Translate this page into:
Do Cognitively Impaired Elderly Patients with Cancer Respond Differently on Self-reported Symptom Scores? A 5-Year Retrospective Analysis
-
Received: ,
Accepted: ,
How to cite this article: Miu DKY, Lam KY, Chan CO. Do cognitively impaired elderly patients with cancer respond differently on self-reported symptom scores? A 5-year retrospective analysis. Indian J Palliat Care 2022;28:167-73.
Abstract
Objectives:
An increasing number of elderly subjects with cancer were admitted to the palliative care unit and they have suffered both distressing symptoms and cognitive impairment. We aim to identify the prevalence of cognitive impairment among elderly cancer patients receiving in-patient palliative care and to examine any difference between patients with cognitive impairment on self-reported symptoms.
Materials and Methods:
Subjects’ age ≥65 admitted to a palliative care unit from 01 September 2015 to 31 August 2020 was included in the study. Exclusion criteria were those with an impaired conscious state, severe cognitive impairment, or language problems that were non-communicable. Variables collected included baseline demographics, cancer diagnosis, cancer stage, mobility state using the modified Barthel index (mBI), and performance status as measured by the palliative performance scale. Cognitive impairment was defined by abbreviated mental test ≤6. Self-reported symptoms scales were measured by the Chinese version of MD Anderson Symptom Inventory and EORTC QLQ C-30 (European Organisation for Research and Treatment of Cancer, Quality of Life Core Questionnaire 30).
Results:
Nine hundred and ninety-one subjects with 1174 admissions were retrieved. Eight hundred and seventy-three admission episodes were included in this study. Three hundred and eight (35%) have cognitive impairment. Cognitively impaired subjects were older, showed worse physical function and performance status, and more often residing in old age homes. Independent predictors of cognitive impairment were age (OR 1.09), mBI (OR 0.96), chair/bed bound state (OR 1.79), and presence of brain metastasis (OR 2.63). They reported lower scores in pain (P < 0.001), distress (P < 0.001), sleep disturbance (P < 0.001) and nausea and vomiting (P = 0.012) in the self-reported symptoms scale.
Conclusion:
Elderly cancer patients with cognitive impairment were older with poorer performance status. They have reported a lower level of pain, distress, and sleep disturbance. Clinicians should be alerted to this phenomenon to tackle the unmet concomitant symptoms.
Keywords
Cognitive impairment
Elderly
Palliative care
Self-reported symptoms
INTRODUCTION
In ageing societies, dementia is increasingly prevalent in cancer patients who were admitted to palliative care. It is estimated that 7–30% of these groups of patients have dementia.[1,2] Cognitive impairment is frequently encountered in palliative care settings. It can affect up to 30% of subjects before chemotherapy and up to 75% during their course of chemotherapy or afterwards.[3,4] The aetiology of cognitive impairment is multifactorial. It can be due to the adverse effect of cancer treatment, especially chemotherapy, pain, medications, or secondary to insomnia, fatigue, or depression.[5-7] Furthermore, impaired cognition can be caused by delirium, in which the course fluctuates. Delirium was reported in 58–88% of patients in the preceding weeks or hours before death.[8]
One cross-sectional study found that nearly 1/3 of cancer patients had possible or definite cognitive dysfunction. Factors associated with this dysfunction included older age, cancer type, low physical performance, short time interval since diagnosis, and higher opioid dose.[9] In a palliative care setting, symptoms assessment is an essential component for the evaluation and management of terminal cancer patients. Cognitively impaired patients may have difficulties in reporting their symptoms. They are less likely to receive inpatient palliative care.[10] They may have impaired ability to report self-aware symptoms which could lead to worse symptoms management. It has also been shown that working and leisure activities and social interaction could be affected by cognitive impairment which will further reduce their quality of life and even the survival of cancer patients.[11] It is necessary to evaluate the accuracy of self-reported symptoms among these cognitively impaired cancer patients with the aim to reduce over- or under-reporting, thus preventing or minimising mismanagement of this group of patients.
This retrospective and case–control study aimed to identify the prevalence of cognitive impairment among a cohort of elderly patients with advanced cancer admitted for palliative care. The secondary aim is to determine any difference in the self-reported measurement of symptoms and quality of life among those with or without cognitive impairment. We aim that thorough evaluation of these self-reported symptoms scales can help palliative care providers to have proper assessment and documentation of cancer symptoms and optimise treatment.
MATERIALS AND METHODS
Hospital records of all patients admitted to a 16-bed palliative care unit during the period from 01 September 2015 to 31 August 2020 were retrieved. Inclusion criteria were patients’ age ≥65 with incurable cancer, participants who agreed to the palliative care approach, and have a do-not-attempt resuscitation status. Exclusion criteria were inability to participate due to impair conscious state, severe cognitive impairment, or language problems that were unable to respond to the questionnaire. Baseline demographics, body mass index, date of cancer diagnosis, comorbidities including chronic obstructive airway disease, heart disease, diabetes, stroke, renal disease, liver disease, dementia, and mild cognitive impairment were recorded. Place of residence was noted. Cancer stage, presence of metastasis, and use of medications including steroid, sedative, anxiolytic, antidepressant, opioids, and other analgesics were collected. Premorbid functional status was measured using the modified Barthel index (mBI). This assessment tool was used to assess patient performance with respect to self-care, sphincter management, transfer, and locomotion. It measures functional disability with higher scores indicating independence in physical functioning. Mobility status was categorised into independent, use of walking aids, and chair/bed bound. The date of death was recorded. Hospital length of stay and in-patient mortality were calculated. The palliative performance scale (PPS) was used for the evaluation of performance status. It is a validated and reliable tool to assess functional performance and to determine progression toward the end of life. It ranges from 0 to 100% where a higher score represents a better performance level.[12] Cognitive function was assessed by the abbreviated mental test (AMT). A score of ≤6 is considered as having cognitive impairment.[13] Quality of life was assessed by the European Organisation for Research and Treatment of Cancer, Quality of Life Core Questionnaire 30 (EORTC QLQ-C30).[14] It includes five functional scales, three symptoms scales, one global health status/quality of life scale, and six single items including dyspnoea, insomnia, loss of appetite, constipation, diarrhoea, and financial difficulties. A higher score represents a higher response level. The Chinese version of MD Anderson Symptom Inventory (MDASI)was used for the measurement of symptoms severity.[15] It consists of 19 items with 13 symptoms and six interference items on a numeric rating scale of 0–10. A higher score means worse cancer symptoms or interference. All patients were interviewed within 3 days after admission. We focused on inpatient episodes of care and reported the outcomes associated with each admission episode.
This study was approved by the cluster Hospital Research Ethics Committee of the Hong Kong Hospital Authority.
Statistical method
Descriptive statistics for baseline demographic variables were reported using mean or median. Subjects were categorised into normal cognition and impaired cognition group based on AMT scores. Those with AMT ≤6 were classified as a cognitively impaired group. Between cognitive function groups, comparisons on continuous data were tested by t-test or Mann–Whitney U-test while χ2 test was used for categorical data. Logistic regression was set up using those variables that were found to have a significant difference between cognitive function groups in the univariate analysis as independent variables and cognitive impairment as dependent variables to identify predictors of cognitive impairment. P < 0.05 is considered statistically significant. SPSS 24 was used for data analysis.
RESULTS
A total of 1281 patients were admitted during the study period. Nine hundred and ninety-one were aged>65 with 1174 admission episodes which were retrieved. Among them, 301 were excluded due to incomplete data given to an overall sample of 873 admission episodes. [Figure 1] shows the consort flow diagram. The mean age was 78.6 (SD 7.89) and 540 (51%) were male. Three hundred and fifty (40.1%) were ambulatory, 365 (41.8%) walk with aids, and 158 (18.1%) were bed/chair bound. The majority of them (709, 80.2%) were living at home. The mean mBI was 39.53 (SD 24.83) and the median PPS was 50 (IQR 40–50). The median AMT score was 8 (IQR 5–10). The most common cancer types were cancer of the digestive tract, lung cancer, and prostate cancer [Figure 2]. Three hundred and eight (35.3%) were cognitively impaired with AMT ≤6. [Table 1] shows the baseline demographics by cognitive status. Cognitively impaired subjects were older, showed worse physical function and performance status, and more often residing in old age homes. Dementia, hypertension, and stroke were more prevalent in those subjects. The cognitively impaired group was having less prescription of sedatives/hypnotics than the cognitively intact group. There was no statistically significant between-group difference on the cancer type, but it was found that the cognitively impaired group has more prevalence of the central nervous system metastasis and less bone metastasis.
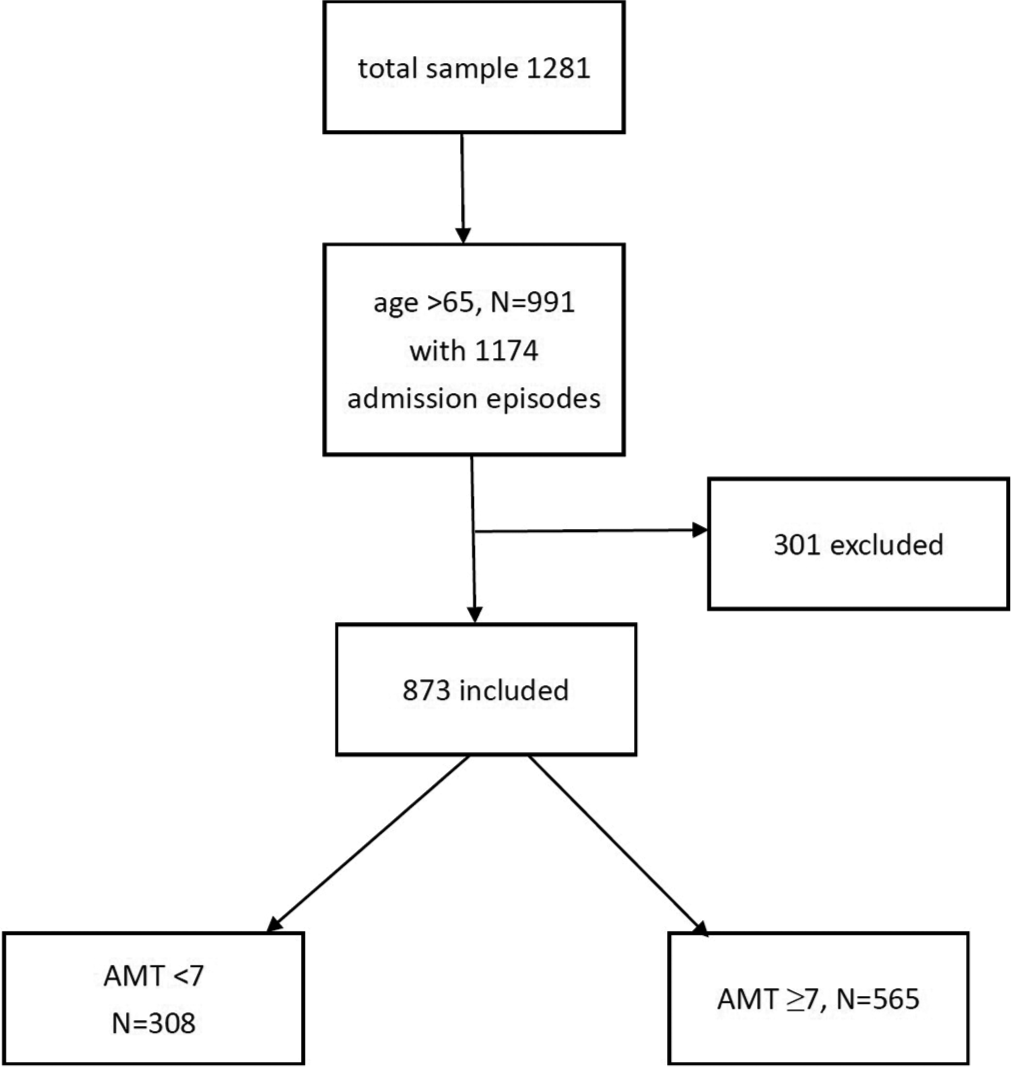
- Consort flow diagram of subjects. AMT: Abbreviated mental test.
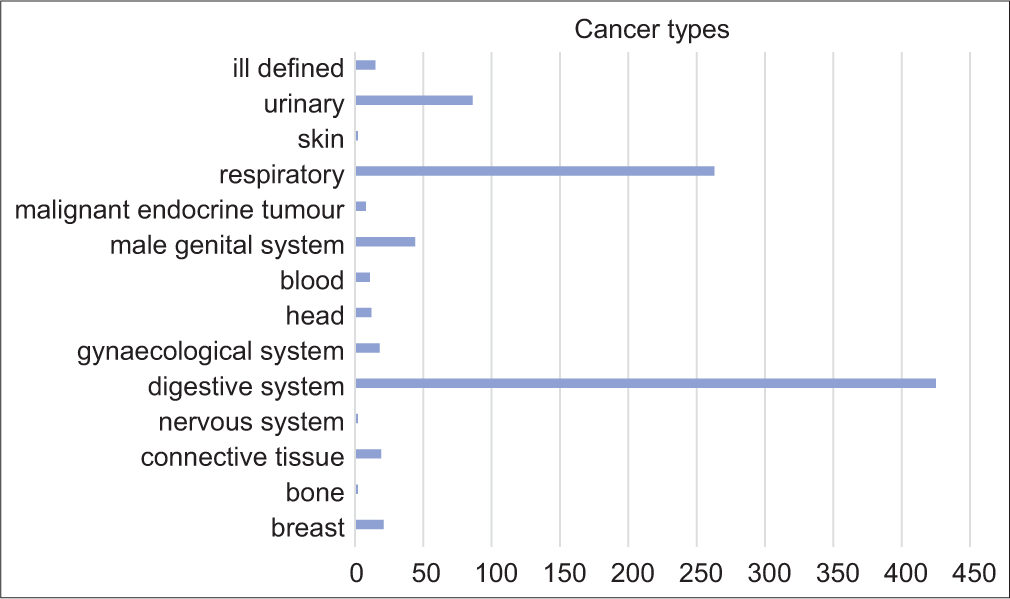
- Cancer diagnosis in all subjects. Each bar represents the number of subjects.
| With cognitive impairment (n = 308) | No cognitive impairment (n = 565) | P-value | |
|---|---|---|---|
| Age (years) | 81.45 (SD 7.3) | 77.07 (SD 7.78) | <0.001 |
| Male sex | 182 (59.1%) | 358 (63.4%) | 0.214 |
| BMI | 19.47 (SD 3.98) | 23.32 (SD 8.45) | 0.43 |
| mBI | 27.11 (SD 19.33) | 46.32 (SD 24.89) | <0.001 |
| PPS (median) | 40 | 50 | <0.001 |
| Length of stay (days) | 24.86 (SD 34.7) | 26.13 (SD 24.53) | 0.528 |
| Mobility | |||
| Independent | 94 (30.5%) | 256 (45.3%) | <0.001 |
| Walk with aids | 130 (42.2%) | 235 (41.6%) | |
| Chair/bed bound | 84 (27.3%) | 74 (13.1%) | |
| Old age home resident | 85 (2736%) | 79 (14%) | <0.001 |
| Time since diagnosis of cancer (months) | 17.18 (SD 24.51) | 16.7 (SD 21.78) | 0.764 |
| In-patient death | 210 (68.18%) | 349 (61.77%) | 0.059 |
| Development of metastasis | 236 (23.49%) | 466 (82.48%) | 0.049 |
| Brain metastasis | 41 (15.6%) | 52 (11.68%) | 0.034 |
| Bone metastasis | 64 (27.47%) | 156 (35.06%) | 0.045 |
| Liver metastasis | 90 (38.63%) | 179 (40.22%) | 0.686 |
| Lung metastasis | 103 (44.21%) | 219 (49.21%) | 0.215 |
| Medications used | |||
| Corticosteroid | 46 (14.94%) | 89 (15.75%) | 0.75 |
| Opioid | 196 (63.64%) | 395 (69.91%) | 0.157 |
| Non-opioid analgesics | 225 (73.05%) | 406 (71.86%) | 0.466 |
| Sedatives/hypnotics | 73 (23.7%) | 198 (35.04%) | 0.001 |
| Antidepressants | 17 (5.51%) | 33 (5.84%) | 0.393 |
| Comorbidity | |||
| Chronic obstructive airway disease | 20 (6.49%) | 43 (7.61%) | 0.542 |
| Heart disease | 49 (15.91%) | 107 (18.94%) | 0.264 |
| DM | 88 (25.97%) | 145 (25.66%) | 0.584 |
| Stroke | 62 (20.13%) | 47 (8.32%) | <0.001 |
| Renal disease | 15 (4.87%) | 18 (3.19%) | 0.212 |
| Liver disease | 6 (1.95%) | 12 (2.12%) | 0.86 |
| Dementia | 40 (12.99%) | 2 (0.35%) | <0.001 |
| HT | 179 (58.12%) | 274 (48.49%) | 0.007 |
BMI: Body mass index, mBI: Modified Barthel index, PPS: Palliative performance status, DM: Diabetes mellitus, HT: Hypertension
Logistic regression found that age (OR 1.09, 95% CI 1.06, 1.12, P < 0.001), mBI (OR 0.96, 95% CI 0.95, 0.97, P < 0.00), bed/chair bound status (OR 1.79, 95% CI 1.07, 3.01, P = 0.027), presence of bone metastasis (OR 0.55, 95% CI 0.36, 0.84, P = 0.005) and brain metastasis (OR 2.63, 95% CI 1.53, 4.5, P < 0.00) were independent predictors of cognitive impairment.
For symptoms severity assessment using MDASI, the cognitive impaired group reported lower scores on pain, nausea, sleeping disturbance, distress, sadness, vomiting, and numbness while the item on difficulty in remember things was more worst [Table 2]. Using EORTC QLQ-C30 for quality of life assessment, the cognitive impaired group reported lower physical function, cognitive function, pain, insomnia, appetite loss, and constipation than the cognitively intact group except for emotional function which was reported to be worse [Table 3].
| With cognitive impairment (n = 308) |
No cognitive impairment (n = 565) |
P-value | |
|---|---|---|---|
| Pain | 1.75 (2.34) | 2.33 (2.66) | <0.001 |
| Fatigue | 3.11 (2.34) | 2.97 (2.3) | 0.407 |
| Nausea | 0.57 (1.23) | 0.92 (1.8) | 0.001 |
| Sleep disturbance | 1.56 (1.61) | 2.19 (2) | <0.001 |
| Distress | 0.75 (1.27) | 1.1 (1.16) | <0.001 |
| Shortness of breath | 1.19 (1.82) | 1.45 (2.06) | 0.056 |
| Difficulty remembering | 2.26 (2.11) | 21.14 (1.56) | <0.001 |
| Poor appetite | 2.48 (2.23) | 2.71 (2.11) | 0.165 |
| Drowsiness | 2.13 (2.16) | 1.91 (2.1) | 0.129 |
| Dry mouth | 2.21 (2.15) | 2.36 (2.24) | 0.342 |
| Sadness | 0.77 (1.36) | 1.11 (1.61) | 0.001 |
| Vomiting | 0.37 (1.04) | 0.65 (1.64) | 0.003 |
| Numbness | 0.47 (1.28) | 0.79 (1.64) | 0.001 |
| General activity | 3.8 (1.18) | 4 (2.8) | 0.359 |
| Mood | 1.93 (2.19) | 2.35 (2.64) | 0.017 |
| Work | 2.82 (3.51) | 2.93 (3.21) | 0.66 |
| Relations with other people | 1.41 (2.12) | 1.43 (2.06) | 0.851 |
| Walking | 4.03 (3.66) | 4.15 (3.66) | 0.621 |
| Enjoy rest of life | 3.13 (3.2) | 3.18 (2.88) | 0.833 |
Data presented as mean (SD). MDASI: Chinese version of MD Anderson Symptom Inventory
| With cognitive impairment (n = 308) |
No cognitive impairment (n = 565) |
P-value | |
|---|---|---|---|
| Global health status | 40.23 (16.22) | 41.56 (31.46) | 0.5 |
| Physical function | 20.11 (25.45) | 28.28 (26.66) | <0.001 |
| Role function | 29.19 (33.8) | 30.94 (37.84) | 0.519 |
| Emotional function | 90.29 (17.29) | 87.63 (17.72) | 0.037 |
| Cognitive function | 65.4 (30.82) | 78.58 (22.18) | <0.001 |
| Social function | 59.77 (35.38) | 63.03 (31.84) | 0.19 |
| Fatigue | 51.19 (30.66) | 47.5 (26.25) | 0.082 |
| Nausea | 10.73 (21.16) | 14.9 (25.89) | 0.012 |
| Pain | 32.08 (32.79) | 34.98 (31.55) | 0.209 |
| Dyspnoea | 28.78 (35.57) | 30.93 (34.1) | 0.388 |
| Insomnia | 31.39 (31.33) | 37.84 (28.98) | 0.003 |
| Appetite loss | 44.03 (33.66) | 43.69 (33.01) | 0.887 |
| Constipation | 17.52 (26.54) | 25.43 (62.63) | 0.039 |
| Diarrhoea | 3.99 (15.48) | 9.63 (15.66) | 0.577 |
| Financial difficulties | 34.71 (34.21) | 30.63 (32.86) | 0.091 |
Data presented as mean (SD). EORTC QLQ C-30: European Organisation for Research and Treatment of Cancer, Quality of Life Core Questionnaire 30
DISCUSSION
We have examined the relationship between cognitive impairment and the ability to self-reporting cancer-related symptoms among patients with advanced cancer who were receiving palliative care. We found that 35% of our study population had cognitive impairment. They were much older with poor physical performance as reflected by a lower mBI and more bed/chair bound status. Their diseases were more severe, with lower PPS and presence of metastasis as compared with those without cognitive impairment. Cognitive dysfunction in palliative care is a frequent phenomenon. A large proportion of patients has different phases of cognitive impairment during their disease course, especially in their past days of life.[16] Fluctuations of cognitive function can occur due to the disease course itself, development of delirium as a result of concurrent medical problems, or the effect of medications or interventions that were used for symptoms relief. Many of them were unable to communicate their problems clearly which could have led to misunderstanding and under treatment.[17]
Logistic regression analysis found that old age with poor physical function and the presence of brain metastasis were independent predictors for the presence of cognitive impairment. It has been well known that the factors of old age and poor mobility were associated with poor cognition. One should also be alerted that brain metastasis will affect the neurocognitive function and its progression will lead to further decline in neurocognitive function. Thus, adjustment of cancer symptoms assessment tools may be needed in patients with brain metastasis even though their mental state examination was still normal in the early course of illness.
In this study, we have explored the relationship between cognitive impairment and symptoms reporting including the most commonly occurred symptoms that affect cancer patients. Pain is one of the most disturbing symptoms among patients receiving palliative care. Proper assessment of pain symptoms helps to reduce suffering and improve quality of life. Some studies suggested that the ability to self-report pain is intact among those with mild-to-moderate cognitive impairment.[18] For MDASI, the cognitive impaired group has scored lower on the pain item. Although there were no significant between-group differences using EORTC QLQ-C30 on pain assessment, the pain score was also lower in the cognitive impaired group. The use of a numeric rating scale in MDASI is more sensitive in identifying pain severity while an ordinal scale in EORTC QLQ C-30 is more easily understandable by cognitive dysfunction subjects. A review of pain management for patients who were unable to self-report found that no reliable tools can adequately reflect their pain feeling.[19] Thus, it is suggested that alternative methods should be considered for pain assessment among the cognitively impaired subjects. Facial expression, mood, and social interaction, and verbal behaviour can be used for patients who cannot communicate efficiently or have poor ability to report their pain symptoms.
An interesting finding from our study is that the cognitively impaired group tends to report lower scores in sleep disturbance and distress so that they were prescribed less frequently with sedatives/hypnotics. We cannot identify any reasons for the phenomena of less sleep disturbances and distress reported by the cognitive impairment group with less use of sedatives/hypnotics. It could be the presence of hypoactive delirium which may be overlooked by healthcare workers or non-pharmacological means to deal with distress and agitation work well in our study subjects. Another reason is that since we use self-reported symptoms score, those with cognitive impairment may have less ability to report on the distressing symptoms. This finding is in contrast to a study that cancer patients with poor cognition have more agitation and apathy as a result of pain. This leads to the prescription of sedatives instead of the necessary pain treatment.[20] As a result of this, cancer patients with impaired cognition will be exposed to a greater risk of untreated cancer pain which, in turn, lead to prolonged suffering.[21] It is recommended that healthcare professionals should concentrate more on the relief of cancer-related pain and other symptoms regardless of the patient’s cognitive status.
Cognitively impaired and dementia subjects have lost their ability of thinking and reasoning. They have a poor expression of their discomfort which will make it very difficult for healthcare providers to provide what is required for them toward their end of life.[22] Their declining ability to express themselves their wishes and concerns will thus result in decision making by their family carers. It would be difficult for their carers to determine which type of end-of-life care is the most beneficial for their beloved ones. This, in turn, will lead to a lower quality of death. As in our study, the emotional functioning component in EORTC QLQ C-30 is greater in the cognitive impaired group. It is suggested that they might have more suffering that was unmet during the in-patient management which could lead to poor quality of life and death. It has been suggested by one studythat we should encourage end-of-life discussion among patients and their family which can reduce the anxiety and stress during the bereavement period.[23] The involvement of family carers of cancer patients with dementia plays a central role in decision-making,[24] which includes the place of care and modality of treatment. A cross-sectional study showed that there was a low-to-moderate agreement between carers and dementia patients on the preference of end-of-life treatment.[25] Patients with moderate dementia were able to participate in decision-making through shared decision-making with their carers.[26] Thus, medical professionals should help to develop shared decision-making strategies and provide support to enhance the decision-making of carers of cancer patients with dementia. Unfortunately, in our study, 1/4 of our subjects were residing in old age homes. The median AMT among the cognitively impaired group was only 4. This group of severe cognitively impaired cancer patients with a lack of family carers makes the decision-making process difficult. Medical professionals should be encouraged to focus more on pain management and to decide the best treatment option to reduce suffering in this group of patients.
There is a hypothesis that links the association between cognition and pain although the underlying mechanism that relates cognitive impairment with fatigue, anorexia, and constipation is not clear. It is suggested that pain may activate the anterior cingulate cortex, insular cortex, and periaqueductal grey matter which compete with other attention-demanding stimuli, interfere with the expression and activity of certain neuromodulators that affect several regions of the brain which involved in cognition.[27] Dong has identified clusters of symptoms as predictors for physical functioning, role functioning, and social functioning using the EORTC QLQ C-30 scale in advanced cancer patients.[28] These may also interfere with cognitive function.
Our study has found that subjects with cognitive impairment have lower physical function and performance status.
Association between cognitive function in cancer patients with physical performance status has been reported. A study comparing cancer patients with a high Karnofsky performance scale (KPS), who were able to perform the normal activity and work with a group of lower KPS, who need assistance for personal care, has demonstrated that those who can carry on the normal activity performed better on sustained attention measurement.[29] Furthermore, in a large study that involved more than 2200 patients with cancer, patients with lower KPS (<40) were more likely to have possible or definite cognitive impairment as measured by MMSE.[6]
Studies have reported that cognitively impaired patients have a more prolonged functional decline with a greater level of impairment and disability.[30] This is in accordance with our study that the cognitively impaired group is more dependent and has a lower level of performance on admission. However, the length of stay and in-patient mortality did not show any significant difference with the normal cognition group. A lower PPS score in patients with cognitive impairment did not translate into a shorter length of stay and higher in-patient mortality. This finding suggested that PPS might not be a satisfactory predictor for prognosis in advanced cancer patients with dementia. We should not rely on PPS for triage of subjects with cognitive impairment for palliative care. In the future, the inclusion of KPS may have added further information for better triage of palliative care.
Among the other commonly assessed symptoms in palliative care, the cognitively impaired group has reported a lower level of nausea, sleep disturbance, distress, feeling of sadness, vomiting, and numbness in the MDASI. This is similar to a recent study that patients with dementia were presented with a lower level of distress for most symptoms, but they have higher level of functional impairment and need more assistance with basic activities of daily living.[31] This indicates that dementia patients have less favourable outcomes in physical, psychological, and spiritual problems. These accentuate the problem encountered in detecting and monitoring symptoms in dementia patients whose cognition and communication are deficient.
For the use of drugs in symptoms management, cancer patients with cognitive impairment have less use of opioids than the cognitively intact group. This is similar to a previous study that cancer patients with dementia have received fewer analgesics or opioids for cancer-related pain.[21] This echoed our previous discussion that pain assessment remains a major challenge in palliative care among cognitively impaired patients with poor communication and cognitive ability. There is no statistically significant difference in the use of corticosteroids among the two groups of patients. A study had shown that there was a lower risk of developing cognitive impairment among patients who were not receiving any steroids.[11] Corticosteroids may have an effect on the brain that interferes with mental function. This may affect some specific functions of cognition.[32] However, in our study, there is no relationship between corticosteroid use and cognitive function. We cannot exclude that some patients with cognitive impairment may have been prescribed corticosteroids in the earlier course of their disease trajectory and hence the effect of corticosteroids on cognition cannot be detected.
Several limitations existed in this study. Cognitive assessment was based on AMT during admission into our palliative care unit. A 1-time brief assessment might not be sensitive enough to detect subtle cognitive impairment and made it difficult to stratify our study population in different stages of cognitive impairment. Patients may develop delirium as a result of the disease itself, medical comorbidities, complications developed during hospitalisation, and the treatment given may have contributed to a low AMT score. Moreover, fluctuation in cognitive function is common. All these can lead to misclassification bias.
For symptoms assessment, some studies supported the use of self-rated quality of life scales in cognitively impaired participants.[33] However, this may not apply to those severe cognitively impaired subjects.[34] During the assessment of symptoms, patients with cognitive impairment might not be able to remember their recent symptoms and thus score falsely low or over rate their quality of life. This reflects the deficiency of self-reported measurements in people who are cognitively impaired with the third-person observation assessment.
Three hundred and one admission episodes were excluded due to inability to communicate or failure to respond to the self-reported symptom questionnaire. This group of subjects may be too ill to participate, too confused, or cognitively severely impaired. Further investigation to explore the symptomatology among them is warranted.
This study reflects the difficulty of assessing patients with cognitive impairment who cannot report what they feel. The influence of cognition on their responses might lead to misunderstanding of the questions asked and being unable to provide the most appropriate answer. Both the MDASI and EORTC QLQ C-30 have been well validated in general palliative care, but they have not been well tested specifically in cognitively impaired subjects. They may underestimate the true needs of this special group of patients. Furthermore, proxy reporting by carers may further lead to the underestimation of distress symptoms. Staff-assessed measurements for patient groups that were unable to self-report might be necessary to avoid under-reporting of symptoms.[35]
CONCLUSION
Cognitive impairment is common among elderly cancer patients receiving in-patient palliative care. Patients with cognitive impairment were older with poorer performance status. They have reported a lower level of pain, distress, and sleep disturbance. This special phenomenon observed in this study needs to be addressed. Clinicians involved in palliative care should be alerted to this high prevalence of cognitive impairment with poor performance status and unmet concomitant symptoms.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Neurocognitive deficits in older patients with cancer. J Geriatr Oncol. 2018;9:482-7.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressiveness of end-of-life care for hospitalized individuals with cancer with and without dementia: A nationwide matched-cohort study in France. J Am Geriatr Soc. 2016;64:1851-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairment and pain among nursing home residents with cancer. J Pain Symptom Manage. 2018;55:1509-18.
- [CrossRef] [PubMed] [Google Scholar]
- An update on cancer and chemotherapy related cognitive dysfunction: Current status. Semin Oncol. 2011;38:431-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive effects of chemotherapy and cancer-related treatments in older adults. Am J Geriatr Psychiatry. 2017;25:1415-26.
- [CrossRef] [PubMed] [Google Scholar]
- The panorama of opioid-related cognitive dysfunction in patients with cancer: A critical literature appraisal. Cancer. 2002;94:1836-53.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence, mechanisms and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102-13.
- [CrossRef] [PubMed] [Google Scholar]
- Delirium prevalence, incidence and implications for screening in specialist palliative care inpatients settings: A systematic review. Palliat Med. 2013;27:486-98.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and predictors of cognitive dysfunction in opioid treated cancer patients: A multi-national study. J Clin Oncol. 2011;29:1297-303.
- [CrossRef] [PubMed] [Google Scholar]
- Do cancer patients with dementia receive less aggressive treatment in end-of-life care? A nationwide population-based cohort study. Oncotarget. 2017;8:63596.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of the predictors of cognitive impairment in patients with cancer in palliative care: A prospective longitudinal analysis. Support Care Cancer. 2017;25:941-9.
- [CrossRef] [PubMed] [Google Scholar]
- Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5-11.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the abbreviated mental test (Hong Kong version) in the elderly medical patients. HKMJ. 1995;1:207-11.
- [Google Scholar]
- Quality of life of breast cancer patients in Taiwan: Validation of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23. Psychooncology. 2013;12:729-35.
- [CrossRef] [PubMed] [Google Scholar]
- Chinese version of the MD Anderson symptom inventory. Cancer. 2004;101:1890-901.
- [CrossRef] [PubMed] [Google Scholar]
- Delirium, confusion and agitation at the end of life. J Palliat Med. 1998;1:177-86.
- [CrossRef] [PubMed] [Google Scholar]
- Scales for evaluation of end-of-life care in dementia. Alzheimer Dis Assoc Disord. 2001;15:194-200.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility and reliability of four pain assessment scales and correlation with an observational rating scale in hospitalized elderly dementia patients. J Gerontol A Biol Sci Med Sci. 2005;60:524-9.
- [CrossRef] [PubMed] [Google Scholar]
- Facial expression and pain in the critically ill noncommunicative patient: State of science review. Intensive Crit Care Nurs. 2010;26:343-52.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbid dementia and cancer in residents of nursing homes: Secondary analyses of a cross-sectional study. Cancer Nurs. 2018;41:E13-20.
- [CrossRef] [PubMed] [Google Scholar]
- Pain and hospice care in nursing home residents with dementia and terminal cancer. Geriatr Gerontol Int. 2013;13:1018-25.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of advance care planning on end of life care in elderly patients: Randomized controlled trial. BMJ. 2010;340:c1345.
- [CrossRef] [PubMed] [Google Scholar]
- End of Life: Helping with Comfort and Care. 2016. Available from: https://www.order.nia.nih.gov/sites/default/files/2017-07/end_of_life_508.pdf [Last accessed on 2021 Jul 02]
- [Google Scholar]
- Cancer-related information needs and treatment decision making experiences of people with dementia in England: A multiple perspective qualitative study. BMJ Open. 2018;8:e020250.
- [CrossRef] [PubMed] [Google Scholar]
- Sampson EL. Advance care planning in dementia: Do family carers know the treatment preferences of people with early dementia? PLoS One. 2016;11:e0159056.
- [CrossRef] [PubMed] [Google Scholar]
- Shared decision making for people living with dementia in extended care settings: A systematic review. BMJ Open. 2018;8:e018977.
- [CrossRef] [PubMed] [Google Scholar]
- Cognition and pain. Curr Opin Support Palliat Care. 2014;8:130-6.
- [CrossRef] [PubMed] [Google Scholar]
- Symptoms clusters in advanced cancer patients: An empirical comparison of statistical methods and the impact on quality of life. J Pain Symptom Manage. 2016;51:88-98.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropsychological performance in cancer patients: The role of oral opioids, pan and performance status. Pain. 2000;86:237-45.
- [CrossRef] [Google Scholar]
- The trajectory of functional decline over the last 4 months of life in a palliative care population: A prospective consecutive cohort study. Palliat Med. 2019;33:693-703.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of people with dementia vs other conditions on admission to inpatient palliative care. J Am Geriatr Soc. 2020;68:1825-33.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of corticosteroids in short-term and long-term memory. Neurology. 2005;64:335-7.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing quality of life of nursing home residents with dementia: Feasibility and limitations in patients with severe cognitive impairment. Int Psychogeriatr. 2013;25:1687-95.
- [CrossRef] [PubMed] [Google Scholar]
- How different are quality of life ratings for people with dementia reported by their family caregivers from those reported by the patients themselves? J Alzheimers Dis. 2017;55:259-67.
- [CrossRef] [PubMed] [Google Scholar]
- Pain assessment using self-reported, nurse-reported and observational pain assessment tools among older individual with cognitive impairment. Pain Manag Nurs. 2015;16:595-601.
- [CrossRef] [PubMed] [Google Scholar]







