Translate this page into:
Low-dose Oral Ketamine as a Procedural Analgesia in Pediatric Cancer Patients Undergoing Bone Marrow Aspirations at a Resource-limited Cancer Hospital in India
Address for correspondence: Dr. Mikael Segerlantz, Palliative Care and Advanced Home Health Care, Sankt Lars väg 90, 221 85 Lund, Sweden. E-mail: mikael.segerlantz@skane.se
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
Many pediatric cancer patients undergo repeated bone marrow aspirations (BMAs) for diagnostic and treatment evaluation purposes. Full anesthesia is the standard of care during this procedure in high-income countries. At hospitals with low resources in low/middle-income countries many children undergo these painful procedures without sufficient pain relief. This study aimed to evaluate the usefulness of low-dose oral ketamine as a procedural analgesic in a low-resource pediatric cancer care department.
Materials and Methods:
Pediatric patients, 4–15 years of age, who underwent BMAs between September 31 and November 30, 2018, were invited to participate. The study was designed as a placebo-controlled, single-blinded trial with three trial groups. Group K received 1.0 mg/kg of ketamine and Group KM received 1.0 mg/kg ketamine with an addition of 0.2 mg/kg midazolam, mixed in juice 30 min before procedures. Group P received placebo consisting of plain juice. All three groups also received the hospital's current standard treatment for procedural pain in BMAs. Patients and caregivers assessed the procedural pain, as did the performing doctors. For the patients, Faces Pain Scale – Revised was used and the Numeric Rating Scale-11 for caregivers and doctors.
Results:
A total of 87 patients were included in the study distributed with 29 in Group K, 29 in Group KM, and 29 in Group P. Seven patients were excluded, one patient denied participation and the remaining did not meet the inclusion criteria. There was no significant difference between the pain reported by the groups. A total of 69% patients in Group KM and 35% in Group K had somnolence reported as a side effect compared to 14% in Group P.
Conclusion:
We found no significant effects on the procedural pain in any of the treatment groups compared to placebo. There were only mild side effects. The doses of ketamine might be insufficient for this painful and stressful procedure.
Keywords
Low-dose oral ketamine
pediatric cancer
procedural analgesia
INTRODUCTION
Bone marrow aspirations in pediatric oncology
Bone marrow aspirations (BMAs) are common procedures in pediatric oncology. Patients often undergo BMAs as a part of diagnostics and repeated BMAs is essential in the evaluation of the tumor-treatment. In high-income countries (HICs), full anesthesia is a standard practice to fully alleviate the pain and stress of the procedure.[1] This practice is, however, resource-demanding; both in terms of qualified personnel, equipment, drugs, and facilities. Full anesthesia also requires the available resources to deal with any adverse effects. As a result, many patients in low-/middle-income countries (LMICs) like India have to undergo the procedure with insufficient or no anesthesia.[2]
Pain assessment in pediatric patients
Pain assessment tools that are based on the patients’ self-reported pain is considered the gold standard for both clinical practice and research. Tools for self-reported pain can be used in children as young as 4 years old but are less reliable in patients <5 years. Although there are many pain tools in use, the Faces Pain Scale – Revised (FPS-R), developed by the International Association for the Study of Pain in 2001 from the older FPS, is considered the most reliable and useful scale to use in a research setting.[3456] Scales similar to FPS-R, such as the Wong-Baker FPS, are often used in clinical work but are not as reliable in a research setting.[345] Children 6 years and upward, can utilize visual or verbal numerical scales that can be used by adults such as the Numeric Rating Scale-11 (NRS-11) to self-report pain, but younger children may have trouble using these type of scales.[6] A major weakness with self-reported pain is the risk of children, especially younger children, not being able to separate pain from fear and stress.[78]
Proper pain management in children is essential both from a humanitarian and from a medical perspective. Patients who have undergone painful procedures without sufficient pain-relief are more likely to experience higher levels of procedural related fear and anxiety when undergoing other painful procedures.[9] Unmanaged procedural pain has been shown to play a role in decisions by patients and their caretakers to stop adhering to or even abandoning treatment.[1011]
Ketamine
Ketamine is a dissociative anesthetic agent, an excellent analgesic, comparable to morphine, and exhibits low incidence of respiratory depression, making it suitable for safe pain-relief both in prehospital care and in the Emergency Department, as well as in palliative care.[121314] In children, ketamine is used for a variety of briefly painful and emotionally disturbing medical procedures.[151617]
Ketamine is commonly administered intravenously or orally. In a low-resource setting, oral administration has the advantage of easy and resource-saving as well as entailed with a low risk of adverse effects. Bioavailability is between 8% and 24% when administered orally, due to a significant first-pass metabolism. Maximum blood levels are reached between 30 and 55 min after ingestion, but variation occurs between individuals.[141819] Administration of oral ketamine for analgesia is recommended at least 30 min before commencing a painful procedure.[20]
Adverse effects include hypertension, nausea, and psychological effects such as agitation, confusion, and hallucinations.[12] The adverse effects are dose-dependent and are uncommon with sub-anesthetic, low-dose administration.[121721]
Ketamine can be used by itself or in a combination with other drugs. When combined it is usually with a benzodiazepine or an opioid to gain a synergistic pain-relieving and sedative effect.[182223] The combination with benzodiazepines also reduces the risk of psychological side effects.[1823]
Study setting at a low-resource cancer-hospital
The study was performed at a governmental tertiary cancer referral center in India with a catchment area of approximately 35 million inhabitants. The hospital registers >10,000 new patients yearly, of which 450–500 pediatric cancer cases, a majority with hematological malignancies. The hospital is short of staff and medical supplies, with crowded wards and an insufficient capacity to monitor adverse reactions and vital signs. Treatment is free of charge at the hospital for patients living below the poverty line. Patients often come from the underprivileged segments of Indian society, illiteracy is common among patients and their families, and the level of preunderstanding of the child's condition is low. Many patients travel from remote parts of the state and even from other states of India, where access to cancer-care is restricted.[24]
Based on earlier studies at the hospital, of pain and pain-management in pediatric cancer, we have shown that pediatric cancer patients undergo painful procedures without sufficient pain relief. In a placebo-controlled study, we have shown that low-dose oral ketamine, at a dose of 0.8 mg/kg body weight is a feasible and effective analgesic for pediatric cancer patients undergoing lumbar punctures (LPs).[25] Thus, the present study continued the previous work on pain-management with low-dose oral ketamine for procedural pain in pediatric cancer patients in a low-resource hospital at a LMIC.
Aim
The primary objective of this study was to assess the effect and safety of low-dose oral ketamine and to compare the analgesic effect of ketamine solely, to a mixture of ketamine and midazolam as a procedural analgesic for pediatric cancer patients undergoing BMAs in a low-resource Indian hospital.
MATERIALS AND METHODS
Study design
This study was designed as a placebo-controlled, single-blinded comparative study. Included patients were divided into three groups, Group K received low-dose oral ketamine, Group KM received a mixture of low-dose oral ketamine with addition of midazolam and Group P received placebo. In addition, all patients received the current standard treatment of topical analgesic and a weak sedative.
The patients were allocated into one of the three groups with a sequential allocation depending on what day the procedure was performed. Measures were taken to ensure equally sized groups. Proper randomization of patients was not possible at the hospital, due to the lack of resources and proper research infrastructure at the hospital.
Pain-rating was collected from patients, with the FPS-R, and from primary caregivers and the pathologist performing the procedure, with NRS-11. Adverse effects were noted by hospital staff, by patients, and by their caregivers.
Baseline data and demographics were retrieved from the patients’ medical records and from interviews.
Inclusion and exclusion criteria
All pediatric patients between the ages of 4 and 15 years, with a scheduled BMA, during the study period from September 31 to November 30, 2018 were invited to participate. Only patients who either gave informed consent or who had caregivers that could give informed consent, were included in the study. Patients older than 15 years of age were excluded since they are considered adults and were treated in the adult ward. Children younger than 4 years were considered unable to accurately self-report their pain. Patients who could or would not report their pain were excluded from the study. Patients who underwent more than one BMA during the period of data collection were included only once.
Bone marrow aspirations
All BMAs were performed in the same room, by experienced pathologists and in a standardized way, from the posterior superior iliac spine. Caregivers together with staff were involved in restraining the child during the BMA. The staff involved had no knowledge of which patients had chosen to participate in the study or which group the included patients were assigned to.
Analgesia protocol
All patients received local standard treatment for patients undergoing BMAs; consisting of a topical analgesic cream (EMLA®) and a weak oral sedative, Triclofos (Pedicloryl®), ahead of the procedure. Just before the BMA, an injection of local anesthesia consisting of 2% lignocaine hydrochloride (LOX®) was given to the area of the procedure by the pathologist performing the BMA.
Group K received 1 mg/kg of ketamine hydrochloride (Aneket®) mixed with mango juice (Maaza®). Group KM received 1 mg/kg of ketamine hydrochloride with an addition of 0.2 mg/kg midazolam hydrochloride (Mezolam®) mixed with mango juice. Group P received an equal amount of regular mango juice. The juice was then administered 30 min ahead of the BMA.
Statistical analysis
A statistical power calculation was carried out before commencing the data collection. Based on previous studies, we assumed the true mean difference between patients having the intervention and those not having the intervention to be 1.5 with a pooled standard deviation of 2. With these assumptions, the groups would require 29 patients each, for a total of 87 patients, to reject the null hypothesis with 80% power. Probability of Type-I-error (alpha) associated with this calculation was 5%.
Statistical analysis of the data was performed with IBM SPSS Statistics version 24, Armonk, NY, USA. The Kruskal–Wallis test was utilized to calculate the significance of the difference between the groups since the data on self-reported pain collected was independent samples on an ordinal scale with limited values. A value of P < 0.05 was considered statistically significant.
Ethical approval
Ethical approval for this study was granted by the Local Ethics Committee at the hospital. A consent form approved by the local ethics committee was used and signed by the participating patients’ caregiver. All patients and caregivers were given both oral and written information about the study. The written information was translated into the local language Telugu, as was the consent form. Additional information about the study was available in both English and Telugu on request. The patients were given age-appropriate information. If illiterate, the information was given orally, with a thumb-imprint on the consent form. All participation was voluntary, and the participants were informed that they could withdraw from the study at any time, without consequences.
RESULTS
Study population
A total of 87 patients aged 4–15 years, together with their caregivers, met the inclusion criteria and accepted to participate in the study. Each group; K, KM, and P were allocated with 29 patients. Seven patients were excluded; one patient denied participation and the remaining did not meet the inclusion criteria.
Three BMA procedures originally included in the study were excluded from Group KM since, due to an administrative error, the patients had already participated in the study. One patient was excluded from Group K due to a communication error where the procedure was performed immediately after administration of the juice mix.
The majority of the patients (n = 43, 51%) performed their BMA as a diagnostic procedure. The most common diagnosis was acute lymphoblastic leukemia (n = 30, 35%). There were no significant differences between groups regarding weight and age. Median age, in the entire cohort, was 8 (4–15) years. Group P had a majority of females (55%), whereas in both Group K and Group KM, the majority were male patients (55% and 59%, respectively). The median average number of previous BMAs was 1 (0–5). None of the included patients in the study received any other pain-medications on the day of the procedure [Table 1].
| Patient data | Categories | Group K (n=29) | Group KM (n=29) | Group P (n=29) | Total (n=87) |
|---|---|---|---|---|---|
| Sex, n (%) | Female | 13 (45) | 12 (41) | 16 (55) | 41 (47) |
| Male | 16 (55) | 17 (59) | 13 (45) | 46 (53) | |
| Age | Mean, years | 9.1 | 8.1 | 8.0 | 8.4 |
| Median (minimum-maximum), years | 9 (4-14) | 7 (4-14) | 8 (4-15) | 8 (4-15) | |
| Weight | Mean, kilos | 22.2 | 20.5 | 20.2 | 21.0 |
| Median (minimum-maximum), kilos | 20 (10-43) | 15 (10-47) | 19 (11-36) | 20 (10-47) | |
| Diagnosis, n (%) | Unknown | 11 (38) | 17 (61) | 15 (54) | 43 (51) |
| ALL | 15 (52) | 5 (18) | 10 (36) | 30 (35) | |
| AML | 2 (7) | 2 (7) | 1 (4) | 5 (6) | |
| Other | 1 (3) | 4 (14) | 2 (7) | 7 (8) | |
| Previous number of BMAs | Mean | 1.1 | 1.3 | 0.8 | 1.1 |
| Median (minimum-maximum) | 1 (0-3) | 1 (0-5) | 1 (0-3) | 1 (0-5) | |
| Ongoing pain medication | Patients with on-going pain medication, n (%) | 3 (10) | 0 (0) | 0 (0) | 3 (3) |
BMAs: Bone marrow aspirations, ALL: Acute lymphoblastic leukemia, AML: Acute myeloid leukemia
Demographics
Mothers were the most common primary caregivers, in 83% of the patients. A majority of patients (61%) lived in rural areas and 66% of the patients had to travel more than 100 km to reach the hospital. The monthly average family income was 8665 Indian Rupees, which equals about 4 United States Dollars per day. Sources of income were mainly day labor, unqualified labor, and agricultural work. Illiteracy was common, reported by a third of the primary caregivers and in another third, only a primary educational level was reached. A majority of patients were below school age or in their age-appropriate grade, while 13% had fallen behind one or more grades [Table 2].
| Demographic variable | Categories | Group K (n=29) | Group KM (n=29) | Group P (n=29) | Total (n=87) |
|---|---|---|---|---|---|
| Primary caregiver, n (%) | Mother | 22 (76) | 25 (86) | 24 (86) | 71 (83) |
| Father | 6 (21) | 1 (3) | 4 (14) | 11 (13) | |
| Other | 1 (3) | 3 (10) | 0 (0) | 4 (5) | |
| Distance from hospital (km), n (%) | 0-100 | 7 (27) | 9 (31) | 11 (44) | 27 (34) |
| 101-500 | 14 (54) | 15 (52) | 11 (44) | 40 (50) | |
| >500 | 5 (19) | 5 (17) | 3 (12) | 13 (16) | |
| Rural or urban, n (%) | Rural | 19 (66) | 17 (63) | 15 (56) | 51 (61) |
| Urban | 10 (35) | 10 (37) | 12 (44) | 32 (39) | |
| Family monthly income, INR (USD), n (%) | Mean | 8804 | 9208 | 8037 | 8665 |
| <5000 (~ 70) | 5 (18) | 6 (25) | 7 (26) | 18 (23) | |
| 5000-10,000 (~70-140) | 16 (57) | 12 (50) | 13 (48) | 41 (52) | |
| 10,001-15,000 (140-210) | 6 (21) | 5 (21) | 7 (26) | 18 (23) | |
| >15,000 (210) | 1 (4) | 1 (4) | 0 (0) | 2 (3) | |
| Patient education level, n (%) | Not in school due to low age | 6 (21) | 10 (35) | 7 (25) | 23 (27) |
| Age appropriate grade | 19 (66) | 14 (48) | 18 (64) | 51 (59) | |
| Age inappropriate grade | 4 (14) | 4 (14) | 3 (11) | 11 (13) | |
| Not enrolled in school | 0 (0) | 1 (3) | 0 (0) | 1 (1) | |
| Caregiver education level, n (%) | Illiterate | 9 (32) | 7 (25) | 12 (43) | 28 (33) |
| Grade 1-8 | 10 (35) | 12 (43) | 6 (21) | 28 (33) | |
| Grade 9-12 | 7 (25) | 6 (21) | 8 (28) | 21 (25) | |
| Tertiary education | 2 (7) | 3 (11) | 2 (7) | 7 (8) |
USD: United States dollar, INR: Indian rupees
Analgesic administration
All patients received a topical analgesic cream, EMLA®, on the area involved in the procedure on average 68.5 min before procedure with no significant differences between the groups. All patients except 9 also received triclofos on an average 65.9 min before the procedure; the patients who did not receive triclofos did not receive the medication due to previous adverse effects. All patients received an injection of local anesthesia, lignocaine hydrochloride, at the site of the BMA before the procedure [Table 3].
| Patient data | Categories | Group K (n=29) | Group KM (n=29) | Group P (n=28) | Total (n=86) |
|---|---|---|---|---|---|
| Interval between EMLA and BMA, min | Mean | 73.2 | 69.9 | 62.1 | 68.5 |
| Median (minimum-maximum) | 71 (31-106) | 64 (35-118) | 60 (28-120) | 64.5 (28-120) | |
| Interval between triclofos and BMA, min | Mean | 66.3 | 69.2 | 62.3 | 65.9 |
| Median (minimum-maximum) | 65 (17-104) | 63.5 (33-114) | 57.5 (38-115) | 62 (17-115) | |
| Interval between juice administration and BMA, min | Mean | 41.3 | 36.7 | 40.0 | 39.4 |
| Median (minimum-maximum) | 41 (29-56) | 36 (23-47) | 39.5 (27-69) | 40 (23-69) | |
| Interval between BMA and interview, min | Mean | 42.8 | 52.3 | 44.9 | 46.7 |
| Median (minimum-maximum) | 38 (10-102) | 40 (21-252) | 40 (8-130) | 39 (8-252) |
BMA: Bone marrow aspiration
All patients took their mixture of mango-juice with drugs, according to their allotted group. The average interval between the administration of the juice and BMA was 39.4 min in the whole study-group, and 41.3, 36.7, and 40 min in Group K, KM, and P, respectively [Table 3].
Patients and caregivers were successfully kept blinded regarding the content of the juice.
Pain score
The median pain score reported by patients was 4 (range: 0–10) in Group K, 4 (0–10) in Group KM, and 3.5 (0–10) in Group P. The difference between the groups is not statistically significant (Kruskal–Wallis P = 0.567). Median pain-score reported by caregivers in Group K was 5 (0–10), 5 (0–10) in Group KM, and 5 (0–10) in Group P and there was no significant difference between the groups (P = 0.727). Finally, the median pain score reported by hospital staff was 4 (1–7) in Group K, 5 (1–6) in Group KM, and 4 (0–8) in Group P with no statistically significant difference (P = 0.548) [Table 4 and Figures 1–3].
| Variable | Categories | Group K | Group KM | Group P |
|---|---|---|---|---|
| Pain score by patient | n | 29 | 28 | 28 |
| Median (Q1-Q3) | 4 (2-6) | 4 (3-6) | 3.5 (1.5-4.5) | |
| Overall P | 0.567 | |||
| Pain score by caregiver | n | 29 | 29 | 29 |
| Median (Q1-Q3) | 5 (4-6) | 5 (2-5) | 5 (3-7) | |
| Overall P | 0.727 | |||
| Pain score by procedure performer | n | 29 | 29 | 29 |
| Median (Q1-Q3) | 4 (3-6) | 5 (3-5) | 4 (2-6) | |
| Overall P | 0.548 | |||
The Kruskal-Wallis test was used for overall comparisons
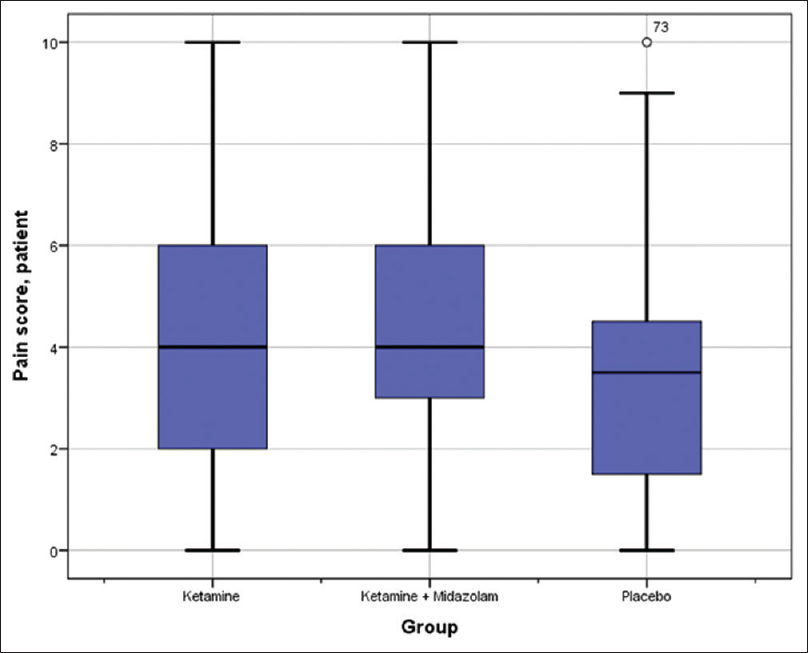
- Box plot diagram of the dispersion of self-reported pain score. Interquartile range (IQR = Q3 - Q1) is represented by the box. Outliers are defined as >1.5 interquartile range from the nearest quartile. Whiskers stretch to the largest/smallest value that is not an outlier
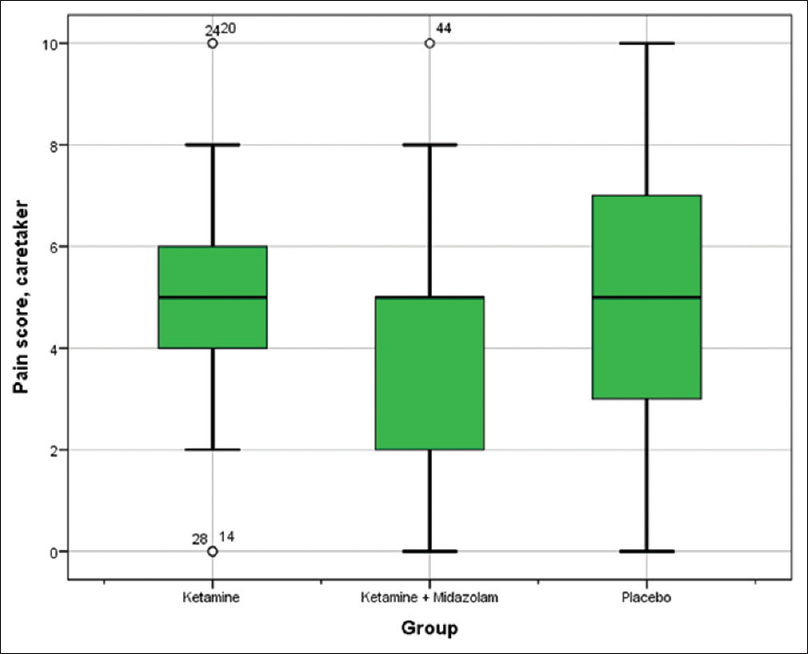
- Box plot diagram of the dispersion of caretaker-reported pain score. Interquartile range (IQR = Q3 - Q1) is represented by the box. Outliers are defined as >1.5 interquartile range from the nearest quartile. Whiskers stretch to the largest/smallest value that is not an outlier
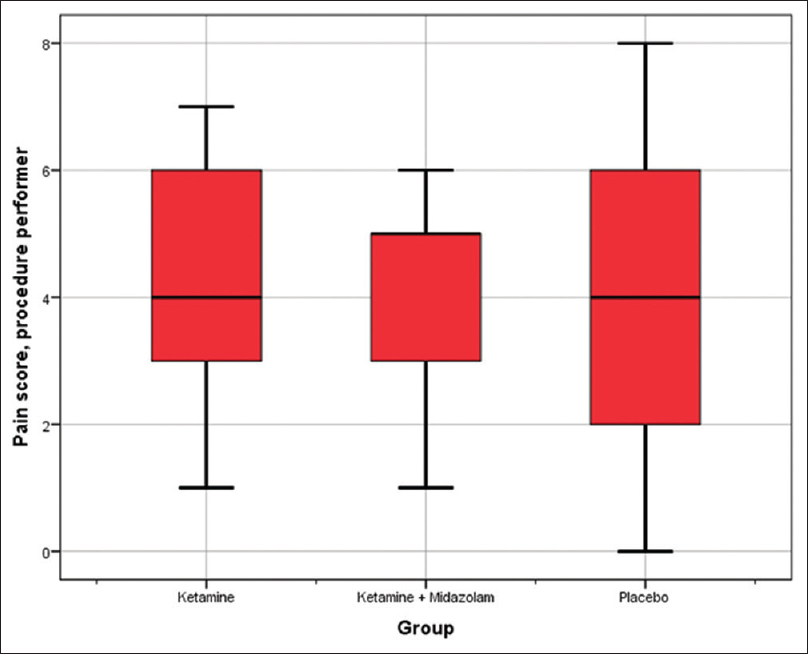
- Box plot diagram of the dispersion of pain score reported by the doctor performing the procedure. Interquartile range (IQR = Q3 - Q1) is represented by the box. Outliers are defined as >1.5 interquartile range from the nearest quartile. Whiskers stretch to the largest/smallest value that is not an outlier
Adverse effects
Somnolence or drowsiness was the most common adverse effects, experienced by 20 (69%) patients in Group KM and 10 (35%) patients in Group K. The patients who reported somnolence were affected for a few hours following the procedure and could be awakened by the light stimulus. Four (14%) patients from Group P also reported somnolence. No psychological side effects, such as agitation or hallucinations were recorded. One patient from Group P left the hospital before being questioned about adverse effects.
DISCUSSION
In the present study, we found no significant effects on the procedural pain during BMA whether placebo, ketamine, or ketamine with an addition of midazolam were administered. This in contrast to our previous study, Rayala et al.,[25] in which significantly reduced procedural pain was seen in patients undergoing LPs receiving oral ketamine, even in a dose lower than in the present study, and compared to patients receiving only local analgesia. No significant side-effects were seen in this study.
The lack of effect in the present study, in contrast to Rayala et al.,[25] may be contributed to several differences between the studies. First, the study by Rayala et al. was conducted on patients undergoing LPs which, although they are painful invasive procedures, does not allow the results from that study to be directly applied to the present study. BMA is a more painful procedure, and as such procedural analgesia requires more potent medications. In HIC, full anesthesia is common practice. Accordingly, we increased the dosage of ketamine and added midazolam to one group of patients to obtain an anxiolytic effect, and thus to decrease the total experience of pain.
In other studies, using oral ketamine as a procedural anesthetic, doses between 2.5 and 5 mg/kg were applied without serious side effects.[20262728] The current literature suggests that the upper limit for giving ketamine in a sub-anesthetic dose, with low physiological impact and low impact on cognition, is considered to be 0.3 mg/kg intravenously. Above 0.3 mg/kg the physiological impact increases with larger doses, reaching a fully dissociative, anesthetic state at ≥1.0 mg/kg intravenous ketamine.[29] Since the bioavailability of oral ketamine is low, only about 8%–24%, a dose of 1 mg/kg is still a relatively low dose.[141819] However, these studies were performed in HIC, on patients with completely different demographic profiles compared to the present study and importantly, access to monitoring vital functions and managing potential adverse effects. Study results based on patients in HIC may not be directly applied to patients in L/MIC settings.[30] Coexisting medical conditions as malnutrition, as well as patients preunderstanding and level of hospital-resources are among important factors that can influence results in medical trials. Thus, one should question the appropriateness of transferring treatment protocols from HIC to LMIC.
In addition, besides more painful, a BMA is also more stressful and traumatic than an LP. Indeed, many of the children were afraid during the procedure. There was no psychological preprocedural preparation and BMAs were carried out in a stressful environment not adjusted to pediatric patients, by an unknown staff, and without direct contact and comfort from their caregivers. In the earlier mentioned study on LPs[26] this procedure was performed by familiar doctors in a well-known environment at the pediatric ward and with the caretaker close and present.[25]
These differences might explain the discordant results between the two studies.
Second, All instructions and all interviews were conducted in local languages (Telugu or Hindi). However, due to the crowded environment, the evaluating interviews could not be conducted either privately or separate for patient and caretaker. This may have influenced the pain reported by patients. However, interviews were performed soon after the procedure, and the interviews were performed in a standardized way by the same staff members, increasing the reliability of the data collected.
A proper randomization protocol would have been the preferable method for assigning patients to different groups to reduce the risk of selection bias. This, however, was not possible at the hospital since many of the hospital personnel involved in the study were unaccustomed to conducting scientific studies. The different groups (K, KM, and P) in this study were large enough to eliminate any significant differences in demographic data.
In the current study, with the given prerequisites at a low-resource hospital, we have probably given too low doses, of both ketamine and midazolam, to achieve sufficient pain-relief in BMA. If adequate analgesia is possible to obtain, with sub-sedative and risk-free doses, needs to be further investigated.
CONCLUSION
In the present study, we found no significant effects on the procedural pain during BMA whether placebo, ketamine or ketamine with an addition of midazolam, in the current low doses, was administered. It is of critical importance that adequate analgesia can be offered to all children undergoing painful procedures, including the underprivileged children in LMICs. Further studies should focus on doses and combinations of drugs, as well as the preprocedural preparation and the physical and psychological environment during the procedure.
Financial support and sponsorship
Travel grant was provided by The Swedish International Development Cooperation Agency.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank MNJIORCC, Hyderabad, for providing resources, and the patients and families participating in the study, making this work possible in the interest of future patients and families. We also would to thank Two Worlds Cancer Collaboration, Canada, and Pain Relief and Palliative Care Society, Hyderabad, India, for providing resources and support.
REFERENCES
- American Academy of Pediatrics Committee on Drugs: Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89:1110-5.
- [Google Scholar]
- A survey of procedural sedation and analgesia practices in pediatric oncology centers in India. Indian J Pediatr. 2012;79:1610-6.
- [Google Scholar]
- Recommendations for selection of self-report pain intensity measures in children and adolescents: A systematic review and quality assessment of measurement properties. Pain. 2019;160:5-18.
- [Google Scholar]
- A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168-98.
- [Google Scholar]
- The faces pain scale-revised: Toward a common metric in pediatric pain measurement. Pain. 2001;93:173-83.
- [Google Scholar]
- Is the verbal numerical rating scale a valid tool for assessing pain intensity in children below 8 years of age? J Pain. 2013;14:297-304.
- [Google Scholar]
- Factors influencing nurses’ pain assessment and interventions in children. J Adv Nurs. 1994;20:853-60.
- [Google Scholar]
- Assessment of acute pain and anxiety in children and adolescents by self-reports, observer reports, and a behavior checklist. J Consult Clin Psychol. 1984;52:729-38.
- [Google Scholar]
- Clinical implications of unmanaged needle-insertion pain and distress in children. Pediatrics. 2008;122(Suppl 3):S130-3.
- [Google Scholar]
- Determinants of treatment abandonment in childhood cancer: Results from a global survey. PLoS One. 2016;11:e0163090.
- [Google Scholar]
- Determinants of parental decisions on ‘drop out’ from cancer treatment for childhood cancer patients. J Adv Nurs. 1999;30:193-9.
- [Google Scholar]
- The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565-75.
- [Google Scholar]
- Intravenous subdissociative-dose ketamine versus morphine for analgesia in the emergency department: A Randomized controlled trial. Ann Emerg Med. 2015;66:222-90.
- [Google Scholar]
- The effects of low-dose ketamine on acute pain in an emergency setting: A systematic review and meta-analysis. PLoS One. 2016;11:e0165461.
- [Google Scholar]
- Ketamine: A review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61-8.
- [Google Scholar]
- Ketamine: A Review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55:1059-77.
- [Google Scholar]
- Efficacy of oral ketamine compared to midazolam for sedation of children undergoing laceration repair: A double-blind, randomized, controlled trial. Medicine (Baltimore). 2016;95:e3984.
- [Google Scholar]
- Low dose ketamine use in the emergency department, a new direction in pain management. Am J Emerg Med. 2017;35:918-21.
- [Google Scholar]
- Benefit and harm of adding ketamine to an opioid in a patient-controlled analgesia device for the control of postoperative pain: Systematic review and meta-analyses of randomized controlled trials with trial sequential analyses. Pain. 2016;157:2854-64.
- [Google Scholar]
- Efficacy of two oral premedicants: Midazolam or a low-dose combination of midazolam-ketamine for reducing stress during intravenous cannulation in children undergoing CT imaging. Paediatr Anaesth. 2010;20:330-7.
- [Google Scholar]
- Low-dose oral ketamine for procedural analgesia in pediatric cancer patients undergoing lumbar puncture at a resource-limited cancer hospital in India. J Palliat Med 2019 doi: 10.1089/jpm.2018.0667
- [Google Scholar]
- Comparison effect of midazolam alone and midazolam combined with ketamine in bone marrow aspiration pain in children. Iran J Ped Hematol Oncol. 2015;5:131-7.
- [Google Scholar]
- Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Paediatr Anaesth. 2005;15:554-9.
- [Google Scholar]
- Midazolam or ketamine for procedural sedation of children in the emergency department. Emerg Med J. 2007;24:579-80.
- [Google Scholar]
- Intravenous sub-anesthetic ketamine for perioperative analgesia. 2016. J Anaesthesiol Clin Pharmacol [Internet]. 321:160-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27275042
- [Google Scholar]
- When can research from one setting be useful in another? Understanding perceptions of the applicability and transferability of research. Health Promot Int. 2013;28:418-30.
- [Google Scholar]






