Translate this page into:
Complementary Role of Intervention Radiology in Palliative Care in Oncology Setting
Address for correspondence: Dr. Ekta Dhamija, Room Number 137, First Floor, Department of Radiodiagnosis, Dr. BR Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi - 110 029, India. E-mail: drektadhamija.aiims@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Owing to advances in treatment of cancer, there has been increase in life expectancy. Palliative care aims at improving quality of life of patients suffering from malignancy and is now recognized as a separate subspecialty. Management of cancer patients needs a multidisciplinary approach, and radiology has a key role to play at every step of it. Interventional radiology has broadened its scope immensely over the last decade with development of newer and less invasive applications useful in oncology and palliative care. The role of interventional radiologists begins from obtaining tissue for histopathological examination and extends to controlling disease spread with ablation or chemoembolization, to managing the tumor-related complications and relieving stressful symptoms such as dyspnea and pain. This article aims to review the interventional radiologist's arsenal in managing patients with malignancies with a special emphasis on palliative care, providing a more holistic approach in improving the quality of life of cancer patients.
Keywords
Fluid drainage
intervention radiology
neurolytic blocks
palliative care
INTRODUCTION
Imaging is an integral and indispensible part of workup of any cancer patient. It encompasses diagnosis, staging, tissue sampling, treatment strategies, and follow-up for response assessment. A component of imaging which extends beyond routine cross-sectional imaging done for staging and response assessment is interventional radiology (IR). The role of latter is crucial and much needed at multiple stages of patient management starting from image guide tissue sampling to executing interventional procedures with curative or palliative intent. Likewise, palliative care is another important front in oncology. It aims at improving quality of life of patients not only physically but also spiritually and emotionally. Both intervention radiology and palliative care work hand-in-hand in more fronts than looked upon commonly.
This article aims at elaborating the scope of IR in oncology and palliative care [Table 1].
| Management of primary malignancy or metastases |
| Percutaneous ablation |
| TACE |
| Management of complications |
| Drainage |
| Obstruction (PCN, PTBD, gastrostomy, cholecystostomy, etc.) |
| Embolization to achieve hemostasis |
| Pain management |
| Miscellaneous: Establishing venous access |
TACE: Transarterial chemoembolization, PCN: Percutaneous nephrostomy, PTBD: Percutaneous transhepatic biliary drainage
MANAGEMENT OF MALIGNANCY OR METASTASES
Percutaneous ablation
IR-mediated tumor ablation causes tumor necrosis by deposition of energy in the tissue by means of radiofrequency ablation (RFA), microwave, ultrasound, laser-induced thermotherapy, cryotherapy, and irreversible electroporation. RFA is considered to be safe, with a mortality rate of 0.3% and a major complication rate of 2.2%.[1] In RFA, electromagnetic energy is administered in a radiofrequency range to the target tissue causing tissue temperature to rise above 60°C with resultant thermal damage and cell death. RFA has gained popularity and is now considered the treatment modality of choice in appropriate liver tumors (hepatocellular carcinoma [HCC]).[2] Few authors have suggested RFA as first treatment option in single liver lesions that are 2 cm or smaller in size.[34] RFA has also been successfully used in unresectable and potentially resectable liver metastases from colorectal cancer[56] [Figure 1]. In palliative care, RFA is used for treatment of painful bone metastases refractory to palliative radiotherapy.[7]
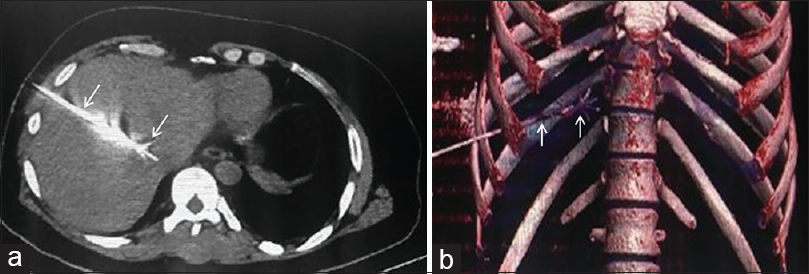
- Radiofrequency ablation of metastatic lesion in liver from colorectal cancer: (a) Axial CT and volume rendered coronal reformatted image (b) showing the course of radiofrequency ablation probe and its expanded prongs in situ
Transarterial chemoembolization
Transarterial chemoembolization (TACE) is used in the treatment of HCCs, where selective hepatic artery cannulation is done and a chemotherapy agent is directly infused in tumor bed followed by an embolic agent that occludes the flow through the catheterized artery. Ischemia produced by the embolic agent makes the tumor cells susceptible to damage by the cytotoxic agents. The advantage of TACE over systemic chemotherapy is that delivery of the chemotherapeutic agent is targeted at the lesion allowing a higher local concentration of the agent and lower systemic doses. The liver tolerates TACE procedure due to its dual blood supply. TACE is the standard treatment in Barcelona clinic liver cancer stage B (intermediate HCC) tumors and is performed with palliative intent in larger unresectable tumors. Overall survival rates for the combination therapy (ablation and chemoembolization) are found to be higher.[8]
MANAGEMENT OF COMPLICATIONS
Drainage of fluid
Fluid accumulation in pleural, peritoneal, or pericardial cavity can lead to significant deterioration in patient's general condition. Malignancies are the third most common cause of pleural effusion and the second most common cause of ascites after cirrhosis.[910] These are among the most debilitating complications of malignancy leading to worsening of quality of life. Drainage of these fluid collections hence becomes important whether it is one-time aspiration or placement of percutaneous catheter.[11]
Malignant pleural effusions are significant cause of morbidity in the oncology patient, presenting with dyspnea, cough, and chest pain. Prevention of recurrent pleural effusions can be achieved by chemical pleurodesis. Before pleurodesis, large effusions require drainage to optimize success rates of pleurodesis and to prevent accumulation of therapeutic agents within the pleural space. Compared to pleurodesis, placement of tunneled pleural catheters has been shown to be feasible and shown to have lower morbidity, lower rates of hospital stay,[12] reduced rates of repeat intervention,[13] and reduction in dyspnea in patients with failed pleurodesis[14] [Figure 2].
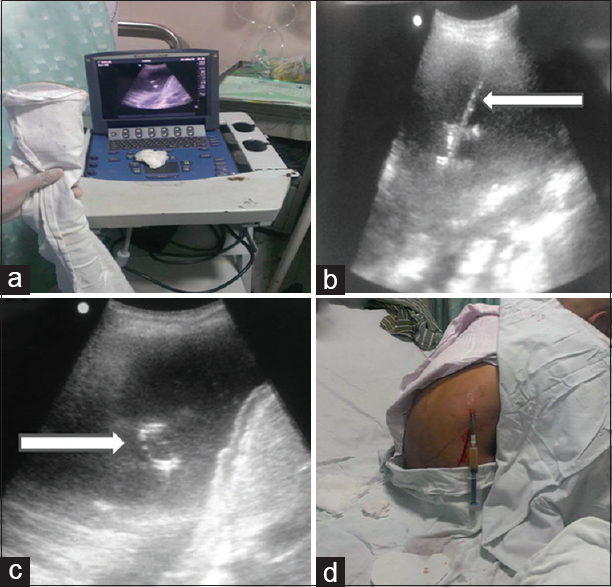
- Drainage of pleural effusion: (a) Ultrasound screen and convex probe wrapped and duly covered using aseptic precautions. (b) Ultrasonography image showing echogenic needle within the pleural fluid (arrow). Subsequently deployed pigtail catheter is seen in situ as it forms loop (arrow in c). The patient sits comfortably after pigtail insertion till the catheter is sutured to skin (d)
Tunneled peritoneal catheters for patients with peritoneal carcinomatosis and refractory ascites have been shown to be safe, cost-effective, and well-tolerated with close to 100% technical success rate.[15] These catheters may also be used to instill chemotherapy without an increase in the rate of infections.[16]
Peritoneovenous shunt (PVS) transfers fluid from the peritoneal cavity into the central venous system through the subclavian or the jugular vein. PVS placement offers several advantages over tunneled catheters. There is no loss of nutrients through drainage of the fluid. Patients may experience a better quality of life as there is no external tube extending out of the patient. It is especially beneficial in chylous ascites following retroperitoneal lymph node dissection, as it may provide complete resolution of ascites.[1718]
Percutaneous interventions in malignant pericardial infusions include pericardiocentesis and catheter drainage, pericardial instillation of sclerosing agents, and placement of a pericardial balloon catheter.[19]
Management of obstructions (hepatobiliary, gastrointestinal, and urinary)
Majority of the patients presenting with malignant biliary obstruction have an underlying carcinoma of the pancreas or gallbladder. Metastatic nodes at hepatic hilar or peripancreatic location may also cause extrinsic compression of proximal part of common bile duct with resultant biliary obstruction. Patients suffer from primary malignancy as well as the severe pruritus caused due to obstruction of the route of biliary drainage. This can be relieved with percutaneous biliary interventions which are preferred in high biliary obstruction whereas endoscopic techniques are preferred in lower biliary duct obstructions. Percutaneous techniques may be indicated in low biliary obstruction when the patient has undergone pancreaticoduodenectomy and in whom endoscopic interventions may be technically challenging.[20] Percutaneous transhepatic biliary drainage involves selective cannulation of the biliary tree with catheter manipulation followed by placement of a catheter or stent to facilitate internal or external drainage of bile and cause slow biliary decompression [Figure 3]. Complications of biliary drainage include cholangitis, hemorrhage, and pericatheter leakage.[21] The likelihood of developing an infection increases with increasing duration of external drain.[22] The ultimate goal of biliary interventions is to provide an internal drainage by stenting, for which self-expandable metallic stents are used.
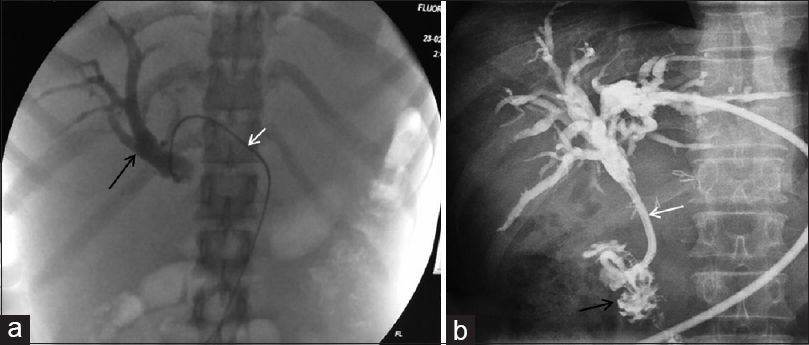
- Percutaneous transhepatic biliary drainage: (a) Fluoroscopic image showing dilated biliary ducts (black arrow) opacified by iodinated contrast injected through the catheter in situ (white arrow). (b) Ring biliary catheter (white arrow) placed with tip in duodenum (black arrow) enabling internal drainage of the bile
Patients with head, neck, or esophageal malignant lesions are frequently unable to tolerate adequate oral intake due to luminal obstruction or swallow impairment and require nutritional support, often by gastrostomy or gastrojejunostomy.[23] Percutaneous image-guided placement of feeding tubes has demonstrated higher technical success rates and is considered safer than endoscopic or surgical placement.[24] Early complications of gastrostomy insertion are infection and mild discomfort on feeding.[25] Tube dislodgement is relatively uncommon; however, if the tract is established for more than 2 weeks, it is frequently possible to access the tract and reinsert the tube without the need for repuncture of the stomach. Nasojejunal tubes can be placed under fluoroscopic guidance and have shown to have an advantage over nasogastric tubes in ill patients.[26]
Decompressive cecostomy can be done under fluoroscopic guidance in cancer patients with large bowel obstruction. It prevents perforation and also provides symptomatic relief.[27] They may also be used as a bridge to more definitive treatment.[28] Colonic stent placement may be done under fluoroscopic guidance alone in patients with unresectable colonic carcinoma or as a bridge to elective surgery with favorable results.[29]
The urinary tract obstruction occurs most commonly at the level of ureters due to pelvic or retroperitoneal malignancies. Image-guided urinary decompression, by means of percutaneous nephrostomy (PCN), is indicated in cases of deteriorating renal function or electrolyte disturbances such as hyperkalemia and metabolic acidosis, pyonephrosis.[30] Image guidance may be provided with fluoroscopy, ultrasound, or often a combination of both modalities.[31] In a study by Wong et al., with malignant ureteral obstruction (bilateral in 68% cases), initial management with PCN achieved successful decompression of the system in 94% of cases.[32] Degree of dilatation of collecting system and the patient's body habitus was the main determinant of the rate of successful completion of PCN in oncology.[33]
Embolization to achieve hemostasis
Approximately 10% patients with lung cancer may have massive hemoptysis. Bronchial artery embolization can be beneficial in these patients. Similarly, embolization can be successfully used in ruptured HCCs, retroperitoneal tumors such as angiomyolipomas and renal cell carcinomas, and severe hemorrhagic cystitis (cyclophosphamide, RT induced) refractory to conservative management.[3435]
Pain management
Pain is a significant source of cancer-related morbidity, particularly in advanced cancer, in which inadequately controlled pain can have a profound impact on the quality of life. Strong analgesics like opiates with considerable side effect profile remain the mainstay of treatment and are used to manage pain in majority of the cancer patients according to the principles of the World Health Organization analgesic ladder.[363738] Neuropathic pain associated with upper abdominal visceral tumors is frequently poorly responsive to analgesic therapy. Celiac ganglion neurolysis and nerve block can achieve successful palliation of pain in the majority of patients, who are refractory to analgesics with pancreatic, gastric, esophageal, and biliary malignancies.[39] It can be performed using fluoroscopic guidance, ultrasonography (USG), or computed tomography (CT) guidance depending on operator's experience. Where fluoroscopy is based on anatomical landmarks, USG provides real-time visualization of needle and CT confirms the location of needle tip in relation to surrounding structures [Figure 4]. Reported minor complications include transient diarrhea in 73% and orthostatic hypotension in 12%.[40] Similarly, peripheral nerve blocks can be used to provide pain relief in various other malignancies.[4142434445]
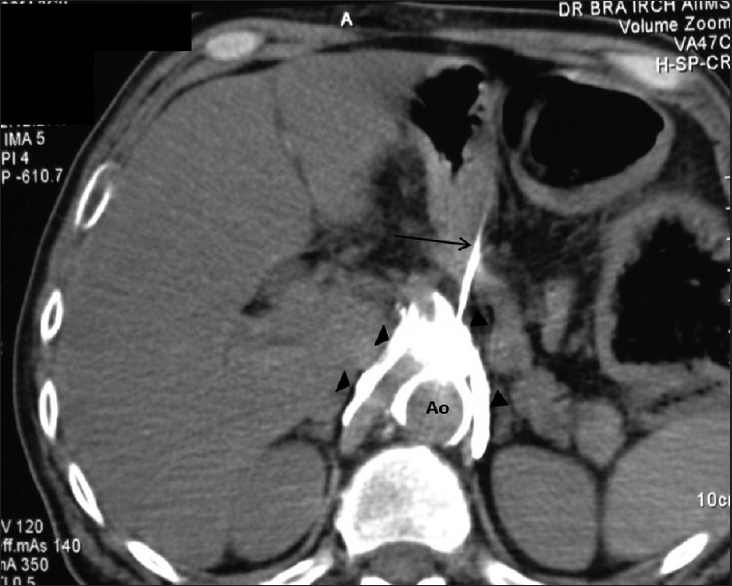
- Computed tomography-guided celiac plexus neurolysis: Needle is placed at the level of celiac axis (arrow) under computed tomography guidance. Uniform spread of contrast-mixed neurolytic agent is seen (arrowheads)
Analgesics, bisphosphonates, chemotherapy, and radiation have been traditionally used to manage metastatic bone pain. Radiotherapy provides partial pain relief in 70% patients while complete pain relief may be seen in up to one-third of patients.[4647] Up to one-third of patients may have refractory pain.[48] According to the latest NCCN guidelines concerning adult cancer pain (v2, 2016), percutaneous ablation for metastatic bone pain may be considered when pharmacologic therapy is inadequate and radiation therapy is contraindicated or not desired by the patient in cases without an oncologic emergency (e.g., pathologic fracture or epidural disease).[49]
Percutaneous cementoplasty provides not only stability but also analgesic effect in painful lytic or mixed bone metastases.[50] It has been reported to provide palliation in extraspinal lytic bone metastases.[51] High-intensity focused ultrasound has been shown to be safe and effective for primary pain palliation and local tumor control.[52]
MISCELLANEOUS
Central venous access and other venous interventions
Cancer patients often require long-term venous access for administration of drugs as well as repeated blood sampling. Permanent venous access is most commonly obtained through the internal jugular vein. “Landmark method” is used to be employed traditionally to locate and puncture the venous sites. Blind or surface marking-guided central venous catheter (CVC) insertions are associated with number of potential complications including pneumothorax, inadvertent injury to arteries, and nerves. USG-guided CVC access has been proven to not only reduce the number of attempts but also reduce arterial puncture and bloodstream infections.[53] There is 4–6 times increased risk of thrombosis in oncology patients as compared with the general population, which is further increased by placement of a CVC.[5455] Peripherally inserted central catheters (PICC) were introduced with the presumed benefit in terms of decreasing these complications. Typically, the cephalic or basilic vein is used as the access site, and the PICC line is advanced into the central veins with its tip lying in the superior vena cava (SVC) or at the cavoatrial junction under US and fluoroscopic guidance [Figure 5].
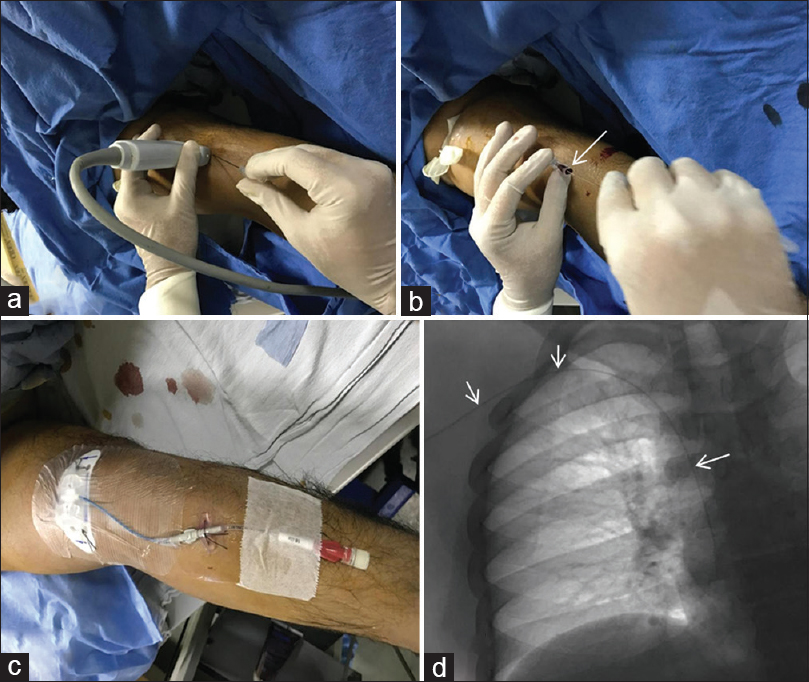
- Peripherally inserted central venous catheter: (a) Venous puncture under ultrasonography guidance is performed followed by establishing access using guidewire (arrow in b). (c) Central venous catheter is placed over the guidewire and position confirmed on radiograph or fluoroscopy spot image (arrows, d)
Malignancy accounts for up to 70% cases of SVC syndrome. Other causes include thrombosis from CVCs and pacemaker leads.[5657] Although no scientific evidence exists, endovascular treatment has been proposed as a first-line treatment for malignant SVC obstruction.[5859] Similarly, obstruction of inferior vena cava (IVC) may result in pedal edema, venous ulcers, renal vein thrombosis, and/or renal insufficiency (when IVC obstruction is at or above the level of renal veins) and Budd–Chiari syndrome (when IVC obstruction is at the level of hepatic veins).[6061] IVC occlusion can be treated successfully using endovascular techniques, and a study by Razavi et al. has reported to have a primary patency rate of 80% at 19 months.[606263] The generally accepted indications of IVC filter placement include failure, contraindication, and complications of anticoagulation.[64] Cancer patients are at an increased risk of venous thrombosis as well as bleeding when on anticoagulation.[65] Placement of IVC filters is an option in these patients for preventing pulmonary embolism.
CONCLUSION
With the expanding application of minimally invasive techniques to the investigation and management of malignancies, the interventional radiologist is assuming a more prominent role in the multidisciplinary team that cares for the patient with cancer. The use of IR techniques in oncology patients should be evidence based to ensure optimal outcome and minimize potential complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology. 2003;226:441-51.
- [Google Scholar]
- Radiofrequency ablation in patients with primary and secondary hepatic malignancies. J Gastrointest Surg. 2006;10:960-73.
- [Google Scholar]
- Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: Long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-92.
- [Google Scholar]
- Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-9.
- [Google Scholar]
- Thermal ablation of liver metastases from colorectal cancer: Radiofrequency, microwave and laser ablation therapies. Radiol Med. 2014;119:451-61.
- [Google Scholar]
- Colorectal cancer with potentially resectable hepatic metastases: Optimizing treatment. Curr Oncol Rep. 2014;16:407.
- [Google Scholar]
- Radiofrequency ablation of osseous metastases for the palliation of pain. Skeletal Radiol. 2008;37:189-94.
- [Google Scholar]
- Radiofrequency ablation combined with transarterial chemoembolization for intermediate hepatocellular carcinoma. Hepatol Res. 2014;44:194-200.
- [Google Scholar]
- Utility and potential of bedside ultrasound in palliative care. Indian J Palliat Care. 2015;21:132-6.
- [Google Scholar]
- A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg. 2013;96:259-63. discussion 263-4
- [Google Scholar]
- Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg. 2012;94:1053-7.
- [Google Scholar]
- Tunneled pleural catheters for treatment of recurrent malignant pleural effusion following failed pleurodesis. J Vasc Interv Radiol. 2010;21:696-700.
- [Google Scholar]
- Repeat large-volume paracentesis versus tunneled peritoneal catheter placement for malignant ascites: A cost-minimization study. AJR Am J Roentgenol. 2015;205:1126-34.
- [Google Scholar]
- Tenckhoff tunneled peritoneal catheter placement in the palliative treatment of malignant ascites: Technical results and overall clinical outcome. Radiol Oncol. 2016;50:197-203.
- [Google Scholar]
- Therapeutic application of percutaneous peritoneovenous (Denver) shunt in treating chylous ascites in cancer patients. J Vasc Interv Radiol. 2016;27:665-73.
- [Google Scholar]
- Symptomatic fluid drainage: Peritoneovenous shunt placement. Semin Intervent Radiol. 2017;34:343-8.
- [Google Scholar]
- Systematic review of percutaneous interventions for malignant pericardial effusion. Heart. 2015;101:1619-26.
- [Google Scholar]
- Palliative percutaneous drainage in malignant biliary obstruction. Part 2: Mechanisms and postprocedure management. J Support Oncol. 2006;4:329-35.
- [Google Scholar]
- Metallic stents in malignant biliary obstruction: Results of a multicenter European study of 240 patients. J Vasc Interv Radiol. 1994;5:279-85.
- [Google Scholar]
- Bacteribilia and cholangitis after percutaneous transhepatic biliary drainage for malignant biliary obstruction. Dig Dis Sci. 1999;44:542-6.
- [Google Scholar]
- Fluoroscopically guided percutaneous gastrostomy with modified gastropexy and a large-bore balloon-retained catheter in patients with head and neck tumors. Acta Radiol. 2004;45:130-5.
- [Google Scholar]
- Radiologic, endoscopic, and surgical gastrostomy: An institutional evaluation and meta-analysis of the literature. Radiology. 1995;197:699-704.
- [Google Scholar]
- Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: A comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005;56:84-90.
- [Google Scholar]
- Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med. 2002;30:586-90.
- [Google Scholar]
- Palliative use of percutaneous endoscopic gastrostomy and percutaneous endoscopic cecostomy tubes. Gastrointest Endosc Clin N Am. 2007;17:795-803.
- [Google Scholar]
- Safety and efficacy of percutaneous cecostomy/colostomy for treatment of large bowel obstruction in adults with cancer. J Vasc Interv Radiol. 2015;26:182-8.
- [Google Scholar]
- Malignant ureteral obstruction: Outcomes after intervention. Have things changed? J Urol. 2007;178:178-83.
- [Google Scholar]
- Redefinitions of indications for percutaneous nephrostomy. Radiology. 1983;147:419-26.
- [Google Scholar]
- Current role of transcatheter arterial embolization for bladder and prostate hemorrhage. Diagn Interv Imaging. 2014;95:1027-34.
- [Google Scholar]
- ABC of palliative care. Principles of palliative care and pain control. BMJ. 1997;315:801-4.
- [Google Scholar]
- Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592-6.
- [Google Scholar]
- A prospective study evaluating the response of patients with unrelieved cancer pain to parenteral opioids. Cancer. 2002;94:3049-56.
- [Google Scholar]
- Celiac plexus block: A palliative tool underused by radiologists. AJR Am J Roentgenol. 2002;179:633-6.
- [Google Scholar]
- Long-term results of celiac ganglia block: Correlation of grade of tumoral invasion and pain relief. AJR Am J Roentgenol. 2004;182:891-6.
- [Google Scholar]
- Efficacy of the anterior ultrasound-guided superior hypogastric plexus neurolysis in pelvic cancer pain in advanced gynecological cancer patients. Pain Med. 2013;14:837-42.
- [Google Scholar]
- Superior hypogastric plexus combined with ganglion impar neurolytic blocks for pelvic and/or perineal cancer pain relief. Pain Physician. 2015;18:E49-56.
- [Google Scholar]
- Continuous tunnelled femoral nerve block for palliative care of a patient with metastatic osteosarcoma. Anaesth Intensive Care. 2010;38:563-5.
- [Google Scholar]
- Continuous brachial plexus block as treatment for the Pancoast syndrome. Clin J Pain. 2000;16:327-33.
- [Google Scholar]
- General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-64.
- [Google Scholar]
- A review of recently published radiotherapy treatment guidelines for bone metastases: Contrasts or convergence? J Bone Oncol. 2012;1:18-23.
- [Google Scholar]
- Interventional options for the management of refractory cancer pain – What is the evidence? Support Care Cancer. 2016;24:1429-38.
- [Google Scholar]
- National CCN. Adult Cancer Pain. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Percutaneous cementoplasty for the treatment of extraspinal painful bone lesion, a prospective study. Diagn Interv Imaging. 2012;93:859-70.
- [Google Scholar]
- Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol. 2013;48:351-8.
- [Google Scholar]
- Ultrasound-guided central venous access: What's new? Intensive Care Med. 2015;41:705-7.
- [Google Scholar]
- Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809-15.
- [Google Scholar]
- Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715-22.
- [Google Scholar]
- Superior vena cava rupture and cardiac tamponade complicating the endovascular treatment of malignant superior vena cava syndrome: A case report and literature review. Semin Intervent Radiol. 2015;32:439-44.
- [Google Scholar]
- The superior vena cava syndrome: Clinical characteristics and evolving etiology. Medicine (Baltimore). 2006;85:37-42.
- [Google Scholar]
- Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2009;193:549-58.
- [Google Scholar]
- Fast-track management of malignant superior vena cava syndrome. Cardiovasc Intervent Radiol. 2004;27:470-3.
- [Google Scholar]
- Results of treatment of inferior vena cava syndrome with expandable metallic stents. Arch Surg. 1998;133:935-8.
- [Google Scholar]
- Redefining Budd-Chiari syndrome: A systematic review. World J Hepatol. 2016;8:691-702.
- [Google Scholar]
- Chronically occluded inferior venae cavae: Endovascular treatment. Radiology. 2000;214:133-8.
- [Google Scholar]
- Clinical outcome after intrahepatic venous stent placement for malignant inferior vena cava syndrome. Cardiovasc Intervent Radiol. 2004;27:129-36.
- [Google Scholar]
- Guidelines for the use of retrievable and convertible vena cava filters: Report from the society of interventional radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17:449-59.
- [Google Scholar]
- Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484-8.
- [Google Scholar]






