Translate this page into:
Profile of Malignant Spinal Cord Compression: One Year Study at Regional Cancer Center
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objectives:
Malignant spinal cord compression is an oncologic emergency, unless diagnosed early and treated appropriately, can lead to permanent neurological impairment and compromised quality of life of patients. We analyzed the epidemiology and the effect of common interventions on the outcome in these patients.
Patients and Methods:
We conducted a prospective study of 77 patients in the year 2014 and recorded relevant patient and disease characteristics. All patients received corticosteroids. Eight patients were operated upon, and radiotherapy was delivered in 62 patients.
Results:
Most of the patients were in the age group of 41–60 years and there was no gender preponderance in patients. Female breast cancer was the most common incident (15.5%) malignancy followed by multiple myeloma, lung, and prostatic carcinoma. Lower dorsal spine was the most common site of compression (35%) followed by lumbar (31%) and mid-dorsal (26%) spine. 70 (91%) patients had cord compression subsequent to bone metastasis while as other patients had leptomeningeal metastasis. In 31 (40%) patients, spinal cord compression was the presenting symptom. Overall, only 26 patients had motor improvement after treatment.
Conclusion:
Grade of power before treatment was predictive of response to treatment and overall outcome of motor or sensory functions. Neurodeficit of more than 10 days duration was associated with poor outcome in neurological function.
Keywords
Cord compression
Motor function
Patient outcome
Radiotherapy
INTRODUCTION
Metastatic malignant spinal cord compression is one of the major causes of morbidity and significantly compromises the quality of life in patients with cancer. Although true incidence of spinal cord compression in cancer patients is unknown, between 5% and 10% of cancer patients will develop metastatic spinal cord compression.[1] It is an oncologic emergency requiring early diagnosis and immediate treatment. The outcome of treatment is often poor, and <50% of patients are ambulatory and about two-fifths require a permanent urinary catheter.[2] Neurological function at presentation is an important prognostic factor for functional outcome. Primary treatment is often selected depending on the patient's performance status, prognosis, and histological type of primary neoplasm. Most patients are not suitable for surgery. External beam radiotherapy has remained the mainstay of treatment in these patients. However, there is no clear consensus on the best radiotherapy dose and fractionation.[3] At our center, we treat approximately four thousand cancer patients per year, and spinal cord compression is the most common oncological emergency.
PATIENTS AND METHODS
We prospectively recruited all patients with spinal cord compression due to malignancy who presented in the year 2014. Only those patients were enrolled who had histological documentation of malignancy (tissue diagnosis before or after cord compression) and magnetic resonance imaging (MRI) documented spinal cord compression. Patients were interviewed at the time of registration with a structured questionnaire including details of the occurrence and time of onset of back pain, paresthesia, weakness, and bladder dysfunction. The date of onset of symptoms of spinal cord compression was recorded by history taking supplemented by cross-checking all patient records. Delays were expressed in terms of whole days. Neurological status was documented when the patients reported to our outpatient department and graded as follows: Motor function (0 - no contraction; 1 - flicker or trace of contraction; 2 - active movement possible only with gravity eliminated; 3 - active movement against gravity but not resistance; 4 - active movement against resistance and gravity; and 5 - normal power). Sensory symptoms and signs along with bladder and bowel function were also recorded. All data related to patients were well-maintained in the files at hospital-based cancer registry (HBCR). All patients received corticosteroids in the form of dexamethasone (16–24 mg in divided doses). All patients were assessed jointly by oncologists and neurosurgeons in the hospital for feasibility of surgery for rapid decompression. Radiation was delivered in three protocols (45 gray in 25 fractions, 30 gray in 10 fractions, and 20 gray in 5 fractions) depending on the overall assessment of the patient and expected outcome. All data were recorded and analyzed in SPSS for Windows version 16.0 (Chicago, SPSS Inc.).
RESULTS
In 2014, we registered 3940 new cancer patients at our Regional Cancer Center (now State Cancer Institute). Seventy-seven patients fulfilled the eligibility criteria for enrollment in this study. Less stringent eligibility criteria could have increased the enrollment (in some cases, there were clinical features of cord compression, but MRI scans were not available/could not be done); hence, those patients were excluded from this study. Most of the patients were in the age group of 41–60 years [Table 1] and there was no sex preponderance in patients [Table 1].

Female breast cancer was the most incident (15.5%) malignancy to cause spinal cord compression [Table 2] which was followed by multiple myeloma, lung, and prostatic carcinoma. Spinal cord compression presented as metastasis of unknown origin in 8 (10%) patients. Carcinoma esophagus was the primary site of disease in 5 (6.4%) patients.
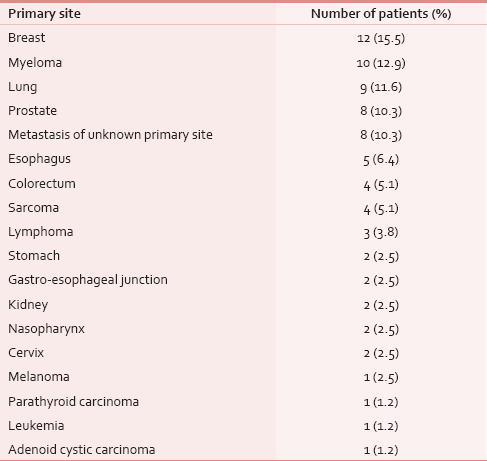
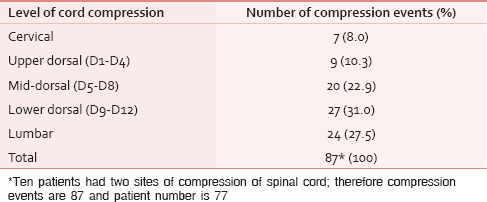
Lower dorsal spine was the most common site of compression (35%) followed by lumbar (31%) and mid-dorsal (26%) spine [Table 3]. Most patients had a single level (87%) of cord compression and only 10 (13%) patients presented with multiple levels of cord compression, usually a combination of mid and lower dorsal spine. There was no relation between primary malignancy and the site of cord compression.
Seventy (91%) patients had bone metastasis and subsequent compression of cord. Other patients had compression due to leptomeningeal metastasis. Eighteen (23%) patients had synchronous liver, lung, and brain metastasis at the time of cord compression. Solitary bone lesion causing cord compression was found in 28 (36%) patients.
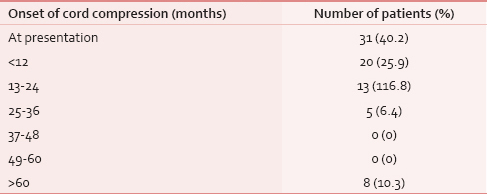
In 31 (40%) patients, spinal cord compression was a presenting symptom (among other symptoms) and among these, in 8 (10%) patients, no site of the primary could be located with routine diagnostic tests [Table 4]. Most compression events occurred in the first 2 years of diagnosis. Most patients presented to our clinic late from the onset of symptoms with a range of 1–90 days (mean delay of 10 days and median delay of 7 days).
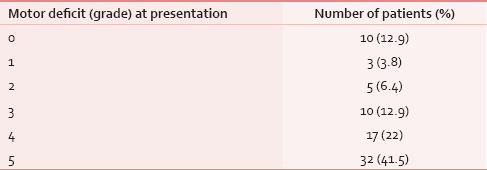
Sixty-one patients (79%) presented with pain localized to the site of metastasis and only 14 (18%) patients presented with weakness of limbs without pain. Complete paraplegia was present in 10 (13%) of patients [Table 5]. Sensory loss was present in only 10 (13%) and bladder involvement in 17 (22%) patients at presentation.
All patients received corticosteroids (16–24 mg in divided doses). A rapid decompression was done only in 8 (10%) patients in Neurosurgery Department [Table 6]. Radiotherapy was delivered in 62 (81%) patients [Table 6]. No surgery or radiation therapy treatment was delivered in 12 patients due to poor prognostic features and low performance score. Three patients defaulted.
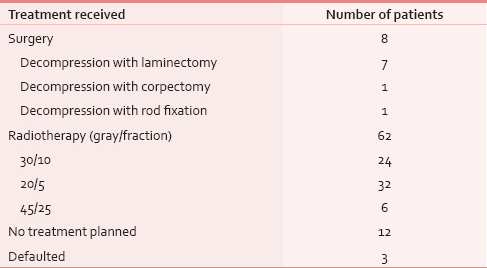
Of 62 patients who received radiotherapy, only 26 patients had motor improvement. An analysis of these patients revealed that there was complete recovery of power in only 13 patients, and further analysis revealed that all these patients had either Grade 3 or Grade 4 power before treatment. A maximum net gain of motor function was three grades of power and was found in two patients. In other words, no patient with Grade 0 or 1 power recovered fully. There was no improvement in eight patients and deterioration was recorded in three patients. Thus, grade of power before treatment was predictive of response to treatment and overall outcome of motor or sensory functions. Delay of treatment by more than 10 days was associated with poor outcome in neurological function.
DISCUSSION
Spinal cord compression due to malignancy is an oncological emergency which, unless diagnosed early and treated promptly, can lead to permanent neurologic impairment and can seriously affect a patient's quality of life.[4] However, most patients report late and land up in permanent damage to the cord. In the current study, we prospectively reviewed the clinical features and treatment outcomes of spinal cord compression due to malignancy. Most compression events occurred in the first 2 years of diagnosis. Most patients (79%) presented with pain localized to the site of metastasis and only 14 (18%) patients presented with weakness of limbs without pain. All 77 patients in our study received high-dose dexamethasone with gastroprotection by proton pump inhibitors. Steroids were started in patients with a high suspicion or immediately after the diagnosis of cord compression. The primary objective of corticosteroid therapy is reduction of edema and inflammation at the site of spinal cord compression. The efficacy of steroids in the management of cord compression in cancer patients has been demonstrated in terms of pain relief and motor function.[56] The distribution of cord compression among various malignancies correlated with the most incident cancers that were registered at our HBCR. Metastatic cord compression was most commonly found in female breast cancer, myeloma, and lung cancer. Our previous work revealed that breast cancer is one of the most common cancers in Kashmiri population among females and lung cancer is the most common malignancy in adult men.[7]
Lower dorsal spine was the most common site of compression (35%) followed by lumbar (31%), and mid-dorsal (26%) spine and only 7 (9%) had compression in cervical spine. The high incidence of compression along lower dorsal and lumbar regions may be due to the high incidence of metastasis at these sites and/or due to progressively increasing weight bearing craniocaudally and subsequent fracture of vertebrae. Moreover, the location of the metastases is also proportional to the volume or mass of bone in each region and is in accordance with other study conducted by Schiff.[8] In 10 (13%) patients, there was a synchronous second site of cord compression.
We found that synchronous liver, lung, brain, and other site metastasis were present in 18 (23%) patients at the time of cord compression. Solitary bone lesion causing cord compression was found in 28 (36%) patients. In many tumors such as breast cancer, bone only metastasis has a better prognosis[9] and visceral metastasis carries a worse outcome. Median survival after spinal cord compression depends on the number of metastases, the type of primary tumor, and the patient's functional status. It has been seen that certain subgroup of patients with spinal cord compression have survival advantage like female gender, radiosensitive tumors with a single metastasis, patients with myeloma, cancer of the breast or prostate.[1011] Patients with multiple metastases, visceral or brain metastases, and lung or gastrointestinal cancers have the shortest survival.[10] Spinal cord compression can be a presenting symptom of malignancy. Our findings reveal that in 31 (40%) patients spinal cord compression was a presenting symptom. We could not locate the site of primary tumor in 8 (10%) patients with routine diagnostic tests. No positron emission tomography scan facility is available at our center and could not be done in any of these patients.
The duration of motor dificult is also an important prognostic factor. Though malignant spinal cord compression is widely recognized to be a medical emergency, but we observed that majority of patients presented late from the onset of symptoms with mean delay of 10 days. Delay in diagnosis and treatment has been associated with poor outcome. We observed that a delay of treatment more than 10 days was associated with poor outcome in neurological function. For physicians, the index of suspicion should be high for spinal cord compression in a known patient of malignancy who presents with pain in the back which may lead to more prompt diagnosis and treatment.
Neurosurgical intervention was done in only 8 (10%) patients. Only those patients were operated in whom cord compression was a presenting symptom. Although most patients were not fit to undergo surgery, but there was general tendency of underutilization of surgical intervention when patients had an established malignancy. Most patients received radiation therapy, and we employed three protocols depending upon the performance status, metastatic pool and expected outcome in neurological function. In majority of patients, we used 20 gray in 5 fractions, and we did not find any difference in outcome with the different fractionation schedules of radiotherapy. Systematic reviews give no clear consensus on the best radiotherapy dose and fractionation.[3] However, Rades et al. showed that compared with short-course, long course radiotherapy resulted in significantly better 1-year local control.[12]
CONCLUSION
Quality of life of patients with spinal cord compression is directly related to their ambulatory status. High index of suspicion, early diagnosis and prompt intervention is the key to achieve a better outcome. Radiotherapy remains an important tool in the management of metastatic cord compression, if promptly instituted, especially in patients with some residual neurological function and duration of <10 days. Research is needed to develop and validate educational tools that will alert patients, their families, and physicians which may lead to early diagnosis and treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Clinical approach to metastatic epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1297-305.
- [Google Scholar]
- Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107:37-43.
- [Google Scholar]
- Metastatic spinal cord compression: Review of the evidence for a radiotherapy dose fractionation schedule. Clin Oncol (R Coll Radiol). 2010;22:222-30.
- [Google Scholar]
- Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39:1255-7.
- [Google Scholar]
- Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: A randomised trial. Eur J Cancer. 1994;30A:22-7.
- [Google Scholar]
- Cancer in Kashmir, India: Burden and pattern of disease. J Cancer Res Ther. 2012;8:243-6.
- [Google Scholar]
- Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favorable prognosis. Cancer. 1986;58:178-82.
- [Google Scholar]
- Prognostic factors in metastatic spinal cord compression: A prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46:1163-9.
- [Google Scholar]
- Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76:1453-9.
- [Google Scholar]
- Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79:524-30.
- [Google Scholar]






