Translate this page into:
Effect of Hypofractionated, Palliative Radiotherapy on Quality of Life in Late-Stage Oral Cavity Cancer: A Prospective Clinical Trial
Address for correspondence: Dr. Shyama Prem Sudha, Additional Professor, Department of Radiotherapy, RCC, JIPMER, Puducherry - 605 006, India. E-mail: shyamagopalakrishnan@yahoo.co.in
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
The study was designed to evaluate the effect of a hypofractionated, palliative conformal radiotherapy regimen of 5250 cGy in 15 fractions in inoperable/incurable oral cavity carcinoma.
Aims:
The primary objective was to assess the change in the quality of life (QOL) with respect to pain and mouth opening pre- and post-radiotherapy using standardized questionnaires. The secondary objective was to assess overall QOL using the same questionnaires and also to assess response rates, survival, compliance, early and late toxicity.
Settings and Design:
This was a single-arm, prospective trial. Patients with incurable oral cavity cancer referred for palliative intent radiotherapy to the Department of Radiotherapy, RCC, JIPMER were recruited into the study.
Subjects and Methods:
Forty-eight patients were recruited and twenty-five patients were given conformal radiotherapy to a dose of 52.5 Gy in 15 fractions. QOL was assessed using the European Organization of Research and Treatment of Cancer (EORTC) questionnaires before and 2 months after the completion of radiotherapy. The response assessment was made using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria 2 months after radiotherapy. The early and late toxicities were assessed at 2 months and 6 months after radiotherapy completion, respectively.
Statistical Analysis Used:
Sample size was calculated to be 53. The Wilcoxon signed-rank test was used to compare QOL scores pre- and post-radiotherapy. Median survival was assessed using the Kaplan–Meier method.
Results:
There was a significant improvement in the pain, mouth opening, speech, social contact, social eating, felt ill items of the EORTC QLQ-H and N35 questionnaire and role functioning, emotional functioning, social functioning, fatigue, pain, insomnia, appetite loss, financial difficulties, and Global QOL subscales of the QLQ-C30 questionnaire. 72% of the patients had grade 3 acute radiation oral mucositis and 36% had grade 3 acute radiation dermatitis. There were no significant treatment breaks due to toxicity. There were no grade 3 late toxicities observed. Overall median survival was 5.1 months. The overall response rate was 47%. The median time to treatment completion was 24 days.
Conclusions:
The improvement in QOL parameters suggests that the regimen of 52.5 Gy in 15 fractions is suitable for palliative intent radiotherapy in late-stage oral cavity cancer for effective palliation for short periods.
Keywords
Conformal radiotherapy
hypofractionated radiotherapy
incurable oral cavity cancer
palliative radiotherapy
INTRODUCTION
According to GLOBOCAN 2012, carcinoma of the oral cavity is the third-most common cancer in India.[1] It is the most common cancer in males and the third-most common nongynecological cancer in females after breast and colorectal cancers.[1] In our institute, it was the third-most common solid malignancy encountered in 2016.
The burden of oral cavity cancer is significantly higher in India when compared to the West with 70% of our patients presenting in locally advanced stages (AJCC Stage III–IV) where chances of cure are dismal.[23] Five-year survival rates are usually around 20%.[4] These patients often have poor general condition, poor nutritional status, and other comorbidities making curative treatments difficult.
Various treatment options are available for these patients. Extensive surgical treatment in patients with large tumors often results in disfigurement, difficulties in speech, and feeding and as a result, poor quality of life (QOL) with little survival benefit. Concerns of tolerability and toxicity with suboptimal response rates make chemotherapy an unpopular treatment option. Palliative radiotherapy has now become a standard palliative modality for symptom management in most areas of the human body. Its’ role in locally advanced head-and-neck carcinoma has been established already by a number of clinical studies.[456789] It is common practice to offer patients curative anti-cancer therapy even in advanced malignancies in the hope that it would maximize local control and cure. However, a significant proportion of these patients default treatment and are noncompliant to such protracted schedules.
In India, the absence of supportive care and nutritional support results in poor compliance with about 30% being lost to follow-up.[9] Intensive curative schedules in such patients make little sense, and the aim should be to identify a schedule which provides effective tumor regression and symptom palliation with acceptable side effects in the minimum possible time.
A standard dose-fractionation scheme is yet to emerge in this setting.[10] A dose of 52.5 Gy in 15 fractions was a novel dose fractionation regimen investigated by us in this subgroup of patients. It had multiple advantages including a near tumoricidal dose of 59 Gy equivalent dose in 2 (EQD2) and a shortened overall treatment time of 19 days. Keeping in mind that an improvement in QOL is the probably the first and foremost intent of treatment in palliative oncology, we used this fractionation regime to assess the impact of palliative radiotherapy on QOL and used the European Organization of Research and Treatment of Cancer (EORTC) H and N 35 and QLQ C30 questionnaires for the same.[1112]
The primary objective was to assess the effect of this hypofractionated, palliative conformal radiotherapy regimen of 52.5 Gy in 15 fractions on QOL in patients with late-stage oral cavity cancer using the EORTC QLQ-H and N35 questionnaire with specific attention to pain and mouth opening subscales (in accordance with the most common symptoms in the patient population).
The secondary objective was to assess the general QOL using the EORTC QLQ-C30 and other parameters in the QLQ-H and N35 questionnaires. Other secondary objectives were to assess the median survival, the response rates (using Response Assessment Criteria in Solid Tumors-RECIST guidelines 1.1), acute and late toxicity during radiation therapy – using Radiation Therapy Oncology Group (RTOG)/Common Terminology Criteria for Adverse Events (CTCAE) grading and compliance to the treatment regimen.[1314]
SUBJECTS AND METHODS
Inclusion and exclusion criteria
Patients with biopsy-proven squamous cell carcinoma of the oral cavity of stage IVA–IVC who were not fit for standard/curative treatment and with no previous history of any anti-oncological treatment or the presence of cancer in any other part of the body were eligible for the study. Pregnant women and those with any medical condition precluding radiotherapy were excluded from the study. Patients of age 18–80 were only considered for the trial.
Rationale for the selected regimen
The selection of 52.5 Gy in 15 fractions was guided by multiple factors. It helped us use a near tumoricidal dose of 59 Gy EQD2 (α/β =10) and the delivery of the same in a relatively shorter duration of around 20 days. This would also prevent accelerated repopulation and shorten the hospital stay of patients. Late toxicities though expectedly higher (68 Gy EQD2 with α/β of 3) would probably be of secondary importance due to the short life span of these patients. The use of conformal techniques would help us control the toxicities further. Finally, a hypofractionated regimen would be logistically better in a center like ours with a heavy patient load.
Study procedure
Forty-eight patients who visited the radiotherapy OPD in RCC, JIPMER from August 2015 to July 2017 and satisfied the inclusion and exclusion criteria were recruited into the study. Twenty-eight patients were started on radiotherapy with the others defaulting treatment due to reasons such as disease progression, anxiety, and poor general condition with 25 completing the scheduled course.
Pretreatment evaluation included a biopsy, necessary imaging, hematologic investigations, and a dental evaluation. Performance status (PS), feeding status, and symptom burden were also evaluated. All patients were optimized before radiotherapy. EORTC QLQ C30 and H and N 35 questionnaires were administered to the patients to calculate the baseline QOL score. Patients were started on radiotherapy after computed tomography (CT) simulation, contouring, and planning.
A dose of 52.5 Gy in 15 fractions was prescribed to the planning target volume. Conformal techniques including 3 dimensional conformal radiotherapy, intensity modulated radiotherapy and volumetric modulated arc therapy were used for planning. Late responding tissues were given appropriate constraints after calculating appropriate equivalent dose. Maximum dose (Dmax) to the spinal cord, brainstem, and brain parenchyma was limited to 32.5 Gy, 39 Gy, and 46 Gy, respectively. Dmax to the lens and optic nerve was limited at 7 Gy and 36 Gy, respectively. The mean dose to the contralateral parotid, inner ear, and eyeball was limited at 20 Gy, 34 Gy, and 27 Gy, respectively. Treatment was delivered on the Varian's Clinac iX daily for 5 days a week using 6 MV photon beams. Treatment was scheduled and delivered on all weekdays with intent to complete treatment within 19 days.
Toxicity assessment, data collection, and analysis
Patients were assessed for acute toxicities during and immediately after treatment using the RTOG and CTCAE v4.03 criteria. Symptomatic management was administered based on standard guidelines. First follow-up after treatment completion/discharge was done at 1 month. EORTC questionnaires were administered at 2 months to assess posttreatment QOL. A repeat contrast-enhanced CT was taken at 2 months to assess treatment response as per the RECIST criteria. Late toxicities were assessed at 6 months.
Statistics
The estimated sample size was 53. The sample size was estimated with an expected improvement of 5 points in EORTC H and N 35 pain subscale (assuming pain to be the most dominant symptom in such patients) with a standard deviation of 12 pretest and 10 posttest, 5% level of significance, and 90% power. Patients were recruited with consecutive sampling.
The median QOL scores of pre- and post-radiation were compared using the Wilcoxon signed-rank test. Survival was assessed using the Kaplan–Meier estimate with the rest of the data represented as counts and percentages. IBMs’ SPSS version 19.0 was used for statistical analysis (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA).
RESULTS
Twenty patients did not report for treatment due to factors including disease progression, worsening general condition, anxiety regarding radiotherapy, and monetary concerns. Twenty-eight patients were started on radiotherapy and 25 patients completed the scheduled treatment and have been considered for data analysis. Three patients defaulted radiotherapy after initiation and were lost to follow-up. Six patients expired before the first scheduled follow-up at 2 months. Seventeen patients completed 2 monthly follow-up and were administered the EORTC questionnaires for a second time. They also had a response assessment CT taken at the time. Six patients completed 6 monthly follow-up. Two patient defaulted follow-up posttreatment, the survival data for whom were collected through telephone. Two patients defaulted 6 monthly follow-up due to progressive disease. The necessary data were collected from the attenders of the patients. Follow-up was done till October 2017. At the time of data analysis, 10 patients were alive and under follow-up. Four had completed 6 months of follow-up and 6 had completed 2 months of follow-up. At the time of writing the article, 5 patients were alive and on follow-up.
Baseline parameters
The study cohort had a median age of 53 with 56% of the patients being females and 80% have buccal mucosa cancer. About 76% had stage IVB malignancy and 24% had stage IVA.
ECOG PS was generally poor with 64% having ≥ 3. No patient had a PS <2. Almost all the patients (95.2%) were tobacco chewers, whereas one-third (32%) were smokers and 60% were alcohol consumers [Table 1].
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 11 (44) |
| Female | 14 (56) |
| Age | |
| 18-40 | 2 (8) |
| 41-50 | 7 (28) |
| 51-60 | 12 (48) |
| 61-70 | 2 (8) |
| 71-80 | 2 (8) |
| Subsite | |
| Buccal mucosa | 20 (80) |
| Lip | 2 (10) |
| Hard palate | 2 (10) |
| Alveolus | 1 (5) |
| RMT, floor of mouth, and tongue | 0 |
| Stage | |
| IVA | 6 (24) |
| IVB | 19 (76) |
| ECOG PS | |
| 2 | 9 (36) |
| 3 | 14 (56) |
| 4 | 2 (8) |
| Habits | |
| Smoking | 1 (4) |
| Tobacco chewing | 17 (68) |
| Alcohol | 15 (60) |
RMT: Retromolar trigone, ECOG: Eastern Cooperative Oncology Group, PS: Performance status
Symptoms
Growth or ulcer was the major symptom in all patients. All patients had pain as a presenting symptom with 80% of the patients having WHO Grade 3 pain. 60% had grade 3 trismus. The other miscellaneous symptoms were ulcer, fistulae, swelling, and puffiness of the face.
Quality of life
The Wilcoxon Signed-rank test was used to compare median scores. There was a universal improvement in QOL scores post radiotherapy [Tables 2 and 3]. We were most concerned with the pain and mouth opening outcomes, which formed our primary objective. Both showed a significant improvement after radiotherapy (P < 0.001 for pain and 0.040 for mouth opening). The other items in the QLQ-H and N35 questionnaire to show a significant difference in scores were the “speech problems” (P = 0.027), “trouble with social eating” (P = 0.001), “trouble with social contact” (P = 0.01), “teeth problems” (P = 0.006), and “felt ill” (P = 0.004) subscales. The “dry mouth” subscale along with the “nutritional supplements” and “feeding tube” subscales showed a significantly higher score (less beneficial) in the QLQ-H and N35 questionnaire at 2 months [Table 2]. This is not surprising as postradiotherapy xerostomia is a well-documented adverse effect. No patient reported weight gain post radiotherapy. Most patients declined to respond to the “Less Sexuality” subscale and results of the same have been omitted from this analysis.
| Subscale | Baseline score | 2 months post-RT score | P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Pain | 50 | 25-83.3 | 25 | 8.3-58.3 | <0.001 |
| Swallowing | 16.6 | 0-50 | 16.6 | 0-50 | 1.000 |
| Speech problems | 44.4 | 0-55.5 | 22.2 | 0-55.5 | 0.027 |
| Senses problems | 33.3 | 0-50 | 16.6 | 0-50 | 0.343 |
| Trouble with social eating | 75 | 33.3-100 | 41.6 | 16.6-75 | 0.001 |
| Trouble with social contact | 60 | 6.6-100 | 26.6 | 0-66.6 | 0.001 |
| Less sexuality | NA | NA | NA | NA | NA |
| Teeth problem | 33.3 | 0-66.6 | 0 | 0-66.6 | 0.006 |
| Opening mouth | 66.6 | 0-100 | 33.3 | 0-100 | 0.040 |
| Dry mouth | 0 | 0-33.3 | 33.3 | 0-100 | 0.005 |
| Sticky saliva | 33.3 | 0-66.6 | 33.3 | 0-66.6 | 0.285 |
| Coughing | 0 | 0-66.6 | 0 | 0-33.3 | 0.131 |
| Felt ill | 66.6 | 0-100 | 33.3 | 0-66.6 | 0.004 |
| Pain killers | 100 | 100-100 | 100 | 100-100 | 1.000 |
| Nutritional supplements | 0 | 0-100 | 100 | 0-100 | 0.020 |
| Feeding tube | 0 | 0-100 | 100 | 0-100 | 0.005 |
| Weight loss | 100 | 0-100 | 100 | 0-100 | 0.317 |
| Weight gain | 0 | 0-100 | 0 | 0-0 | 0.317 |
RT: Radiation therapy, NA: Not available
| Subscale | Baseline score | 2 months post-RT score | P | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Physical functioning | 73.3 | 40-100 | 80 | 53.3-100 | 0.509 |
| Role functioning | 33.3 | 0-100 | 66.6 | 33.3-100 | 0.002 |
| Emotional functioning | 41.6 | 8.3-83.3 | 66.6 | 33.3-100 | 0.001 |
| Cognitive functioning | 83.3 | 33.3-100 | 83.3 | 66.6-100 | 0.170 |
| Social functioning | 33.3 | 0-66.6 | 50 | 33.3-100 | 0.001 |
| Fatigue | 55.5 | 0-66.6 | 33.3 | 0-66.6 | 0.004 |
| Nausea and vomiting | 0 | 0-66.6 | 0 | 0-66.6 | 0.170 |
| Pain | 66.6 | 33.3-100 | 16.6 | 0-66.6 | <0.001 |
| Dyspnoea | 0 | 0-33.3 | 0 | 0-33.3 | 0.157 |
| Insomnia | 66.6 | 0-100 | 0 | 0-33.3 | 0.001 |
| Appetite loss | 66.6 | 0-100 | 33.3 | 0-66.6 | 0.002 |
| Constipation | 0 | 0-100 | 0 | 0-66.6 | 0.713 |
| Diarrhoea | 0 | 0-0 | 0 | 0-66.6 | 0.180 |
| Financial difficulties | 33.3 | 0-100 | 33.3 | 0-66.6 | 0.006 |
| Global Health Status/QOL | 50 | 16.6-83.3 | 66.6 | 33.3-83.3 | 0.005 |
QOL: Quality of Life, RT: Radiation therapy
All functional scale scores showed improvement postradiotherapy. However, only the “role functioning,” “emotional functioning,” and “social functioning” with P values of 0.002, 0.001, and 0.001, respectively, reached significance [Table 3]. There was a significant improvement in some of the symptom scales, namely “fatigue” (P = 0.004), “pain” (P < 0.001), “insomnia” (P = 0.001), “appetite loss” (P = 0.002), and “financial difficulties” (P = 0.006), while others such as “nausea and vomiting,” “dyspnea,” “constipation,” and “diarrhea” showed no significant change. There was a significant improvement in the Global QOL postradiotherapy (P = 0.005) [Table 3].
Survival and response
The median survival of the population was 5.1 months (15 months for IVA and 4.3 months for IVB). The response rate was 47% (12% complete response, 35% partial response). About 35% had stable disease.
Toxicity
Seventy-two percent of the patients had grade 3 acute oral mucositis and 36% had ≥3 acute dermatitis. The severity of acute radiation dermatitis and oral mucositis was expectedly high due to the dose-dense radiotherapy schedule we used. Three patients had treatment breaks due to toxicity. Most patients responded well to supportive care in the postradiotherapy period. At two monthly follow-up no patient had grade 3 mucosal reactions. A total of 3 patients developed oro-cutaneous fistulae – one before the start, one during and one 2 weeks after radiotherapy. The tract persisted in all three patients. Ten patients developed acute radiation pharyngitis during radiotherapy/immediately after it. All of them had odynophagia to varying degrees, which subsided after symptomatic care. Grade 2 laryngeal toxicity and oral candidiasis were found in four patients each, all of whom responded well to supportive treatment.
Only 5 patients completed 6 months follow-up, the others being too sick to report for assessment and as a result, the data on late toxicity are modest. All patients had grade 2 xerostomia and grade 2 subcutaneous fibrosis. One patient had grade 2 dysphagia. 13 of the 20 patients who were offered Ryle's tube feeding accepted the same. All patients with >3 radiation reactions/inadequate nutrition were referred for in-patient care. Fourteen patients received in-patient care during and in the immediate postradiotherapy period.
DISCUSSION
Local control and cure rates are disappointing in locally advanced oral cavity cancer. Untreated patients have modest survival rates of around 100 days.[15] Hypofractionated palliative radiotherapy schedules have shown improvements in QOL and symptomatic burden along with good response rates and acceptable toxicities making them a standard in incurable oral cavity malignancies [Table 4]. The “Christie Trial” from the Netherlands studying 50 Gy in 16 fractions reported a 77% improvement in pain in patients with a Global health score of 71 in the EORTC questionnaires.[5] The Agarwal et al. study, which used 40 Gy in 16 fractions reported >50% pain improvement in 78% of patients.[6] We identified QOL as the most important endpoint in these group of patients as different schedules have shown little improvement in survival and local control over the years. Since buccal mucosa is the most common subsite of cancer of the oral cavity and forms the major subgroup of patients reporting to our OPD, our focus was on this subgroup of patients. Our main objective was to assess QOL scores with respect to pain and mouth opening, two of the most distressing symptoms in this subgroup of patients. Our patients showed a significant improvement in median pain scores 2 months after treatment. The improvement in pain scores was probably a result of good tumor control with all patients reporting an improvement in pain with 59% of the patients reporting >50% improvement. All patients were prescribed pain killers at the onset of evaluation and adherence to the same was strictly monitored with the help of the Palliative Care Clinic staff. About 68% of the patients were on oral morphine at some stage and 16% were on fentanyl patches.
| Study | QOL instrument and results | Dose-fractionation | Overall treatment time | EQD2-acute | EQD2-late | Response rate | Median survival | Toxicity (> Grade 3) |
|---|---|---|---|---|---|---|---|---|
| Our study | EORTC QLQ C-30 and QLQ - HN 35. Significant improvement in pain, mouth opening, social contact and eating, speech problems, social, role, and emotional functioning, fatigue, insomnia, appetite loss, financial difficulties, and Global QOL | 52.5 Gy in 15 fr | 24 days | 57 Gy | 68 Gy | 47% | 5.1 months | Muco - 72% Derm - 36% |
| Al-Mamgani et al.[5] | EORTC QLQ C-30 and QLQ - HN 35. GHS of 71 | 50 Gy in 16 fr | 22 days | 55 Gy | 61 Gy | 73% | 17 months | Muco - 65% Derm - 45% |
| Corry et al.[7] | EORTC QLQ - C30. 44% reported improvement in overall QOL | 42 Gy in 12 fr | 2-58 days | 16-25 Gy | 18-55 Gy | 53% | 5.7 months | Muco - 0% Derm - 0% |
| Porceddu et al.[8] | FACT - HN. 62% improvement in overall QOL, 67% improvement in overall pain, 76% improvement in ability to work | 30 Gy in 5 fr with additional 6 Gy boost | 15-18 days | 39-48 Gy | 54-65 Gy | 75% | 6 months | Muco - 26% Derm - 11% |
| Das et al.[9] | FACT- HN. Significant improvement in Social well-being. Improvement in all other subscales - not significant. HN-specific score showed no significant change |
40 Gy in 10 fr | 30 days | 41 Gy | 56 Gy | NA | 7 months | Muco - 24% Derm - 3% |
| Fortin et al.[16] | EORTC PAL C-15 and EORTC QLQ - HN 35. 83% reported improvement in pain scores, 75% in swallowing scores and 58% reported improvement in physical functioning and global QOL at 6 months | 25 Gy in 5 fr | 5 days | 31 Gy | 40 Gy | NA | 6.5 months | Muco - 7% Derm - 0% |
| Mohanti et al.[4] | NA | 20 Gy in 5 fr - upto 70 Gy EQD2 depending on response | 5 days | 23 Gy | 28 Gy | 47% | 6.6-13 months | Muco - 62% Derm - 14% |
| Agarwal et al.[6] | NA | 40 Gy in 16 fr - upto 50 Gy in 20 fr depending on response | 22-26 days | 41-50 Gy | 44-55 Gy | 73% | NA 1 year PFS - 55.1% | Muco - 66% Derm - 56% |
| Soni et al.[17] | University of Washington. Significant improvement in scores of pain, appearance, activity, recreation, swallowing, mood, anxiety, physical domain, social domain, HRQOL 7 days and overall QOL to various degrees in all the 3 arms | Arm 1-44.4 Gy in 12 fr Arm 2-50 Gy in 16 fr Arm 3-40 Gy in 10 fr |
Arm 1-44 days Arm 2-22 days Arm 3-26 days |
Arm 1-37 Gy Arm 2-55 Gy Arm 3-44 Gy |
Arm 1-60 Gy Arm 2-61 Gy Arm 3-56 Gy |
Arm 1%-83% Arm 2%-80% Arm 3%-77% |
Arm 1-11.5 months Arm 2-10.5 months Arm 3-11 months |
Muco - 37% (1) versus 53% (2) versus 23%(3) Derm - 23%(1) versus 40%(2) versus 20%(3) |
| Laursen et al.[18] | NA | 52-56 Gy in 13-14 fr | 43-46 days | 48-50 Gy | 73-78 Gy | 45% | 5.4 months | Muco - 24% Derm - 15% |
GHS: Global Health Score, QOL: Quality of life, HN: Head and neck, HRQOL: Health-related quality of life, EQD2: Equivalent dose in 2 Gy per fraction, fr: Fraction, muco: Mucositis, derm: Dermatitis, NA: Not available, EORTC: European Organization of Research and Treatment of Cancer
The results from the mouth opening subscale were similarly encouraging with 35% reporting an improvement. The improvement in pain may also have contributed to this end point. Due to paucity of literature on this end point, a standard comparison could not be made.
Other symptom subscales in the QLQ-H and N35 showing a significant improvement [Table 2] was a vindication of the ability of the radiotherapy schedule and associated supportive care to shrink the visible tumor and improve the oral hygiene. Most patients were found to have inadequate nutrition as a result of the treatment/tumor. Although supportive measures in the form of nasogastric feeding were initiated, no patient reported an improvement in weight with most continuing to have weight loss. Other items to show significant worsening in scores were the “dry mouth,” “nutritional supplements,” and “feeding tube” subscales [Table 2]. As xerostomia is well-documented toxicity of radiotherapy, this was not unexpected. The difference in the other two was due to the greater use of feeding tube and nutritional supplements as part of supportive care.
The improvement in functional scales was a direct result of the tumor regression post radiotherapy [Figure 1 and Table 3]. The lack of significant differences in other subscales was probably due to the poor nutritional status and the limited value of the assessed parameter in itself. Further symptomatic improvement was reflected in other subscales [Table 3].
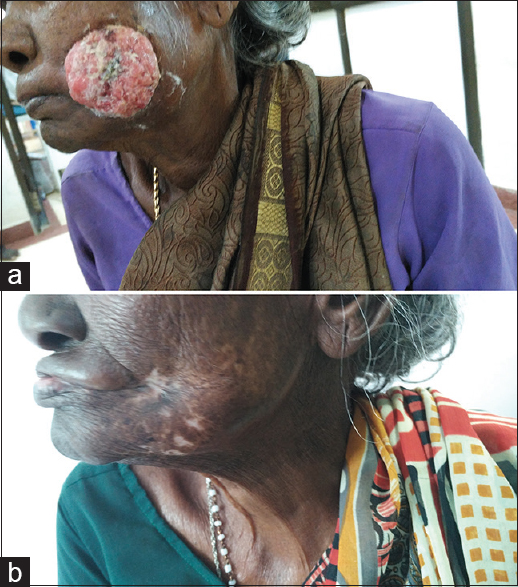
- (a) A patient with Stage IVA Buccal mucosa cancer before receiving radiotherapy. (b) The same patient showing complete clinical response 2 months after radiotherapy completion
The severity of acute dermatitis and mucositis were expectedly high with 36% and 72%, respectively having grade ≥3 radiation reactions. However, this was the by-product of a dose-intense regimen. The three trials closest to ours in terms of total dose and dose per fraction was the Christie trial, the Tata Memorial Hospital (TMH) study and the All India Institute of Medical Sciences (AIIMS) study and had grade 3 mucositis rates of 65%, 66%, and 62%, respectively.[456] The corresponding grade 3 skin toxicity was 45.14% and 56%, respectively in these three studies. The Christie group in the three arm randomized trial had 53% grade 3 acute mucosal toxicity and 40% grade 3 skin reactions.[17] Our results of 36% grade 3 skin toxicity though high was not completely unexpected and was in keeping with the schedule we used and similar to the rates reported in the literature. Even though some trials have reported very minimal toxicity rates using IMRT like the Laursen et al.[18] trial, prolonged overall treatment time and possibly lesser treatment volumes (only 20% with stage IV B) would have also contributed to it. Only six patients could complete the necessary 6 monthly follow-up to record late toxicities, and as a result, the data were not sufficient to draw any meaningful conclusions. However, there were no grade 3 toxicities recorded in any of the patients who completed follow-up.
Twenty patients were offered feeding tube support at some point in the study. Thirteen (65%) patients accepted Ryle's tube, while 7 declined the same. All patients continued Ryle's tube feeding till the end of the study/their death. The 52% Ryle's tube dependence seen in our study was slightly higher than the 29% reported by the Christie trial and 25% reported by the TMH study but was on par with the 42% reported by the Split course trial by Bledsoe et al.[19]
The median survival for the cohort was 5.1 months. This was along expected lines. Most studies have shown survival rates between 5 and 12 months with the Christie scheme study by Al-Mamgani et al. being the notable exception. They reported a median survival of 17 months.
Response rates were assessed using the RECIST criteria. Only two other studies, both from Canada and both reporting 82% response rates – had attempted the same.[2021] The study population had 47% overall response rate. This was similar to the Quad Shot trial and the AIIMS study. The local response rates reported in literature varies between 47% and 100% with both the Christie trial and the TMH study reporting response rates of 73%. However, the complete response rates from the Christie trial was 45% compared to the 10% complete response rates reported by the TMH study. Our patients had a complete response rate of 12%. As more than three-fourths of the patients had T4b disease, response rate, similar to the survival data was expected to be modest. Most patients had tumors with significant local extension resulting in very large treatment volumes. The local control of such extensive tumors is often cumbersome as it proved in our case.
The median time to treatment completion in our study was 24 days (range-19–59). Twenty-three patients (48%) defaulted treatment due to toxicities, financial, and social reasons resulting in prolonged treatment times, the progression of the disease, financial concerns, and other reasons. This was higher than the 27% dropout rates in the CMC, Vellore study[9] and 16% noncompliance rates in the TMH study.[6] The 89% treatment completion rates after initiation of radiotherapy showed that the study was compatible with this patient subgroup.
CONCLUSIONS
The hypofractionated regimen of 5250 cGy in 15 fractions is an acceptable palliative radiotherapy regimen for late-stage oral cavity cancer. Good palliation in terms of pain, mouth opening, speech problems, and social functioning was achieved with this regimen. However, the prolonged periods of palliation we intended to achieve in terms of pain and mouth opening did not come to fruition due to relatively low survival rates. The dismal overall survival rate of 5.1 months was probably due to the inadequacy of dose (BED 59 Gy) and the very advanced stages of the disease the patients had (76% stage IVB). These patients may benefit from newer fractionation schedules, which find an acceptable balance between prolonged tumor control, compliance, and toxicity.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Fact Sheets by Population. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- Indian Council of Medical Research consensus document for the management of buccal mucosa cancer. Indian J Med Paediatr Oncol. 2014;35:136-9.
- [Google Scholar]
- Cancers of the head and neck region in developing countries. Radiother Oncol. 2003;67:1-2.
- [Google Scholar]
- Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol. 2004;71:275-80.
- [Google Scholar]
- Hypofractionated radiotherapy denoted as the “Christie scheme”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. 2009;48:562-70.
- [Google Scholar]
- Hypofractionated, palliative radiotherapy for advanced head and neck cancer. Radiother Oncol. 2008;89:51-6.
- [Google Scholar]
- The ‘QUAD SHOT’ – A phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137-42.
- [Google Scholar]
- Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment-”Hypo trial”. Radiother Oncol. 2007;85:456-62.
- [Google Scholar]
- Hypofractionated palliative radiotherapy in locally advanced inoperable head and neck cancer: CMC Vellore experience. Indian J Palliat Care. 2013;19:93-8.
- [Google Scholar]
- Palliative radiation therapy for head and neck cancer: Toward an optimal fractionation scheme. Head Neck. 2008;30:1586-91.
- [Google Scholar]
- Quality of life in head and neck cancer patients: Validation of the European Organization for Research and treatment of cancer quality of life questionnaire-H and N35. J Clin Oncol. 1999;17:1008-19.
- [Google Scholar]
- The EORTC core quality of life questionnaire (QLQ-C30): Validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer. 1995;31A:2260-3.
- [Google Scholar]
- New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228-47.
- [Google Scholar]
- Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341-6.
- [Google Scholar]
- Palliative radiation therapy for advanced head and neck carcinomas: A phase 2 study. Int J Radiat Oncol Biol Phys. 2016;95:647-53.
- [Google Scholar]
- Comparative evaluation of three palliative radiotherapy schedules in locally advanced head and neck cancer. World J Oncol. 2017;8:7-14.
- [Google Scholar]
- An extended hypofractionated palliative radiotherapy regimen for head and neck carcinomas. Front Oncol. 2018;8:206.
- [Google Scholar]
- Split-course accelerated hypofractionated radiotherapy (SCAHRT): A safe and effective option for head and neck cancer in the elderly or infirm. Anticancer Res. 2016;36:933-9.
- [Google Scholar]
- 0-7-21 hypofractionated palliative radiotherapy: An effective treatment for advanced head and neck cancers. Br J Radiol. 2015;88:20140646.
- [Google Scholar]
- Retrospective study of palliative radiotherapy in newly diagnosed head and neck carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:958-63.
- [Google Scholar]






