9) [Database Record] APA PsycTests 1999
- [CrossRef] [Google Scholar]
- Symptom Clusters in Advanced Cancer. J Pain Symptom Manag. 2011;42:24-31.
- [CrossRef] [PubMed] [Google Scholar]
- A Prospective Study to Determine the Association between Physical Symptoms and Depression in Patients with Advanced Cancer. Palliat Med. 2004;18:558-63.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for Perinatal Depression with the Patient Health Questionnaire Depression Scale (PHQ-9): A Systematic Review and Meta-analysis. Gen Hosp Psychiatry. 2021;68:74-82.
- [CrossRef] [PubMed] [Google Scholar]







