Translate this page into:
Blood Transfusions in Patients with Advanced Cancer at the End-of-Life: Are They Really Beneficial?
*Corresponding author: Steven Callaghan, Cancer Center of Excellence, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia. Stevenc108@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Callaghan S, Sadler K, Abudari GA, Almutairi M, Almusaed SM, Alqahtany B, et al. Blood Transfusions in Patients with Advanced Cancer at the End-of-Life: Are They Really Beneficial? Indian J Palliat Care. doi: 10.25259/IJPC_356_2024
Abstract
Objectives
Anaemia is prevalent in individuals with advanced cancer and may contribute to adversely affecting their quality of life. In palliative care (PC), red blood cell (RBC) transfusions are regularly administered to address bothersome symptoms such as fatigue and dyspnoea. However, they are not without risks and adverse effects in individuals who are often frail at the end of life. Within this context, RBC transfusion benefits and drawbacks have yet to be demonstrated. This study aimed to assess RBCs transfusion-targeted indications, practices and clinical outcomes at the end-of-life in hospitalised patients with advanced malignancy.
Materials and Methods
A retrospective cohort design was used. All adults with a cancer diagnosis admitted to a PC Unit in a tertiary care centre in Saudi Arabia and who received at least one RBC transfusion between 1 January 2020 and 1 January 2024 were included in the study. Data were retrieved from their medical records (demographics, clinical and transfusion episode information) and included the Charlson Comorbidity Index.
Results
A total of 84 patients were included, comprising a total of 159 episodes of transfusion. Patients had a mean age of 47 years (19.4%). The most frequent location of cancer was the gastrointestinal system (34.6%). For most patients, a low haemoglobin level was the main indication for the transfusion, without precise symptoms being targeted. There was no improvement following several transfusion episodes (82.4%). A moderate negative correlation was observed between age and survival days post-transfusion.
Conclusion
There is a need for further studies to better understand the benefits of RBC transfusions at the end of life. In addition, more attention is warranted to establish targeted clinical outcomes pre-transfusion rather than relying on abnormal laboratory values. Validated self-reported tools should be used to ensure the benefits of an intervention that involves such a limited and valuable resource.
Keywords
Blood transfusion
End-of-life
Palliative care
Quality indicators
Saudi Arabia
INTRODUCTION
Anaemia is common in individuals with advanced cancer at the end of life, with studies reporting a prevalence rate as high as 50% for any palliative care (PC) admission to 90% in the last admission before death.[1] The World Health Organization defines anaemia with haemoglobin level under 12 g/dL in women and 13 g/dL in men respectively.[2] Anaemia aetiology includes chronic inflammation, erythropoietin deficiency, bone marrow infiltration, bleeding events and treatment side effects.[1] While several types of anaemia exist, iron deficiency anaemia (IDA) is most commonly linked to malignancy.[3] IDA implies a lack of sufficient mineral iron to help the body produce red blood cells (RBCs). However, research carried out by Natalucci et al. (2021) suggests that the majority of cancer-related anaemia is, in fact, multifactorial.[4] In an endof-life context, RBC transfusions are regularly administered to address bothersome symptoms such as fatigue, dyspnoea, or in patients with active bleeding.[5] Recent reviews found that most patients report symptomatic benefits that can extend even to a few weeks, with minimal adverse events.[1] However, there are still no baseline predictors of who will most likely benefit.[1] The decision to transfuse patients in PC can be complex. In this context, goals of care are individualised, and clinical endpoints usually involve the short-term alleviation of symptoms rather than the restoration of health. Thus, the transfusion decision thresholds differ from the one used in non-palliative patients.[6] Transfusions are not without risks, including adverse reactions, fluid overload, and infection.[7] Furthermore, benefits seen in non-palliative patients cannot be extrapolated to patients at the end of life, given their disease extent, cachexia, and poor functional status at baseline.[7] Factors such as other underlying comorbidities, poor nutritional intake and deficiencies, deconditioning and expected survival time all confound usual RBCs transfusion risks and benefits assessments.[6] Multiple unresolved questions remain about the place of RBC transfusions in the therapeutic arsenal to alleviate symptoms in PC. What are the symptomatic benefits? What are the effects on the overall quality of life? What is the impact on the overall survival post-RBC transfusion? Evidence of the risks and benefits of RBCs in a PC setting remains scarce.[1,6,8]
In addition, RBCs are a valuable and limited resource.[6] In emergencies, they are regularly urgently required for their life-saving effect, thus raising ethical considerations in balancing the availability of resources, patient’s needs, and the wider population requirements.[1]
There are currently no clinical practice guidelines or randomised controlled trials to evaluate the effectiveness of transfusions or to determine which groups of patients with advanced cancer are most likely to benefit.[9] This study aimed to examine the indications, practices and outcomes of RBC transfusions at the end-of-life in hospitalised patients with advanced cancer, with the goal of contributing to the evidence needed for the future development of clinical practice guidelines for RBC transfusions in PC settings.
MATERIALS AND METHODS
Participants and setting
A retrospective cohort study assessed transfusion indications and practices of RBCs in a specialized PC unit. All adult patients with a histologically confirmed cancer diagnosis, admitted under the care of the PC Service, who received at least one RBC transfusion, were included in the study for a period between 1 January 2020 and 1 January 2024. There were no exclusion criteria. During the 4-year study period, if a patient had more than one transfusion episode, each episode was treated separately and included in the analyses. The study was conducted at King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, a 1200-bed tertiary care and cancer centre. The PC team comprises specialized physicians and nurses supported by dedicated allied healthcare professionals. PC services range from complex symptom control to psychological, social, and spiritual support. PC services are provided across inpatient and outpatient settings. Fifteen beds are allocated for patients with cancer at the end of life (usually with a prognosis of <6 months), a Do-Not-Attempt-Resuscitation order, and who are no longer receiving disease-modifying treatments.
The organisation’s Institutional Review Board approved the study in July 2024 (Reference #2241222), and due to the minimal risks involved, a waiver for informed consent was granted.
Data sources
Data were retrieved from patients’ medical records (demographics, clinical and transfusion episode information). Demographic information includes age, sex, and residence location. Clinical information includes primary diagnosis, Charlson Comorbidity Index, resuscitation status, presence of disease-modifying treatment, and medication history (specifically, treatments to prevent or mitigate anaemia (e.g., iron) and medication that could cause or exacerbate bleeding taken up to 72 h’ pre-transfusion). Transfusion episode information includes the nonexclusive indications for the RBC transfusion, who requested the transfusion, haemoglobin and platelet levels pre-transfusion, presence of active bleeding, urgency for the transfusion, number of RBC unit(s) given per episode, history of RBC transfusion in the past 6 months, clinical outcomes, adverse reactions, survival time post-transfusion and length of stay. The Charlson Comorbidity Index predicts the mortality and disease burden by looking into the primary diagnosis, comorbidities, and age. It considers 17 diagnosis types, such as cancer or heart disease. Each diagnosis is assigned a score of 1, 2, 3, or 6, depending on the mortality risk associated. Incremental additional points are added for individuals over 50 years. Scores are then summed up. The higher the score, the higher the predicted mortality rate.[9] The final score can help decide if a treatment is worth it regarding benefits versus risks.
A data abstraction form was built using the Redcap™ software hosted in the organisation. Team members were met to review the form and data extraction process and ensure data reliability.
Statistical analysis
Descriptive statistics were used to present the cohort, using means/standard deviations (SDs) and median/interquartile ranges for continuous variables and counts/percentages for categorical variables. A Pearson correlation was used to assess the association between the Charlson Comorbidity Index score and the patient’s demographic, clinical, and transfusion episode characteristics. P < 0.05 was considered significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 21.
RESULTS
Baseline characteristics
A total of 84 patients were included, comprising a total of 159 episodes of transfusion. Patients’ mean age was 46.7 years (SD = 19.4) (Median = 48; 95% confidence interval [CI] [43.82, 49.88]), with a slight female predominance (n = 55; 65.4%). The most common diagnosis was gastrointestinal cancer (n = 27; 34.6%), followed by musculoskeletal cancer (n = 14; 18.2%) [Table 1]. The mean Charlson Comorbidity Index score was 7.8 (SD = 2.3). All patients had a do not attempt resuscitation (DNAR), and none were receiving disease-modifying treatments.
| n | % | |
|---|---|---|
| Sex | ||
| Male | 29 | 34.6 |
| Female | 55 | 65.4 |
| Primary tumour | ||
| Gastrointestinal | 27 | 34.6 |
| Musculoskeletal | 14 | 18.2 |
| Reproductive organs | 10 | 11.9 |
| Renal | 10 | 11.9 |
| Breast | 9 | 11.3 |
| Lung | 6 | 4.4 |
| Haematological | 3 | 3.1 |
| Ear-Nose-Throat | 2 | 1.9 |
| Brain | 1 | 1.3 |
| Other | 2 | 1.9 |
| DNAR | 84 | 100 |
| Curative treatments | 0 | 0 |
n=84 patients. Age: M=47 (19), Mdn=48; 95% Confidence interval (CI) (34, 62), DNAR: Do not attempt resuscitation
Medication history pre-transfusion
Medication supplements used to prevent or mitigate anaemia were uncommon. Only ten patients regularly took folic acid (6.3%), and nine took iron tablets (5.7%). However, several medications increasing the risks of bleeding as potential adverse effects were used, such as anticoagulants (n = 57; 35.8), anti-inflammatory drugs (n = 43; 27%), selective serotonin recapture inhibitors (n = 16; 10.1%) and antiplatelet agents (n = 8; 5%).
RBCs transfusion indications and practices
The primary non-exclusive indications for the RBCs transfusion were a low haemoglobin level (n = 151; 95%), followed by shortness of breath (n = 31; 19.5%), tiredness (n = 28; 17.6%) and weakness (n = 24; 15.1%). Other less common indications included paleness, dizziness, irregular heartbeat, chest pain, and headache [Figure 1]. PC physicians requested most transfusions (n = 109; 68.5%). The mean pretransfusion haemoglobin level was 64.5 g/L (9.3) and ranged from 40 to 87 g/L. The mean platelet count was 229 × 109/L (205), ranging from 1 to 1322 × 109/L. Active bleeding was present in 50 participants (31.4%). The mean time between the prescription and transfusion initiation was 5.6 h (SD = 3.9). Most of the transfusions were considered non-urgent. Most patients received either 1 (n = 75; 47.2%) or 2 (n = 83; 52.2%) RBCs unit(s) per transfusion episode, with only one patient (0.6%) receiving three units. 144 patients (90.6%) had a transfusion history in the past six months.

- Frequency of nonexclusive indications for blood transfusions. n = 159 transfusion episodes. X axis represents symptoms and Y axis represents frequency (%).
Transfusion outcomes
Considering all episodes of RBC transfusion (n = 159), for 82.4% (n = 131) of these, there was no documentation of symptomatic relief post-transfusion. The symptomatic benefit was evaluated by reviewing the physician’s written documentation and patient reports, either through verbal feedback regarding the effects following the RBC transfusion or, on occasion, using the validated Arabic Questionnaire for Symptom Assessment (AQSA) tool. Among the remaining, improvement in tiredness (n = 9; 5.7%), shortness of breath (n = 7; 4.4%), bleeding (n = 6; 3.8%), paleness (n = 2; 1.3%), and dizziness (n = 2; 1.3%) were recorded [Figure 2]. For 6 patients (3.9%), the documentation indicated a ‘clinical improvement’ without specifying its nature. Only one transfusion adverse reaction was recorded as a febrile nonhaemolytic transfusion reaction.
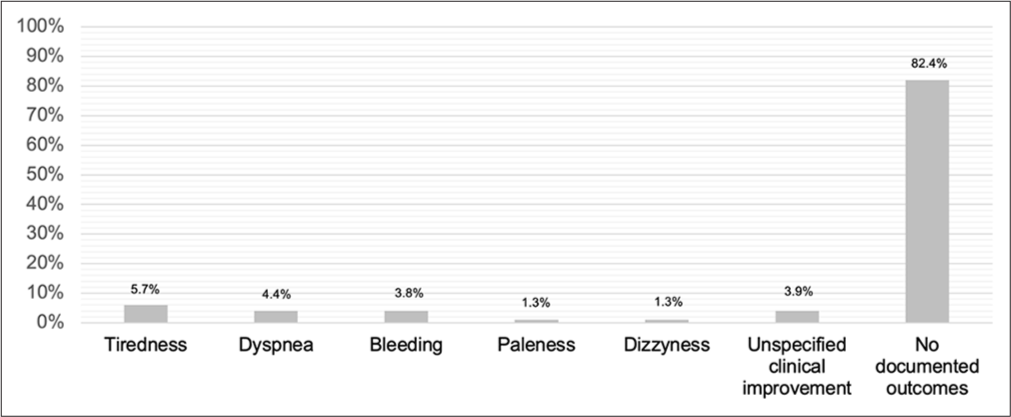
- Post-blood transfusion episode symptomatic outcomes. n = 159 transfusion episodes. X axis represents symptoms and Y axis represents frequency (%).
There were no significant correlations between documented indications and documented symptomatic relief post-transfusion; for example, the Indications for transfusion (shortness of breath) and symptomatic benefit post-transfusion (improved shortness of breath) were pearson correlation test (r) = −0.022.
Survival analysis
The mean survival time post-transfusion was 31 days (SD = 33.2), with a median of 18 days with 95% CI (25, 36). In 25% of the RBC episodes, the patient survived for 7 days or less, and 50% survived for 18 days or less [Figure 3]. No significant differences in survival were found based on sex (t-test [t] = −0.3833, P = 0.7023) or primary diagnosis (t = 1.3029, p-value [P] = 0.2421). A moderate negative correlation was observed between age and survival days (r = −0.303), suggesting that older patients tended to have a shorter survival time post-transfusion. The Charlson Comorbidity Index showed a weak positive correlation with survival days (r = 0.134). The mean length of stay (LOS) during the transfusion episode was 33 days (SD = 41). Most patients (n = 132; 83%) died during the same hospitalisation that the transfusion was administered. For the remaining, some were discharged to a local hospital (n = 3; 1.9%), home (n = 1; 0.6%), or had an unknown disposition (n = 23; 14.5%). The survival probability decreases progressively over time, indicating that patients tend to experience a decline in survival as the number of days post-transfusion increases [Figure 4]. A sharp decline in survival probability is observed during the initial days post-transfusion. This suggests that a significant proportion of patients face death shortly after the transfusion. A smaller subset of patients survives beyond 100 days post-transfusion.

- Distribution of the number of survival days’ post-blood transfusion episode.

- Kaplan–Meier survival curve.
DISCUSSION
This study aimed to assess RBC transfusion indications, practices, and outcomes at the end of life in a cohort of patients with advanced cancer admitted to a specialized PC unit. The results of this study indicate that the mean age of patients receiving a transfusion was 46.7 years. This mean age is significantly lower than in similar studies in Portugal and Canada.[9,10] Saudi Arabia’s relatively young population could potentially explain these results.
In this study, a third of the RBC transfusion episodes occurred while the patient had active bleeding. Several medications increased the risks of bleeding, such as anticoagulants (35%) and anti-inflammatory drugs (27%), were found to be taken by patients. The place of such medications in an end-of-life context is still poorly understood, with scarce evidence regarding their benefits.[11] Physicians often have mixed reviews about prescribing, pursuing, or discontinuing these medications in a PC setting.[11]
The reasons guiding their choice to pursue these drugs were the lack of evidence supporting their discontinuation in this context, uncertainty about patients’ life expectancy, and the fear of harming patients.[11] A retrospective review in Sweden investigated bleeding as a side effect of antithrombotic treatments in the last year of life.[12] The researchers mapped the timing of de-prescribing these drugs (n = 1501 charts).[12] The results highlighted the lack of clear guidance regarding the pursuit or discontinuation of antithrombotic agents in a PC settin g. They found these drugs to be associated with a high risk of bleeding, especially in the last years of life.[12]
In this study, for almost all the RBC transfusion episodes, the primary indication was a low haemoglobin value, followed in a much smaller proportion by shortness of breath, fatigue, and general weakness. If the proportion of patients who were administered RBCs due to a low haemoglobin level is much higher than found elsewhere, the other listed indications are similar to studies globally.[4,10] Two retrospective chart reviews of RBC transfusion practices in a PC service in Canada indicated that RBC transfusions rarely occur.[4,10] Similarly to our study, when administered, blood products are primarily used in patients with cancer to address fatigue and dyspnoea, with most patients reporting benefits.[4,10] A study conducted in Portugal also reported fatigue as the primary indication, with dizziness and shortness of breath following.[9] RBC transfusions were beneficial for 36% of patients.[9] Fatigue was the most commonly targeted symptom in a large prospective series of RBC transfusions in PC.[1] If 49% of patients reported symptomatic relief, no clear predictors of response were identified.[1] Another retrospective cohort study reported that the most common indications for RBC transfusion were fatigue/lethargy (41/44, 93%), followed by shortness of breath (7/44, 16%), light-headedness (3/44, 7%) and active bleeding (6/44, 14%).[7] The majority of patients (94%) reported symptomatic improvements.[7]
Surprisingly, in this study, no outcomes in symptomatic relief post-RBC transfusion were documented for most patients. In other studies, clinicians commonly report improvement following RBC transfusion in 65–89% of patients.[7] However, clinicians’ assessments are not exempt from biases. They can be overconfident in their ability to objectively assess their patients’ clinical situations, not systematically feeling the need to ask patients to self-assess their symptoms. Another interesting finding is the poor improvement in average scores recorded using validated symptom measurement tools such as the Resource Utilisation Groups-Activity of Daily Living, Australia Modified Karnofsky Scale, or Symptom Assessment Scale-Breathing and fatigue scales in studies.[7] These tools are possibly not well adapted to assess the needs and benefits of RBC transfusions in the end-of-life context.
This study’s mean survival time post-transfusion was approximately 1 month, and in 75% of the RBC transfusion episodes, patients survived <2 or 5 weeks [Figure 3]. A similar result was found in a study assessing RBC transfusion’s impact on survival in patients with advanced cancer in the terminal stage.[13] Anaemic patients who were transfused compared with anaemic patients who were not transfused had a survival time (15 days vs. 8 days, P < 0.001). In contrast, other studies have found longer survival times than our study. In a large retrospective cohort study (n = 885 patients transfused/6980 patients referred to PC between 2014 and 2018), the median survival post-transfusion was 56 days, 95% CI (19, 200).[14] Another similar study reported a 30-day survival rate of 57% post-RBC transfusion.[9] Among those surviving more than 30 days, having received transfusions was associated with a higher likelihood of death in a hospital setting.
The decision to administer an RBC transfusion is challenging, requiring a balance between symptomatic benefits and available resources. An alternative approach is to discuss the benefits, risks and prognosis of transfusions with patients and families to ensure alignment with their preferences. In addition, prioritizing RBC resources for patients who will gain significant comfort and avoiding transfusions with minimal benefit is suggested. A study on transfusion practices among PC physicians in Canada revealed that most physicians felt a lack of evidence to guide transfusion therapy and often relied on judgment and experience when making transfusion decisions.[15]
In our study, older patients tended to have a shorter survival time post-transfusion. In a retrospective review exploring the 1-year mortality rate in 897 heart failure patients, the Charlson Comorbidity Index was used.[16] However, in our study, the mean Charlson Comorbidity Index score was 7.8 (SD = 2.3), compared with a similar study (9), which showed a mean score of 8.9 (SD = 2.3). These findings can be explained by the marked difference in the mean age of the two studies; with this study’s mean age of 46.7 versus 80.2, a higher Charlson Comorbidity Index score is associated with older age.
Even if studies rarely report adverse effects of RBC transfusions, the high rate of deterioration linked to the disease progression observed in patients despite these transfusions still raises questions about their actual effect at this very end of life.[17]
Limitations
Inherent to retrospective studies, the analysed data are tributary to available data in medical charts. A suboptimal report of symptom assessment, taking into consideration a patient’s self-reported assessment, was noted. A limitation of the study is the limited use of the.[11] tool to assess pre- and post-RBC transfusion symptoms, which could have provided more accurate outcomes. This was likely due to patients being too unwell to complete the AQSA, leading to reliance on physician assessments. It is recommended to implement such tools for future use to gather valuable data on RBC effectiveness. A benefit of using validated patient-reporting tools is that they provide direct feedback from the transfusion recipients, reducing potential bias from clinician judgment. Certain demographic or clinical variables, like nutritional status, may not have been included in the study because they were not the primary focus, and including them could have complicated the analysis. In addition, reliable data on nutritional status may not have been consistently available across all participants. Another source of bias is the relatively low proportion of patients with haematological malignancies in the total sample when we know that these patients are among the most frequent users of transfusion.[17] This can be explained by the much lower rate of referrals to PC services and a tendency for continued aggressive care until the endof-life observed in these patients.[18] Consequently, the current study better represents the RBC transfusion practices in patients with advanced solid tumors. Finally, another limitation of this study.
CONCLUSION
This study found limited benefits of RBCs transfusion in terms of symptomatic relief and increased survival time in hospitalised patients with advanced cancer. As illustrated in a systematic review, there is a lack of high-quality studies supporting the benefit of RBC transfusions in a PC context. In addition, a lack of validated pre- and post-transfusion assessment tools does not enable the recommendation of the practice at this stage. An optimal outcome assessment would ideally incorporate self-reported measures of specific symptoms and overall quality of life, including functional status. RBCs transfusions should not be indiscriminately offered to patients with anaemia, but rather given to those predicted to have the most significant benefit. This advice is consistent with recommendations from international guidelines.
Ethical approval
The research/study was approved by the Institutional Review Board at King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia, number 2231429, dated 25th February 2025.
Declaration of patient consent
Due to the minimal risks involved, absence of identifying information, and retrospective nature of the study, a waiver for informed consent was granted by the IRB.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- The Prospective Evaluation of the Net Effect of Red Blood Cell Transfusions in Routine Provision of Palliative Care. J Palliat Med. 2017;20:1152-7.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative Care. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/palliative-care [Last accessed 2024 Oct 10]
- [Google Scholar]
- Biological and Immunological Aspects of Iron Deficiency Anemia in Cancer Development: A Narrative Review. Nutr Cancer. 2018;70:546-56.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-Related Anemia: An Integrated Multitarget Approach and Lifestyle Interventions. Nutrients. 2021;13:482.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Transfusions in Palliative Care: A Method to Improve Quality of Life or Double-Edged Sword? A Mini-Review. Palliat Med Pract. 2023;17:245-7.
- [CrossRef] [Google Scholar]
- Transfusion as a Palliative Strategy. Curr Oncol Rep. 2019;21:92.
- [CrossRef] [PubMed] [Google Scholar]
- Can We Detect Transfusion Benefits in Palliative Care Patients? J Palliat Med. 2016;19:1110-3.
- [CrossRef] [PubMed] [Google Scholar]
- Blood Transfusion Practice in the UK and Ireland: A Survey of Palliative Care Physicians. BMJ Support Palliat Care. 2019;9:474-7.
- [CrossRef] [PubMed] [Google Scholar]
- Transfusion Practices in Patients with Advanced Cancer: A Retrospective Study in a Palliative Care Service. Porto Biomed J. 2022;7:e195.
- [CrossRef] [PubMed] [Google Scholar]
- A Retrospective Chart Review of Transfusion Practices in the Palliative Care Unit Setting. Am J Hosp Palliat Med. 2019;36:185-90.
- [CrossRef] [PubMed] [Google Scholar]
- Physicians' Opinions on Anticoagulant Therapy in Patients with a Limited Life Expectancy. Semin Thromb Hemost. 2021;47:735-44.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with Antithrombotics in the Last Year of Life-Incidence of Bleeding and Side Effects After Deprescribing. J Palliat Med. 2024;27:1310-7.
- [CrossRef] [PubMed] [Google Scholar]
- Use of blood Transfusion at the End of Life: Does it have any Effects on Survival of Cancer Patients? Asian Pac J Cancer Prev. 2014;15:4251-4.
- [CrossRef] [PubMed] [Google Scholar]
- Red Blood Cell Transfusion and Associated Outcomes in Patients Referred for Palliative Care: A Retrospective Cohort Study. Transfusion. 2021;61:2317-26.
- [CrossRef] [PubMed] [Google Scholar]
- Red Blood Cell Transfusion in Palliative Care: A Survey of Palliative Care Physicians. J Palliat Med. 2019;22:1139-42.
- [CrossRef] [PubMed] [Google Scholar]
- Transfusion in Palliative Cancer Patients: A Review of the Literature. J Palliat Med. 2014;17:88-104.
- [CrossRef] [PubMed] [Google Scholar]
- Transfusion Dependence, Use of Hospice Services, and Quality of End-of-life Care in Leukemia. Blood. 2018;132:717-26.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative and Endof-Life Care for Patients With Hematologic Malignancies. J Clin Oncol. 2020;38:944-53.
- [CrossRef] [PubMed] [Google Scholar]







