Translate this page into:
Bleomycin in Hodgkin's Lymphoma – A Boon or a Bane? – A Retrospective Study of Bleomycin Pulmonary Toxicity in Hodgkin's Lymphoma
Address for correspondence: Dr. Karthik S Udupa, Department of Medical Oncology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal - 576 104, Karnataka, India. E-mail: udupa.k@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Hodgkin's lymphoma (HL) is one of the most curable malignancies with cure rates of above 85% across all stages. Bleomycin containing regimen is routinely employed in the treatment of HL. Pulmonary toxicity due to this drug is the most feared side effect in these regimens where the mortality rate is approximately 2%–3%. We have conducted this study to assess the genetic susceptibility among the Indian HL patients to bleomycin pulmonary toxicity (BPT).
Materials and Methods:
In a retrospective study conducted at a tertiary care hospital from South India between January 2013 and May 2019, we reviewed 100 HL patients who were treated with bleomycin-containing regimen (adriamycin, bleomycin, vinblastine, and dacarbazine or cyclophosphamide, vincristine, procarbazine, and prednisone/adriamycin, bleomycin, and vinblastine) for BPT.
Results:
A total of 100 patients with HL who had received bleomycin-containing regimen were analyzed, which included 23 females and 77 males. Twenty-nine patients had BPT and five deaths were attributed to the same. Radiology reports showed that 15 patients had acute BPT and eight patients had chronic changes. Four patients had rare findings of bleomycin-induced lung damage and computed tomography of the chest could not be done for two patients, whose chest X-ray showed features suggestive of BPT.
Conclusion:
The incidence of bleomycin induced pulmonary toxicity and mortality was significantly higher in our study compared to that of other Western studies. This could be probably due to the increased susceptibility of the Indian patients to bleomycin induced lung damage. In a highly curable cancer such as HL, it is unacceptable to have such a high life-threating toxicity. Hence, an alternative chemotherapy regimen without bleomycin is to be explored which would prevent toxicity and hence the compromise on survival.
Keywords
Bleomycin
Hodgkin's lymphoma
pulmonary toxicity
INTRODUCTION
The curative systemic treatment of Hodgkin's lymphoma (HL) has been voted as one of the best five achievements in oncology over the past five decades.[1] The cure rate of HL in the 1970s was around 57%, which has now gone past 85% owing to the multiple well-conducted randomized control trials and improvement in imaging over the last four decades.[2]
Combination chemotherapy with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) regimen is the most commonly used regimen for the treatment of HL. Since the last decade, more intensive escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone regimen has shown better survival results in advanced disease and has become popular among some of the HL study groups.[3] The most feared toxicity of these regimens containing bleomycin is the pulmonary toxicity which approximately manifests in 10%–20% of all patients of HL with 2%–3% mortality rates.[4] This study was conducted to assess the bleomycin pulmonary toxicity (BPT) among the Indian HL patients.
MATERIALS AND METHODS
We conducted a retrospective study at a tertiary care center from South India, from January 2013 to May 2019. Patients diagnosed as HL who were treated with bleomycin containing chemotherapy regimens (ABVD or cyclophosphamide, vincristine, procarbazine, and prednisone/adriamycin, bleomycin, and vinblastine) were analyzed for bleomycin lung toxicity. The Institutional Ethics Committee clearance and patients’ consent were obtained before including them in the study.
All the patients with signs or symptoms of BPT such as dry cough, dyspnea at rest or exertion, chest pain, crackles on examination, or hypoxia were subjected to high-resolution computed tomography (HRCT) chest or chest X-ray whenever HRCT chest was not possible. Images were analyzed by two independent radiologists, and bleomycin lung toxicity was confirmed when both the radiologists were in consensus.
HL patients who were not treated with bleomycin containing regimen and those who were suspected to have BPT but did not undergo imaging were excluded from the analysis.
Patients also underwent blood investigation and staging evaluations. The correlation analysis was done using the Mann–Whitney test to know if any association existed between pulmonary toxicity and parameters such as age, sex, hemoglobin, serum albumin, creatinine, lactic acid dehydrogenase (LDH), cumulative dose of bleomycin, and stage of the disease.
RESULTS
A total of 100 patients with HL were analyzed for BPT which included 23 females and 77 males. The study population also included 10 pediatric patients. The detailed demographic profile of the study population is shown in Table 1.
| Characteristics | Values |
|---|---|
| Adult patients (n) | 90 |
| Pediatric patients (n) | 10 |
| Mean adult age (years) | 41.54±16.66 |
| Mean pediatric age (years) | 8.4±4.03 |
| Male (n) | 77 |
| Female (n) | 23 |
| Stage of the disease (n) | |
| 1 | 10 |
| 2 | 24 |
| 3 | 34 |
| 4 | 32 |
| IPSS stage (n) | |
| 0 | 2 |
| 1 | 25 |
| 2 | 27 |
| 3 | 19 |
| 4 | 18 |
| 5 | 6 |
| 6 | 3 |
| 7 | 0 |
| Mean hemoglobin (g/dL) | 11.6±2.24 |
| Mean LDH (IU/L) | 303±155 |
| Mean albumin (g/dL) | 3.76±0.66 |
| Mean absolute lymphocyte count (n) | 1608±1101 |
| Mean WBC (cells/microliter) | 9.12×103±5.3 |
| Mean creatinine (mg/dl) | 0.80±0.37 |
| Mean cumulative dose of bleomycin in patients with bleomycin pulmonary toxicity (IU/m2) | 68.62±32.30 |
| Patients receiving ABVD chemotherapy | 97 |
| Patients receiving COPP/ABV chemotherapy | 3 |
IPSS: International Prognostic Scoring System, LDH: Lactic acid dehydrogenase, WBC: White blood cell, ABVD: Adriamycin, bleomycin, vinblastine, and dacarbazine, COPP: Cyclophosphamide, vincristine, procarbazine, and prednisone, ABV: Adriamycin, bleomycin, and vinblastine
Of the 100 patients analyzed, 29 had BPT and five deaths were attributed to the same. Fifteen patients showed acute phase of toxicity in the form of nonspecific interstitial pneumonia, acute interstitial pneumonia, ground-glass opacification (GGO), and areas of consolidation with or without interstitial septal thickening [Figures 1 and 2]. Eight patients in chronic phase had usual interstitial pneumonia (UIP) and pulmonary fibrosis pattern and two of them had both diffuse alveolar damage (DAD) and fibrosis [Figure 3]. Another four cases showed rare findings such as few nodules in the lungs, subpleural atelectatic bands, and pleural effusion. BPT was diagnosed in two of the patients on X-ray findings of diffuse areas of nodular and reticular opacities [Figure 4].
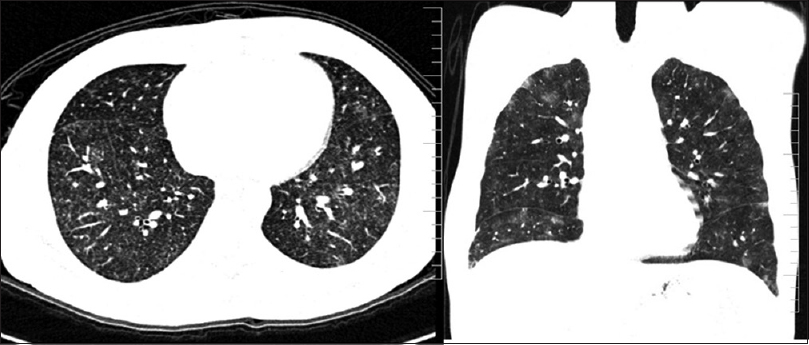
- HRCT chest with bleomycin pulmonary toxicity showing multiple nodular ground glass areas diffusely involving both the lungs representing mild and early changes of lung injury
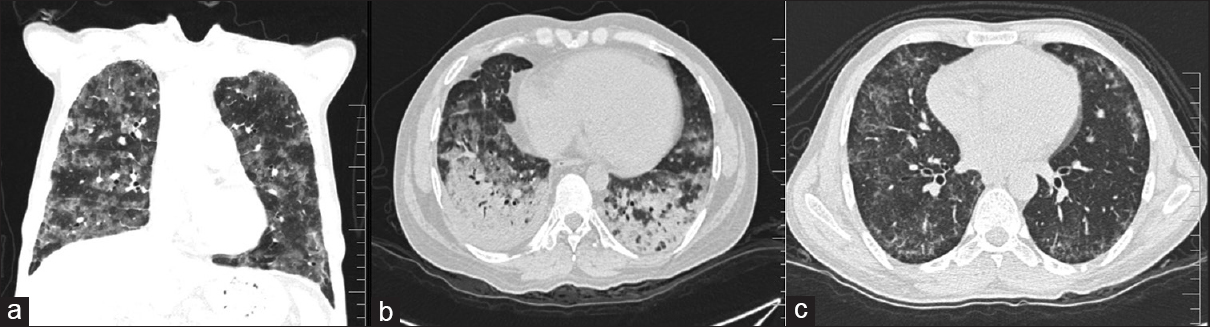
- HRCT showing classical appearance of lung toxicity as extensive diffuse alveolar damage in bilateral lungs seen as a) Diffuse ground glass opacities and b) Ground glass opacity and areas of basal consolidation c) NSIP pattern
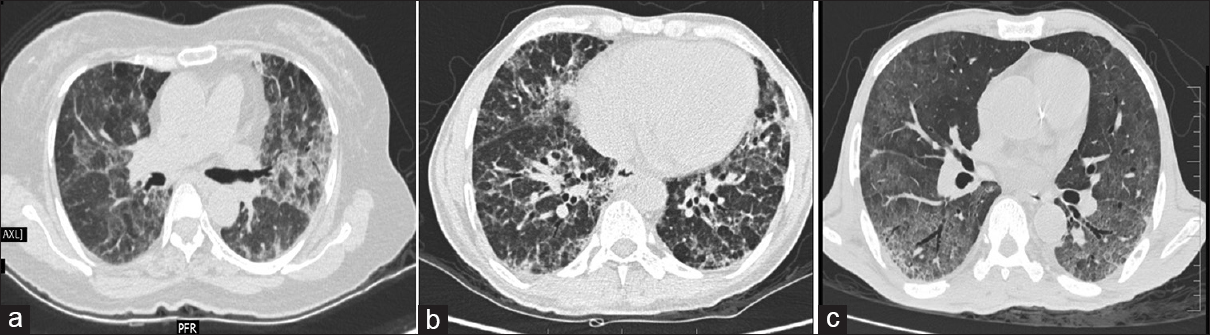
- HRCT changes representing sequel of bleomycin toxicity, a) Fibrotic type of NSIP b) DAD with bronchiectasis and septal thickening c) UIP pattern
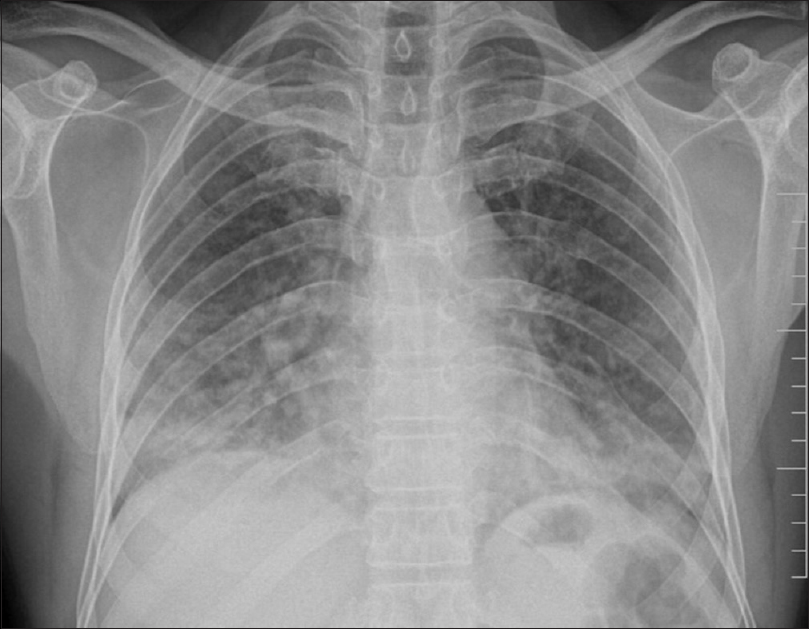
- Chest X-ray showing nodular and patchy opacities predominantly in lower zones
DISCUSSION
Bleomycin is an antitumor antibiotic which was first extracted from the fungus Streptomyces verticillus in 1966.[5] The susceptibility of the lungs and skin to bleomycin toxicity can be explained by the fact that the drug is inactivated by an enzyme bleomycin hydrolase (a cytosolic aminopeptidase) which has its lowest activity in these organs.[6] This drug is predominantly excreted through kidneys, and hence the toxicity is increased in renal failure. Other postulated risk factors for bleomycin toxicity described from various clinical trials include cumulative dose of bleomycin >300 to 500 U, age >40 years, radiotherapy to the chest, exposure to high concentration of oxygen therapy, concomitant administration of growth factors and chemotherapeutic agents such as cisplatin.[7891011]
In our study, there was no statistically significant association of pulmonary toxicity with age, sex, albumin, creatinine, LDH or stage of the disease. The mean cumulative dose for bleomycin toxicity in our study is 68 IU/m2, which is significantly lesser than that quoted in other Western literature.[71213] This could be due to increased susceptibility of the Indian patients toward bleomycin.
BPT will have a varied appearance on HRCT. The most common is DAD which appears as diffuse ground-glass asymmetrical opacities in both the lungs which on progression can form areas of consolidation. Predominant ground-glass opacity involving subpleural region along with septal thickening gives nonspecific interstitial pneumonia pattern. Sometimes the lungs can show small nodules measuring 5 mm to 3 cm along with ground-glass areas. Early or suppurative phase will have alveolar damage. As the disease stage progresses, there will be extensive reticulations, tractional bronchiectasis, development of honeycombing, and areas of fibrosis giving the UIP pattern. Honeycombing, extensive reticulations and fibrosis are the features of chronic bleomycin toxicity and are representative of nonreversible stage.[1415]
In our study, the incidence of BPT and related mortality was much higher (29% and 5%, respectively) and also the cumulative dose of bleomycin to produce pulmonary toxicity was much lesser compared to the Western literature which may be due to the increased susceptibility of the Indian patients to bleomycin. All the patients who manifested features of BPT did not receive the drug in subsequent cycles after their recovery. Seventeen of them recovered spontaneously after bleomycin was withdrawn. Twelve patients who had severe toxicity were treated with oral prednisolone at a dose of 1 mg/kg/day till they recovered completely, and then the dose was tapered and stopped over next 2 weeks. The mean duration of prednisolone treatment was 4.4 weeks. Five patients did not show improvement with steroid treatment, and all five of them died even after mechanical ventilation.
Important drawbacks of our study were that pulmonary function tests and diffusion capacity of the lungs for carbon monoxide (DLCO) were not performed at presentation as well as during treatment. Many centers withdraw bleomycin if the DLCO drops by >25% during treatment, and hence some asymptomatic patients with early bleomycin toxicity could have been missed in our study.[16]
Whether bleomycin can be omitted from the ABVD regimen without compromising the overall survival was answered by HD-13 trial. This trial clearly showed that even in early favorable HL, omitting bleomycin completely resulted in compromised survival.[17] Some of the other strategies that are being tried presently to decrease bleomycin toxicity is to incorporate anti-CD30 monoclonal antibody brentuximab vedotin along with AVD chemotherapy in the front-line setting. One more approach is to discontinue bleomycin after two cycles of ABVD chemotherapy if the interim positron emission tomography shows a complete response, thus decreasing the cumulative dose of bleomycin exposure.[1819]
CONCLUSION AND RECOMMENDATIONS
The increased incidence of bleomycin pulmonary toxicity in our study is probably due to higher genetic susceptibility of Indian patients to bleomycin.
The future challenge will be to conduct larger and well-designed prospective randomized controlled trials among the Indian HL patients which would accurately detect the genetic susceptibility to bleomycin and to incorporate newer strategies to overcome bleomycin toxicity without compromising the survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Long-term survival after treatment for Hodgkin's disease (1973-2002): Improved survival with successive 10-year cohorts. Br J Cancer. 2012;107:531-6.
- [Google Scholar]
- Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348:2386-95.
- [Google Scholar]
- Pulmonary function in long-term survivors of testicular cancer. J Clin Oncol. 2009;27:2779-86.
- [Google Scholar]
- Serum creatinine level during chemotherapy for testicular cancer as a possible predictor of bleomycin-induced pulmonary toxicity. Jpn J Clin Oncol. 1998;28:546-50.
- [Google Scholar]
- Bleomycin associated pulmonary toxicity: Is perioperative oxygen restriction necessary? J Urol. 1998;160:1347-52.
- [Google Scholar]
- Interstitial lung disease in lung cancer: Separating disease progression from treatment effects. Drug Saf. 2005;28:103-13.
- [Google Scholar]
- Pulmonary toxicity in patients with advanced-stage germ cell tumors receiving bleomycin with and without granulocyte colony stimulating factor. Chest. 1997;111:657-60.
- [Google Scholar]
- Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function and symptoms in early-stage Hodgkin's disease. J Clin Oncol. 1996;14:1297-305.
- [Google Scholar]
- Effect of treatment for Hodgkin's disease on pulmonary function: Results of a prospective study. J Clin Oncol. 1994;12:297-305.
- [Google Scholar]
- Clinical, radiologic, and histopathologic studies on pulmonary toxicity induced by treatment with bleomycin (NSC-125066) Cancer Chemother Rep. 1972;56:343-56.
- [Google Scholar]
- Pulmonary function in patients with germ cell cancer treated with bleomycin, etoposide, and cisplatin. J Clin Oncol. 2016;34:1492-9.
- [Google Scholar]
- Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin's lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418-27.
- [Google Scholar]
- Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374:2419-29.
- [Google Scholar]
- Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378:331-44.
- [Google Scholar]






