Translate this page into:
Practical Guide for Using Methadone in Pain and Palliative Care Practice
Address for correspondence: Dr. Gayatri Palat, Department of Pain and Palliative Medicine, MNJ Institute of Oncology and RCC, Red Hills, Hyderabad - 500 004, Telangana, India. E-mail: gpalat@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Since the 2014 Amendment to the NDPS Act methadone has been released in India for pain management. The methadone is supplied as racemic mixture with R & S methadone with benefit in pain management and associated adverse effects. Physicians need to be aware of adverse effects so that methadone can be administered safely. Similarly, patients and families need to store and use methadone carefully and experience the benefits and not increase the risk of further morbidity. Considerable amount of literature on methadone is available and sometimes conflicting, hence the article is attempting to guide a physician to use methadone safely to acquire experience and expertise over time.
Keywords
Drug interaction
equianalgesic dose
methadone
neuropathic pain
opioid rotation
INTRODUCTION
Methadone is a synthetic opioid and classified as WHO Ladder Step 3, strong opioids such as morphine and fentanyl. Methadone was introduced as an analgesic in the mid-1940s but soon lost favor because of its side effect profile. A better understanding of pharmacokinetics and dynamics resulted in reemergence of methadone in the 1980s. Oral methadone as an analgesic was included in the 20th edition, WHO model list of essential medicines in 2017.[1]
In India, methadone was introduced as a substitution therapy medication for opioid dependence treatment in the year 2012.[2] It became commercially available for pain management in 2014.[3] In 2015, the Government of India listed methadone as an “Essential Narcotic Drug” for medical and scientific use under the modified NDPS Act 2015.[4]
PROPERTIES OF METHADONE OF CLINICAL SIGNIFICANCE
R-Methadone is a Mu & Delta agonist, while S-Methadone is an NMDA antagonist, Serotonin and Norepinephrin re-uptake inhibitor. Methadone as racemic mixture has unique properties as a pharmaceutical, to improve complex chronic noncancer or cancer pain with nociceptive and neuropathic components.[56]
Some basic pharmacokinetic and pharmacodynamic properties of methadone, which may have a significant bearing on clinical practice [Table 1]:[6]

INDICATION OF METHADONE AS AN ANALGESIC
According to the WHO analgesic ladder,[7] pain may be classified as mild, moderate, or severe. The step of the ladder and the analgesic is chosen according to severity of pain [Figure 1].
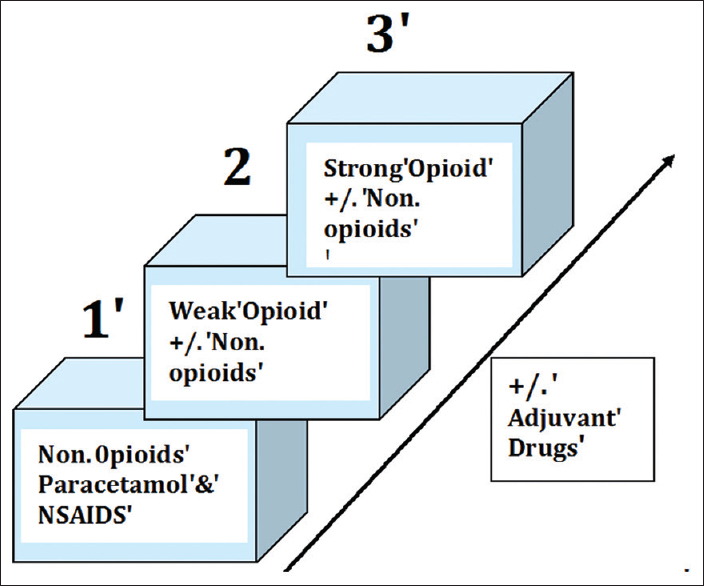
- WHO 3-steps analgesic ladder for cancer pain in adults.
When pain cannot be controlled with an opioid despite adequate dose titration, or when adverse effects become intolerable, [Table 2], the opioid can be switched to another opioid, a practice called opioid rotation or switch.[8]

Methadone is commonly used as a replacement (substitution or conversion) medication when patients develop opioid toxicity, opioid tolerance, allergic, or are unable to tolerate the side effects of morphine or other strong opioids [Table 2].[69]
Special consideration in Indian context
Methadone is very useful for managing complex chronic pain syndromes (combination of nociceptive and neuropathic), which are commonly seen in the top three cancers in India,[10] namely, cervical, head and neck, and breast cancers. The typical examples are cervical cancer with lumbosacral plexopathy and progressive oral cancer with compression on the branches of trigeminal nerve. Methadone can play a significant role if long-acting medication is needed or if the availability of morphine or cost become an issue.
ADVERSE EFFECTS OF METHADONE
S-Methadone has unique adverse effects like QT/ QTCprolongation and Serotonin syndrome. R-Methadone, a Mu agonist, exhibits similar adverse effects as other opioids, but nausea, constipation, and confusion are less common than other opioids [Table 3].[6]

How to recognize opioid-induced neurotoxicity
Opioidinduced neurotoxicity (OIN) causes symptoms such as hyperalgesia (exaggerated sensitivity to the existing pain), allodynia (normally nonnoxious stimuli resulting in a painful sensation), delirium with hallucinations, and sometimes myoclonus, leading to seizures.[111213] OIN results from the accumulation of toxic metabolites of opioids, for example, morphine-3-glucuronide in the case of use of morphine. OIN can be precipitated by factors such as impaired renal function, dehydration, electrolyte imbalances, and high-dose parenteral administration of morphine. The management of OIN is to consider hydration and if needed, opioid rotation.
METHADONE CONTRAINDICATIONS
Methadone should not be used in conditions such as patients who are hypersensitive to the active substance,[14] severe liver[15] and respiratory failure,[16] pain conditions such as mild, intermittent, or short duration pain that can be managed with other analgesics and in acute unstable pain, patients taking monoamine oxidase inhibitors (or within 14 days of such therapy) [Table 4].[17]

Use caution when using methadone in the following:
-
Severe chronic obstructive pulmonary disease
-
Significant sleep apnea
-
Prolonged QTc interval: >450 ms[1]
-
Concurrent administration of some drugs that might result in significant drug interactions or known to impact the clearance and/or protein binding
-
Drinking alcohol (may result in increased plasma levels and fatal overdose) and benzodiazepines.
METHADONE DOSING: INITIATION, TITRATION, AND MAINTENANCE
There are no conclusive prospective and comparative studies available to recommend one single best dosing strategy. Based on limited research evidence and clinical experience, the practical advice is to initiate methadone at low doses, individualize the treatment based on indication and prior opioid exposure status, titrate slow, and monitor patients for adverse effects such as sedation.[16] Before starting methadone, complete an individualized medical and behavioral risk/benefit evaluation.
Initiating methadone in opioid-naïve patients
Initiating methadone as a firstline opioid in an opioid-naïve patient is not a common practice. It is strongly recommended to consult with a pain and palliative care consultant in such situations.[1819]
Method
-
“Start low and go slow” method
-
Starting dose: Start with 2.5 mg orally every 12 or 8 h. In elderly or frail patients, start as low as 1 mg orally once per day.
-
Titration: In the outpatient setting, the dose can be increased not more 5 mg every 5–7 days, depending on the patient's response
-
Children:[20] Starting dose of 100 mμ/kg (maximum 5 mg/dose initially) every 6–8 h. Caution: A more cautious initiation of methadone is warranted in children with chronic pain, particularly in nonhospital settings. The panel suggests the use of short-acting opioids for breakthrough pain or if more rapid initial pain control is needed, rather than loading doses of methadone.
-
Titration: Increases once every 5–7 days by no >50% of the current methadone dose or based on the amount of breakthrough pain medications needed to maintain pain control.
-
Initiating methadone on patients already on opioids
-
In patients on lower doses of other opioids (e.g., <40–60 mg morphine equivalents/day), the dose initiation and titration is similar to as recommended for opioid-naive patients
-
In patients on higher doses of other opioids.
The conversion of other opioids when on higher doses to methadone should be performed carefully.[2122] Consider consultation with an experienced colleague until you have done many of these conversions. In all cases, have a colleague make the calculation as a cross-check?
Dose conversion
-
Step 1: Determine the oral morphine equivalent daily dose (MEDD). This will include the sum of baseline immediate-release/the long-acting medications as well as short-acting medications used for breakthrough pain. The total in 24 hours is the daily oral morphine equivalent dose.
-
Step 2: The MEDD is noted in a patient and appropriate conversion ratio is used to arrive at the daily methadone requirement in each patient.
Note: Methadone dose conversion is not a linear process.[23]. [Table 5]. For example, 60 mg MEDD would have a conversion ratio 4:1 (15 mg of methadone/day) and 180 mg MEDD would have a conversion ratio of 8:1 (22.5 mg methadone/day).[24]

-
Step 3: It is recommended to reduce the calculated methadone dose to further 30% to account for the incomplete cross-tolerance, especially for patients on higher MEDDs (even though the equianalgesic dose ratio (EDR) tables account for cross-tolerance).[25]
-
Opioid switch to methadone for side effects from an existing opioid and uncontrolled pain, Consult with an experienced clinician is warranted.
-
“Stop-start”/rapid switch method: Start with one-third of the calculated methadone (Step 3) dosage every 8 h. Reduce the dose of previous opioid dose by 50% and continue simultaneously with the methadone for the first 24 h. Observe the patient closely and adjust the dose as appropriate. Stable dosing may be achieved in 3–5 days. Inpatient supervision is recommended.
Or
-
Three-step slow switch method: The three-step approach is to convert the calculated equianalgesic dose of methadone gradually over 3 days. Most opioid-induced side effects are dose-related and will start to improve when only part way through the switch, often within 24 h of first 1/3 reduction. A short-acting form of the previous opioid should be available for breakthrough pain or for rescue dosing in case the anticipated methadone dose proves to be inadequate.
Calculate methadone total daily dose equivalent (Step 1, 2, 3)
-
Day 1: Reduce original MEDD by 1/3, add 1/3 as calculated methadone dose, and use original analgesic for rescue
-
Day 2: Reduce the existing MEDD analgesic by 2/3, use 2/3 of calculated methadone dose in 3 divided doses, and use original analgesic for rescue.
-
Day 3: Give total dose of methadone calculated in 3 divided doses and methadone as rescue analgesic 10% of 24 methadone dose, q3 h p. r. n.[26]
-
Other Methods[2728]: There are various other methods reported in the literature such as Edmonton method and “add low-dose methadone to existing regimen by ‘Start low and go slow’ approach (1–2.5 mg PO q8 h)” but it is strongly recommended that only experienced physicians should try such methods and only if they are comfortable.
MAINTENANCE DOSE OF METHADONE
Once patients are on a stable methadone dose, the dose should be adjusted by increments of 10%–20% every 5–7 days and not earlier. For elderly patients or patients with impaired liver function, this adjustment period should be increased.[29]
BREAKTHROUGH DOSE WHEN ON METHADONE THERAPY
Adequate treatment of breakthrough pain during the conversion and dose-titration period using agents such as nonsteroidal anti-inflammatory drug or acetaminophen or a short-acting opioid such as morphine should be considered. Nonpharmacological interventions (radiation therapy, occupational therapy, consulting, or physiotherapy consultation to explore less painful ways of movement) can reduce the need for opioid breakthrough medications.
Methadone as a breakthrough medication is not advised for both baseline therapy and incident pain because of the potential for accumulation and inadvertent methadone overdose. However, when other opioids are contraindicated, methadone is rarely used as a breakthrough medication. It is important to remember that when methadone is considered for breakthrough pain, the patient and family should be adequately trained, specially, if they are otherwise used to morphine.
Calculation of dose of breakthrough methadone
Calculate p.r.n doses as a 10% of the total daily dose. There should be no more than three breakthrough doses per day to avoid accumulation.
Example: Patient is on methadone 5 mg PO q8H (15 mg/day), 10% is 1.5 mg. The breakthrough dose would be 1.5 mg q4 h prn, maximum 3 doses per day.
MONITORING
Monitor for sedation, lethargy, confusion, and respiratory depression every 6 h for 3–6 days after initiation or dose change and daily monitoring until at least day 10. Respiratory depression risk is reported greatest from day 4 to day 6.[30]
Points to remember
The variable half-life of methadone means that some patients may not reach steady state (5 half-lives) for over 3 weeks.
Always, an effective breakthrough dose must be available.
Resist the temptation to reduce the breakthrough dose in parallel with reductions in the original opioid. Even though the original opioid is being reduced, methadone is being added, and the breakthrough dose must reflect the total opioids (i.e. the initially calculated morphine daily dose).
During the titration phase, close outpatient follow-up, daily telephone progress reports by the patient, family members, home health nurses, or hospice personnel are recommended. Patients should be informed that several titrations might be necessary to reach optimal pain control.
Outpatient initiation of methadone and rotation for cancer pain is safe and exhibits high success rates.[31]
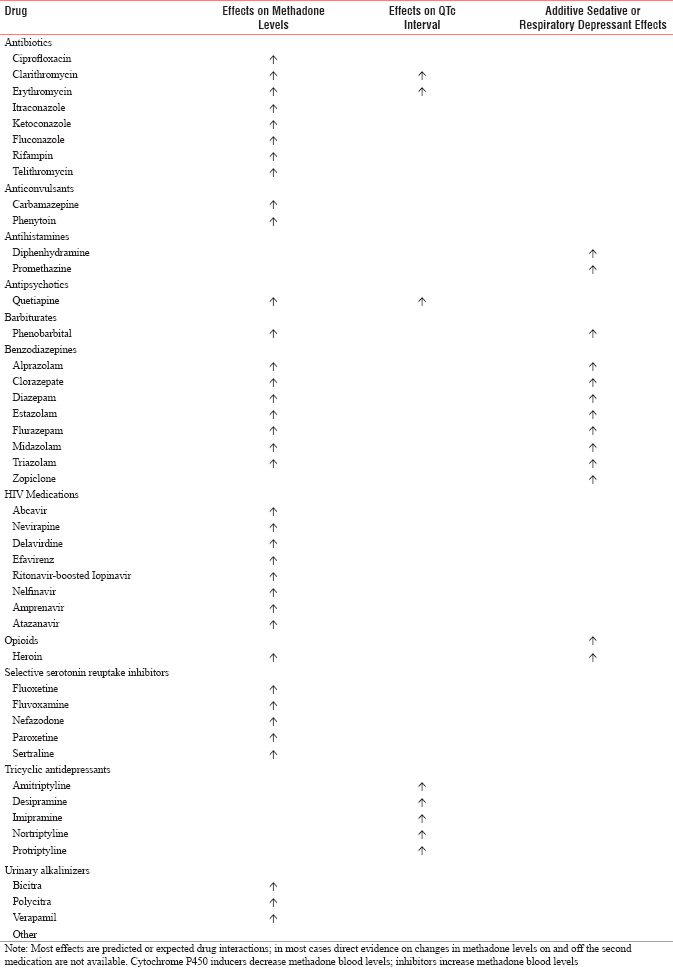
DRUG INTERACTION OF METHADONE WITH OTHER MEDICATIONS
It is recommended that clinicians review patient medications before initiation of methadone and consider discontinuation or dose reduction of medications with potential interactions or additive side effects. Drugs that can increase risk and require attention as below:
-
Alter methadone absorption, metabolism, and/or excretion, thereby changing “free methadone in circulation”
-
Additive or synergistic sedative or respiratory suppressant effects
-
Prolong QTc intervals
-
Affect the “free methadone” because they are CYP inhibitors (leading to increased levels) or CYP inducers (leading to decreased levels) [See Appendix 1].[32]
QTC INTERVAL SCREENING AND MONITORING WHEN USING METHADONE FOR PAIN MANAGEMENT
Methadone in moderate-to-high doses can prolong the QTc interval and may induce torsades de pointes (TdP). A QTc interval of >450 ms is associated with increased risk. It is recommended not to use methadone in patients with a baseline QTc interval >500 ms.[1633]
Risk factors for QT/QTc prolongation when on methadone
-
Electrolyte abnormalities such as hypokalemia or hypomagnesemia (e.g., a side effect of cis-platinum therapy or malnutrition)
-
Structural heart disease (congenital heart defects or a history of endocarditis or heart failure)
-
History of bradycardia or long QT syndrome
-
Genetic predisposition of prolonged QT syndrome or family history of prolonged QT syndrome
-
Concomitant use of drugs associated with prolongation of the QT/QTc interval
-
Concomitant use of some drugs which may raise the serum methadone level, either through reduced methadone clearance or its displacement from plasma proteins
-
Impaired liver function
-
High doses of methadone.
Guidance for QTc interval screening
In patients without new risk factors for QT/QTc interval prolongation, an electrocardiogram (ECG) within the previous 3 months with a QTc < 450 ms can serve as the baseline study.[1633]
Consider baseline ECGs when
-
Patient has risk factors for QTc interval prolongation
-
Any previous ECG shows a QTc >450 ms
-
A history of ventricular arrhythmia.
Monitoring and follow-up ECGs when
-
There are baseline ECG findings
-
Methadone dose is changed
-
There are risk factors for QTc interval prolongation such as the addition of a medication which can prolong QTc interval.
If the QTc interval is found to be ≥500 ms
-
Switch to a different opioid
-
Immediately lower the methadone dose
-
Evaluate and correct reversible causes of QTc interval prolongation
-
Repeat the ECG after lowering the methadone dose.
If the QTc interval is found to be ≥450 ms but < 500 ms
-
Consider switching to an alternative opioid
-
Lowering the methadone dose (otherwise, discuss with the patient the potential risks of continuing methadone therapy)
-
Evaluate and correct reversible causes of QTc interval prolongation
-
Repeat the ECG after lowering the methadone dose.
Electrocardiograms monitoring when using methadone in advanced illness situation
In advanced illness and end-of-life-care situations, when the goal of care is comfort care, there should be informed discussion with the patient and the family members about the potential risk for cardiac arrhythmia, and it is recommended to initiate/continue with methadone treatment without subjecting the patient to unnecessary investigations which may affect the quality of life.
METHADONE OVERDOSE
Methadone toxicity may manifest many hours or days after a dose change. Main protection against overdose is vigilance and education. Methadone overdose occurs under the following circumstances:
-
Intentional overdose
-
Unintentional, for example, drug interaction, error in dose calculation, rapid dose escalation, and frequent use of breakthrough doses.
Diagnosis
Suspect methadone overdose when patient exhibits excess somnolence, pinpoint pupils, respiratory rate < 8/min, systolic blood pressure < 90 mmHg, or 20% less than baseline.
Management
Methadone overdose is reversed with naloxone.
Dose
Mix one ampule (0.4 mg) of naloxone in 9 ml of sterile normal saline (0.04 mg naloxone/ml). Administer 1 ml of diluted naloxone every 2–4 min until patient recovers from clinical signs of overdose. Once a conscious level is achieved, repeated naloxone dosing (or an infusion titrated to effect) may be required for some time due to the slow clearance of methadone.
Caution
Care should be taken to administer minimum safe dose. Complete reversal of methadone may precipitate pain crisis.
SWITCHING METHADONE TO ANOTHER OPIOID
Switching methadone to alternative safe opioids may be required in the event of unacceptable side effects or unresolved pain. The equivalent dose ratio when switching from methadone to another opioid is not a reverse process. There is no acceptable standard conversion strategy for switching from methadone to another opioid. A proposed safe and conservative approach is a 1:3 methadone to morphine ratio (10 mg methadone/day = 30 mg oral morphine/day).[253435]
METHADONE FORMULATION IN INDIA
Methadone is available as liquids and tablets. The liquid contains 150 ml and contains 1 mg/ml. The tablet is available as 5 mg strength. It can be administered orally, sublingually, or buccally.
PATIENT EDUCATION AND COUNSELING
Before prescribing methadone, educate and counsel patients about the indications for treatment, goals of therapy, and possible adverse events – either face to face or by phone. There should be a commitment from the patient and the family about the need for adherence to treatment, monitoring, reporting, and follow-up. It is important that the patient or family learn accurate dose measurement using a syringe (for liquid methadone) or the tablet. They should shake bottle of liquid before use and use same, accurate measuring device for liquid methadone. Patients should be instructed to call the prescribing physician if there is a change in status such as the need to use >4 or 5 breakthrough opioid doses/day.[16]
The patient information sheet should carry the following instruction:
-
Methadone must be taken only as per the prescription. Never take extra doses without approval from your doctor
-
Taking methadone frequently as other opioids may prove fatal
-
Use sedating medications such as benzodiazepines with caution and only as directed by your doctor
-
The use of illicit drugs and/or alcohol with methadone may be fatal
-
It usually takes 5–7 days to build up the dose and to see the full effects and drowsiness may appear even after a few days
-
Tell all your doctors that you are taking methadone. Adding medications (including use of grapefruit) or changing dosing of other medications can affect methadone and should be coordinated with the doctor who prescribes methadone
-
Avoid activities requiring mental alertness or coordination (such as driving or using machinery) until the effects of methadone are achieved which usually takes a week or longer
-
Rise slowly from a sitting/supine position, as methadone may cause dizziness initially.
-
Methadone, like other opioids, can cause significant constipation. Take your prescribed laxative as directed
-
Report any of the following symptoms immediately and/or seek urgent/emergent care: dizziness or lightheadedness, irregular heartbeat (palpitations), falls or near falls, chest pain/pressure, and shortness of breath.”
METHADONE STORAGE AT HOME
Patients at home should be instructed to take following precautions:[36]
-
Store methadone in a container clearly labeled
-
Keep container in a safe place and away from children and pets
-
Keep liquid methadone solutions in a locked box in the fridge or at room temperature as appropriate for the formulation.
SUMMARY
Methadone is an effective analgesic for the management of chronic complex pain such as chronic neuropathic pain syndromes seen in cancer when not responding to other strong opioid medications such as morphine or fentanyl. The distinct pharmacokinetic and pharmacodynamic properties of methadone require caution when a patient is initiated, titrated, and maintained on methadone. The equianalgesic dose of methadone shows variability. Use MEDD to methadone conversion ratios or nomogram before titration. Methadone interacts with many medications, resulting in serious adverse reactions and be cautious. There have been reports of sudden deaths with the use of methadone due to its QT prolongation and TdP properties, particularly in higher dosage. Health-care professionals prescribing methadone should undergo special training in its usage and should strictly adhere to recommendations to avoid serious adverse events.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Available from: http://www.palliumindia.org/2014/05/good-newsabout-methadone/
- Available from: http://www.cbn.nic.in/html/END05052015.pdf
- Methadone as an additional opioid for a cancer patient with severe neuropathic and bone pain not responsive to other opioids and adjuvant analgesics. J Palliat Care. 2013;29:119-21.
- [Google Scholar]
- 1986. World Health Organization. WHO's Pain Relief Ladder. Available from: http://www.who.int/cancer/palliative/painladder/en/
- Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “best practices” for opioid rotation: Conclusions of an expert panel. J Pain Symptom Manage. 2009;38:418-25.
- [Google Scholar]
- End-stage renal disease: Symptom management and advance care planning. Am Fam Physician. 2012;85:705-10.
- [Google Scholar]
- Pain & Analgesics. In: Downing GM, Wainwright W, eds. Medical Care of the Dying (4th ed). Victoria, B.C. Canada: Victoria Hospice Society Learning Centre for Palliative Care; 2006. p. :189-251.
- [Google Scholar]
- Opioid side effects and special situations. In: Managing Cancer Pain – The Canadian Health Care Professional's Reference. Toronto, Ontario: Healthcare & Financial Publishing, Rogers Media; 2005. p. :42-8.
- [Google Scholar]
- Cancer pain. 2003. JAMA. 290:2476-9. 14.15 Available from: http://www.medicine.iupui.edu/clinpharm/ddis/clinical-table/
- [Google Scholar]
- Methadone for relief of cancer pain: A review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73-83.
- [Google Scholar]
- Acute pain management pharmacology for the patient with concurrent renal or hepatic disease. Anaesth Intensive Care. 2005;33:311-22.
- [Google Scholar]
- Methadone safety: A clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15:321-37.
- [Google Scholar]
- Available from: http://www.medicine.iupui.edu/clinpharm/ddis/clinical-table/
- Evidencebased Recommendations for Medical Management of Chronic Nonmalignant Pain: Reference Guide for Physicians. The College of Physicians and Surgeons of Ontario; November. 2000
- [Google Scholar]
- Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-30.
- [Google Scholar]
- World Health Organization: Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses. Available from: http://www.who.int/medicines/areas/quality_safety/guide_perspainchild/en/
- [Google Scholar]
- Morphine to methadone conversion: An interpretation of published data. Am J Hosp Palliat Care. 2011;28:135-40.
- [Google Scholar]
- Switching from morphine to oral methadone in treating cancer pain: What is the equianalgesic dose ratio? J Clin Oncol. 1998;16:3216-21.
- [Google Scholar]
- Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-87.
- [Google Scholar]
- Primer of Palliative Care (6th ed). Chicago, IL: American Academy of Hospice and Palliative Medicine; 2014.
- Dose ratios between high dose oral morphine or equivalents and oral methadone. J Palliat Med. 2013;16:947-50.
- [Google Scholar]
- Role of methadone in the management of pain in cancer patients. Oncology (Williston Park). 1999;13:1275-82.
- [Google Scholar]
- Low-dose methadone has an analgesic effect in neuropathic pain: A double-blind randomized controlled crossover trial. Palliat Med. 2003;17:576-87.
- [Google Scholar]
- Available from: https://www.cpsbc.ca/files/pdf/MMP-Analgesia-Guidelines.pdf
- On Behalf of the CPSBC. Recommendations for the Use of Methadone for Pain. Vancouver, BC, Canada; February 2010
- College of Physicians and Surgeons of BC. Recommendations for the Use of Methadone for Pain; March. 2005
- [Google Scholar]
- Methadone initiation and rotation in the outpatient setting for patients with cancer pain. Cancer. 2010;116:520-8.
- [Google Scholar]
- Available from: https://www.cpsbc.ca/files/pdf/MMP-Analgesia-Guidelines.pdf
- Available from: https://www.cpsbc.ca/files/pdf/MMP-Analgesia-Guidelines.pdf
- Demystifying Opioid Conversion Calculations: A Guide to Effective Dosing. Bethesda, MD: ASHP; 2010.
- [Google Scholar]
- Switching from methadone to a different opioid: What is the equianalgesic dose ratio? J Palliat Med. 2008;11:1103-8.
- [Google Scholar]
- Safe storage of methadone in the home – An audit of the effectiveness of safety information giving. Harm Reduct J. 2005;2:9.
- [Google Scholar]
Appendix 1: Selected Methadone Drug-Drug Interactions