Translate this page into:
Assessment of Cancer-related Fatigue among Cancer Patients Receiving Various Therapies: A Cross-sectional Observational Study
Address for correspondence: Dr. Harminder Singh, Department of Pharmacology, Baba Farid University of Health Sciences, G.G.S. Medical College, Faridkot - 151 203, Punjab, India. E-mail: dr_harminderchahal@rediffmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The objective of this cross-sectional, noninterventional 3-month observational study was to analyze the prevalence of the cancer-related fatigue (CRF) in cancer patient populations with correlation of CRF with different treatment modalities.
Materials and Methods:
A descriptive study was carried out jointly by the pharmacology and oncology departments of a tertiary care center in the Malwa region of Punjab. The data collection was performed by administering the validated Brief Fatigue Inventory (BFI) after obtaining the informed consent.
Results:
One hundred and twenty-six cancer patients were recruited with the mean age of 49.13 years ± 14.35 (standard deviation). There are statistical correlations found between fatigue and chemotherapy agents such as vinblastine, dacarbazine, and cyclophosphamide.
Conclusion:
We observed that CRF is a symptom that is experienced by majority of cancer patients, irrespective of the diagnosis, or type of treatment received. In addition, assessing CRF before and after treatment will facilitate health-care practitioner to treat this symptom.
Keywords
Brief Fatigue Inventory
cancer-related fatigue
chemotherapy
INTRODUCTION
Constant fatigue is documented as one of the most frequent, ongoing symptom description by the patients following cancer treatment. Cancer-related fatigue (CRF) is a distressing, continual, and the personal sense of physical, emotional, and/or cognitive fatigue or tiredness related to cancer or cancer therapy that is hindered with normal performance.[1] Fatigue linked with cancer or its treatment is dissimilar from the characteristic fatigue that most people experience as a result of usual daily life. Distinct to classic fatigue in normal persons, CRF is inconsistent with physical exertion level and is not releived by rest or sleep.[2]
As CRF is a subjective sense of distressing, persistent emotional, and/or a cognitive tiredness; majority of cancer patients will acknowledge some level of fatigue during their treatment and about one-third will have constant fatigue several years posttreatment. Conversely, before presuming that fatigue is related to cancer, treatable causes of this symptom should to be ruled out, such as anemia, thyroid dysfunction, pain, depression, and lack of sleep.[3]
Since this is a known fact that a large proportion of cancer patients experience cancer treatment-related fatigue that includes physical and mental symptoms and it is a one of the most recurrently reported symptoms among cancer patients.[45] The prevalence of CRF may vary with an estimate of 60%–96% in cancer patients who are undergoing treatment experiencing fatigue.[67]
The consequence of CRF on a patient's capability to function is significant; therefore, this symptom is very hurtful as reported by patients. CRF can carry on for months or even years after the termination of cancer treatment. As the life expectancy of people with cancer increases with the evolution of new drugs and new therapeutic modalities’ at the same time the burden associated with CRF also grows many folds.[89] CRF also has an effect on cancer treatment as it may compromise the timing or finishing point of treatment regimens either because fatigue is a dose-limiting adverse effect or because it reduces the patient's willingness to adhere to treatment.[9]
The mechanisms accountable for this situation are poorly understood. Some pathophysiologic theories have been projected for CRF such as disrupted circadian rhythms, chronic stress response mediated through the hypothalamic-pituitary axis, systemic inflammatory response, and pro-inflammatory cytokines.[10] The role of inflammatory cytokines may be based on several lines of evidence such as nononcologic patients with chronic fatigue syndrome have increased levels of pro-inflammatory cytokines such as IL1 beta, IL 1 receptor antagonist, and tumor necrosis factor-alpha (TNF-α),[11] and fatigue is a major side effect of cancer patients receiving interleukins, TNF-α, and interferon.[12] Several studies have revealed an association between fatigue and different types of oncological therapy. It is known that fatigue is the common side effect of chemotherapy and radiotherapy. it is reported that 65%–100% of patients undergoing radiotherapy,[131415] and up to 82%–96% of those receiving chemotherapy suffer from fatigue during their treatment and nearly all patients experience CRF those receiving the biologic modifiers’ interferon or interleukin.[161718]
Only in recent times, as the clinical picture of fatigue has been integrated into the field of oncology, regardless of its high occurrence and latent negative effect on patients’ activities and emotional comfort, research in CRF is still underdeveloped and there are scarcely very few studies reporting CRF among the Indian population. Without a doubt, its estimation is still often not included among the parameters usually used to demonstrate the toxicity of chemotherapy.
Different methods of evaluating and measuring the CRF have been projected or launched and the Brief Fatigue Inventory (BFI) is one of the methods developed to study CRF.[19] This instrument assesses fatigue over 24 h using a scale from 1 to 10 (1 indicates the absence of and 10 the worst imaginable fatigue).[19] Studies have shown that values of 7 or over are robustly connected with a clinically applicable level of CRF.
Aims and objectives
With the above-said concern, we have designed a study with following objective:
-
The estimation prevalence of CRF in cancer patient populations
-
To study the correlation CRF with different treatment modalities, i.e., chemotherapy, radiation, biologic modifiers, or combination of any
-
Correlation of CRF severity with personal and demographic parameters
-
Mapping of predictors of CRF and determination of various factors that are barrier to pain management in cancer patients.
MATERIALS AND METHODS
This is a cross-sectional, noninterventional, study that has been carried out in hospitalized and outpatient department cancer patients from the oncology department at Guru Gobind Singh Medical College, Faridkot. We recruited 126 patients with advance cancers over 3-month period.
Inclusion criteria
-
Diagnosed with cancer and receiving, i.e., chemotherapy, radiation, or a combination of both
-
No history of other chronic disease such as diabetes or heart disease
-
No known history of psychiatric illness or being treated with psychotropic drugs.
A questionnaire containing demographics, disease description/stage/extent of treatment (chemotherapy/radiotherapy/surgery or any combination, etc.) were completed from the patient interview and case records. The BFI scale questionnaire for estimation of CRF was provided to cancer patients in their respective language (Punjabi/Hindi). Ethics approval was obtained from the Institutional Ethics Committee.
Data collection
Data collection was done in two parts, part one contained demographic information such as age, gender, education, occupation, marital status, monthly income, and cancer specific information such as type of cancer, stage of cancer, treatment history, duration of cancer, remission, and failure. The second part was a structured questionnaire taken from validated BFI scale questionnaire. The IBM® SPSS® Statistics 19 student version (IBM Corp., Armonk, NY) software used for data compilation.
Brief Fatigue Inventory
The BFI is a screening tool which measures the severity of fatigue over the previous 24 h. The BFI has only nine items, with the items measured at 0–10 numeric rating scales. Three items ask patients to rate the severity of their fatigue at its “worst,” “usual,” and “now” during normal waking hours, with 0 being “no fatigue” and 10 being “fatigue as bad as you can imagine.” Six items assess the amount that fatigue has interfered with different aspects of the patient's life during the past 24 h. The interference items include general activity, mood, walking ability, normal work (includes both work outside the home and housework), relations with other people, and enjoyment of life. The interference items are measured on a 0–10 scale, with 0 being “does not interfere” and 10 being “completely interferes”. Fatigue was categorized using the BFI as either severe (score 7–10) or no severe (score 0–6), with the latter further subcategorized into moderate (score 4–6) and mild (score 0–3).
Statistical analysis
Baseline distinctiveness (demographic, cancer-specific parameter, and BFI scoring) was summarized by descriptive statistics. Frequency, mean, percentages were calculated wherever appropriate. BFI scoring and its relation to different parameter and demographic characteristics were compared with the Chi-square statistical test. All P ≤ 0.05 was considered as statistically significant.
RESULTS
A total of 126 patients diagnosed with cancer, including 46 males and 80 females, which consist of 36.5% and 63.5%, respectively, who participated in this observation study. 83.3% of patients experienced fatigue according to BFI scale. The mean age was 49.13 years with the leading age group (57.1%), being 40–60 years. Among 126 patients, about 93.7% shown a negative family history of any tumor or malignancy. The most prevalent malignancy was genitourinary cancer 36 (28.6%), followed by breast cancer 27 (21.4%) and head and neck cancer 23 (18.3%) as shown in Table 1.
The Figure 1, described the CRF, which interferes with the daily routine activities of the patient. Among them, normal activities affected worst and relation with others was least hindered. There is statistical significance fatigue relation with anemic patients shown in Table 1. Fatigue percentages with chemotherapy and radiotherapy shown in Figure 2.
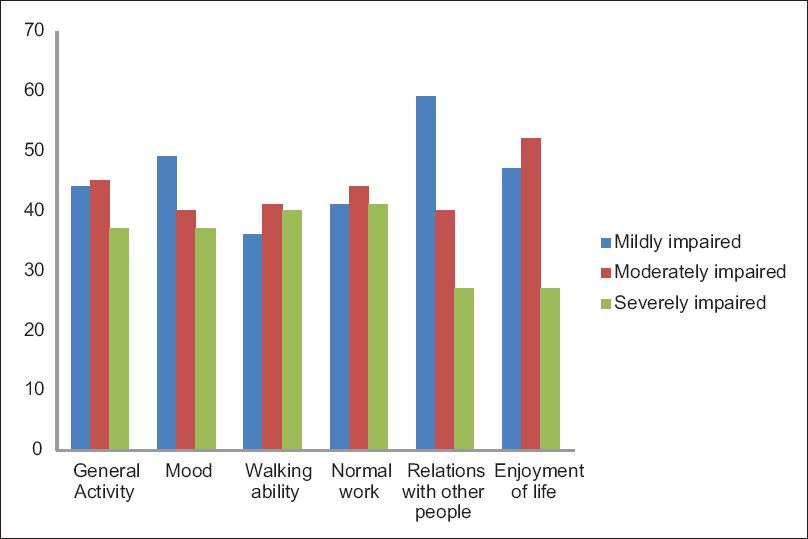
- Fatigue interferes with normal activities of cancer patients.
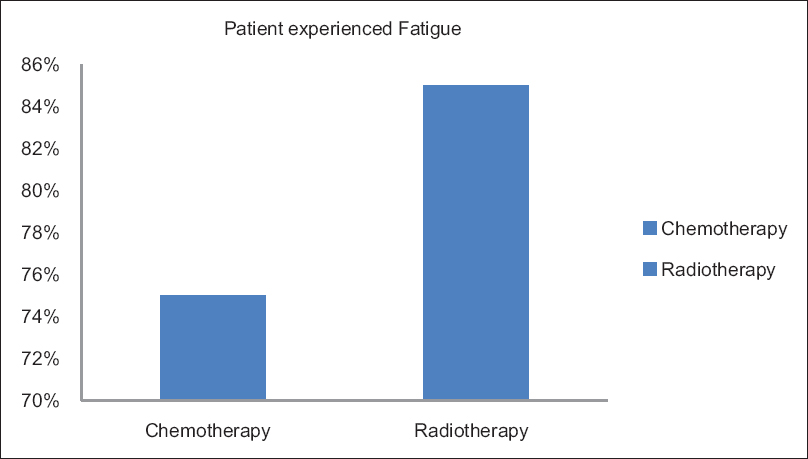
- Prevalence of fatigue in chemotherapy and radiotherapy.
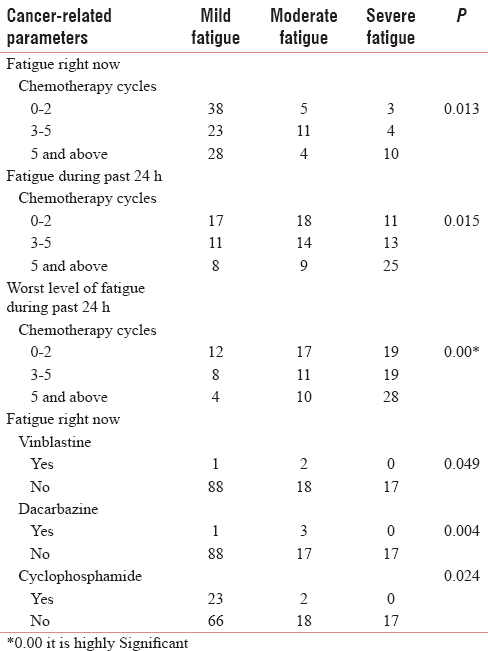
There is a statistical correlation between fatigue and chemotherapy cycle and fatigue with specific drugs such as vinblastine, dacarbazine, and cyclophosphamide described in Table 2.
DISCUSSION
CRF is the most widespread observable fact, in individuals with cancer who receive radiation therapy, cytotoxic chemotherapy, or biological response modifiers.[4] It is a multifactorial, multidimensional phenomenon, which consists of physical, psychological, social, cognitive, and behavioral characteristic.[20]
Fatigue is more common during chemotherapy, usually persist for more than 2 weeks; furthermore, it has been shown to have the maximum and most long-lasting impact after chemotherapy.[21] Almost every patient go through some kind of fatigue during cancer treatment and in the scientific literature, it is about 99% reported during the treatment course.[5] In spite of mounting confirmation, concerning the fatigue occurring due to a range of anticancer treatments and how CRF influence patient's quality of life, determining its severity is undervalued among the cancer patients suffering from this stressful symptom. The current study focuses on measuring the prevalence rate of fatigue (severity of fatigue) among the cancer patients receiving the various anticancer treatments.
The findings of this study revealed that as many as 80% of the participants experienced fatigue during their course of treatment, irrespective of the diagnosis. These scores appear to be in line with the prevalence rates of fatigue cited in other literature studies, i.e., 30%–90% during the course of treatment.[22] Females have more fatigue scores than males. Anemic patients noticed higher fatigue scores than nonanemic patients. Patients who received vinblastine, dacarbazine, and cyclophosphamide-based chemotherapy had statistically higher scores of fatigue. A study by Donovan et al. provides the first evidence that among women with early stage breast cancer, chemotherapy is related to more severe fatigue than radiotherapy, which is similar to our results.[23]
No significant correlation was found between the tumor site and amount of fatigue both during and after treatment in the current study. The results were contrary to the findings by Hickok et al., who studied the pattern of fatigue longitudinally in a sample consisting of breast, prostate, lymphoma and ovarian cancer, and prostate cancer patients experienced more fatigue.[24]
This study had certain other limitations in terms of sample size as it was relatively small and unequal between the groups. Nevertheless, the fact we did not examine potentially correctable etiologies for patients’ fatigue is a limitation of this study.
CONCLUSION
We observed that CRF is a symptom that is experienced by majority of cancer patients, irrespective of the diagnosis, or type of treatment received. The study showed high prevalence of fatigue in cancer patients, and in addition, results confirmed by our observation were similar to those cited in literature. Assessment of CRF should begin once the patient is diagnosed with cancer before patient receives anticancer treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840-50.
- [Google Scholar]
- Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640-50.
- [Google Scholar]
- Cancer-related fatigue: Evolving concepts in evaluation and treatment. Cancer. 2003;98:1786-801.
- [Google Scholar]
- The impact of fatigue on patients with cancer: Overview of fatigue 1 and 2. Oncologist. 2000;9:125-7.
- [Google Scholar]
- Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16:1689-96.
- [Google Scholar]
- Fatigue and psychiatric morbidity among Hodgkin's disease survivors. J Pain Symptom Manage. 2000;19:91-9.
- [Google Scholar]
- Symptoms in advanced cancer: Relationship to endogenous cortisol levels. Palliat Med. 2003;17:503-8.
- [Google Scholar]
- Toxicity and efficacy of escalating dosages of recombinant human interleukin-6 after chemotherapy in patients with breast cancer or non-small-cell lung cancer. J Clin Oncol. 1995;13:2585-93.
- [Google Scholar]
- Fatigue. In: Carrieri VK, Lindsey AM, West CM, eds. Pathophysiological Phenomena in Nursing: Human Response to Illness. London, UK: Saunders; 1993. p. :279-302.
- [Google Scholar]
- Correlates of fatigue in people with breast or lung cancer. Oncol Nurs Forum. 1991;18:81-7.
- [Google Scholar]
- Side effects expected and experienced by women receiving chemotherapy for breast cancer. BMJ. 1991;302:272.
- [Google Scholar]
- Factors contributing to emotional distress during cancer chemotherapy. Cancer. 1982;50:1020-7.
- [Google Scholar]
- The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer. 1999;85:1186-96.
- [Google Scholar]
- Determinants of chronic fatigue in disease-free breast cancer patients: A cross-sectional study. Ann Oncol. 2002;13:589-98.
- [Google Scholar]
- Cancer-related fatigue: The scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
- [Google Scholar]
- Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373-80.
- [Google Scholar]
- Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772-8.
- [Google Scholar]






