Translate this page into:
Scrambler Therapy Enhances Quality of Life in Cancer Patients in a Palliative Care Setting: A Randomised Controlled Trial
*Corresponding author: Sushma Bhatnagar, Department of Oncoanaesthesia and Palliative Medicine, Dr. B. R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India. sushmabhatnagar1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kashyap K, Singh V, Dwivedi SN, Gielen J, Bhatnagar S. Scrambler therapy enhances quality of life in cancer patients in a palliative care setting: A randomized controlled trial. Indian J Palliat Care 2022;28:287-94.
Abstract
Objectives:
Given the known side effects of opioids and the negative impact of these side effects on quality of life (QOL), there is a need for therapies that can reduce opioid intake and improve QOL in patients suffering from cancer pain. Scrambler therapy (ST) is a neuromodulatory therapy that has been shown to reduce cancer pain, but its effect on QOL is not well understood. This study intended to evaluate the efficacy of ST for enhancing QOL in cancer patients through minimising pain and opioid intake.
Material and Methods:
This was a randomised controlled trial including 80 patients with head, neck and thoracic cancer. In both arms, patients were given pain management drugs following the WHO analgesic ladder for ten consecutive days. In the intervention arm each day ST was given. Pain, morphine intake, and QOL (WHOQOL-BREF) were assessed.
Results:
All domains of QOL improved significantly in the intervention arm in comparison to the control arm. In comparison to baseline, pain improved in both the intervention and the control arm on day 10 and at follow-up. However, QOL significantly improved in the intervention arm, while morphine intake decreased. In the control arm, QOL deteriorated, while morphine intake increased.
Conclusion:
ST significantly improved QOL. Since the increase in QOL took place along with a significantly lower morphine intake, the improvement in QOL may not only be explained by lower pain scores but, also, by a reduced intake of morphine, because the lower dosages of morphine will decrease the likelihood of side effects associated with the drug.
Keywords
Scrambler therapy
Quality of life
Breast cancer
Head and neck cancer
Lung cancer
INTRODUCTION
Cancer and its treatment can cause pain and symptoms that have a debilitating effect on patients’ lives. When pain and symptoms are properly managed, patients may regain lost capacities such as performing activities of daily living, a sound sleep, or simply enjoying life. From this perspective, it is not surprising that quality of life has been suggested as an important outcome measure in studies related to pain management.[1] This is in line with the concept of total pain which includes attention to social, psychological, and spiritual aspects of pain in addition to physical pain and the effect of all these aspects on quality of life.[2]
Assessment of the quality of life is of particular importance within the context of head and neck and thoracic cancer which are known to often have a severe negative impact on quality of life. For instance, cancer of the head and neck can lead to severe disability which can have a substantial impact on the patient’s quality of life. In general, head and neck cancer (HNC) patients have impaired quality of life (QOL) around the time of diagnosis. Functioning domains and symptoms show considerable deterioration with treatment, followed by a pattern of some recovery after 3–6 months.[3] Investigators have found that even after cancer treatment there is a considerable prevalence of problems related to swallowing, speech, chewing, pain, aesthetics, social eating and dryness of the mouth.[3] Moreover, there is a clear association between impaired physical functioning and psychological distress as seen in depressive symptoms, social anxiety and perceived neck function.[4] Besides that, major depressive disorder in patients with HNC ranges from 11 to 52%.[5] Outcomes such as these (social anxiety, depression and physical functioning) have a strong bearing on the quality of life. Similarly, the negative impact of thoracic cancers such as lung cancer and breast cancer on quality of life is well known and has been studied and described in populations across the world.[6-10]
Cancer pain is an important contributing factor to decreased QOL. As pain increases, it starts to interfere with various aspects of daily life, such as appetite, sleep, mood, or basic task such as walking. When the impact of pain on life continues and increases, the feeling of discomfort and the inability to do things that the patient considered important, may lead to depression.[11] The main effect of pain may be on the quality of life (QOL) and several studies have shown that increased pain is associated with deteriorating scores in all dimensions of QOL.[12,13] However, this does not mean that pain management, even when successful in defeating physical pain, will necessarily improve quality of life. Opioids, which remain the most effective drug for cancer pain management,[14] illustrate this. Many cancer patients and cancer survivors require chronic opioid therapy which lasts for more than 3 months.[14] Morphine is the most favoured for severe pain followed by tramadol for mild to moderate and Non steroidal anti-inflammatory drugs (NSAIDs), adjuvants for mild pain.[15] Unfortunately, chronic opioid therapy has been associated with side effects such as the increased risk of depression, constipation, impaired wound healing, fuzzy-headedness, nausea, sedation, dizziness, vomiting, physical dependence, tolerance and respiratory depression.[16,17] Lesser-known side effects are delayed gastric emptying, hyperalgesia, immunologic and hormonal dysfunction, muscle rigidity, and myoclonus.[16] Side-effects such as these can have a profound negative impact on quality of life and because of these side-effects of opioids, the search for non-pharmacological treatment modalities has intensified.[14] Neuromodulatory techniques such as transcutaneous electrical nerve stimulation and scrambler therapy (ST) have gained popularity in recent times. Unfortunately, scientific data supporting these methods is still limited.[18,19]
ST was introduced in the early 2000s and has been used in the treatment of cancer pain since then. ST uses a device, which produces sensations (signals) with the help of five artificial neurons that appear to be “self ” generated within the body. These artificially generated signals replace pain signals before they reach the central nervous system, by artificially generated non-pain signals.[20] The non-invasive treatment nature, ease of use, excellent safety profile and very limited side effects make it a very desirable complementary technique for cancer pain treatment. Pain relief associated with ST has been found significant and long-lasting among various groups of patients,[14] including cancer patients.[21-23] The large majority of the available studies on ST for the management of cancer pain have concluded that it is an effective therapy.[21,23] A randomised controlled trial among patients suffering from thoracic and HNC, recently came to the same conclusion.[24] However, data on the effect of ST on quality of life, particularly among cancer patients, are more sketchy.[25-28] Therefore, this study intended to assess the effect of ST on the quality of life of patients suffering from thoracic and HNC. The secondary aim of this study was to understand the potential impact of quality of life by studying it along with evolutions in physical pain and morphine intake. The hypothesis that informed this study was that, in comparison to the control arm, QOL outcomes in the intervention arm would be significantly better due to a more substantial decrease in pain and/or a decrease in morphine intake. The emphasis of this study is on the important impact of ST on QOL rather than pain, which has been studied elsewhere.[24]
MATERIAL AND METHODS
This was an open-label parallel design randomised control trial. The study was conducted in 2016–2017 in the Palliative Care Unit of Dr. B.R.A. IRCH, All India Institute of Medical Sciences, New Delhi (India) and was approved by the ethical committee (IRB) of the institute. The study was registered in the clinical trial registry in India (CTRI acknowledgment number Ref/2015/08/009516). The protocol of this study including the power of study, randomisation and blinding, has been described in detail elsewhere.[24] Included patients who had been diagnosed with head, neck and thoracic cancers (breast cancer and lung cancer) and were experiencing persistent pain of oncological origin, with a numerical rating scale (NRS) of more than 4. A total of 80 patients suffering from cancer pain, with pain intensity, measured more than four on the NRS scale were included in the study with 40 each in the intervention and control arm. Patients were randomised into the arms using a computer-generated random sequence. In both the arms, patients received pain management drugs for 10 days, as per standard protocol based on the WHO ladder. In the intervention group, in addition to the standard treatment, patients received ST for 40 min on each of these days.
The investigators assessed three outcome measures: Physical pain, intake of tramadol and morphine and QOL at baseline, on the 10th day of therapy, and at follow-up (FU). Before each treatment session, the outcome measure of physical pain was assessed with the NRS, which is a numeric scale on which patients indicate pain intensity by mentioning a number ranging from 0 to 10 with 0 meaning “no pain” and 10 meaning “worst possible pain.”[29] The investigators also recorded the prescribed dose of oral opioids. For each patient, the total dose (mg) per day was calculated for morphine and tramadol.
QOL was assessed with WHOQOL BREF on the 1st day, 10th day and at FU (1 week after the last therapy session). WHOQOLBREF is a briefer version of the extensive WHOQOL-100 and is recommended in situations where it is essential to reduce the burden on respondents. WHOQOL-BREF comprises four domains: Physical health (seven items), psychological health (six items), social relationships (three items) and environment (eight items). Based on a participant’s answers a score is computed for each domain. The domains are preceded by two individual items that enquire about, respectively, how the participants rate their quality of life and how satisfied they are with their health. WHOQOL-BREF is a widely used measure for quality of life, including that of cancer patients.[30-32] An international field trial, which included data from New Delhi, observed that the measure has good psychometric characteristics.[33] Previously, a study in which the Hindi version of WHOQOL-100 was assessed in New Delhi, had already concluded that WHOQOLBREF in Hindi is an adequate measure.[34]
Statistical analysis
To find out the difference in QOL between the arms, the t-test/Wilcoxon test was used. Differences in QOL over time (baseline, 10th day, FU) were assessed through repeated measure Analysis of variance (ANOVA). To find the correlation of changes in Morphine dose with changes in QOL, Pearson’s correlation or Spearman rank correlation was used. P < 0.05 was considered statistically significant. STATA/ SE version 14.2 (StataCorp LP, College Station, TX, USA) was used for the statistical analysis.
RESULTS
There were no clinically relevant baseline differences between the patients in both arms.[24] The differences between both arms regarding the four WHOQOL-BREF domains and overall QOL measured at baseline, day 10, and FU was compared using a two-sample t-test/t-test or Wilcoxon rank-sum test. The results of these tests are presented in [Table 1] along with the mean and median quality of life in all four domains and overall QOL. The table shows that the differences in quality of life at baseline were not statistically significant. However, the table also shows that the differences in quality of life on the 10th day were highly significant for all domains (with P < 0.001 for domains 1, 2 and 4 and P = 0.03 for domain 3) and overall QOL (P < 0.001). At FU, the differences in quality of life at FU were highly significant for all domains and overall QOL (P < 0.001).
| WHO QOL baseline | Control | Intervention | P-value |
|---|---|---|---|
| Domain 1 | |||
| Mean (SD) | 36.696 (12.990) | 34.107 (8.784) | 0.2996 |
| Median (Q-range) | 39.286 (28.57–39.286) | 32.143 (28.57–32.140) | |
| Domain 2 | |||
| Mean (SD) | 41.458 (14.246) | 39.791 (10.838) | 0.5577 |
| Median (Q-range) | 41.666 (31.25–41.666) | 41.666 (33.33–41.666) | |
| Domain 3 | |||
| Mean (SD) | 55.417 (14.805) | 50.833 (11.448) | 0.1255 |
| Median (Q-range) | 50 (50–50) | 50 (41.666–50) | |
| Domain4 | |||
| Mean (SD) | 46.64 (10.14) | 44.921 (8.028) | 0.4033 |
| Median (Q-range) | 46.875 (40.625–46.875) | 46.875 (40.625–46.875) | |
| Overall | |||
| Mean (SD) | 72.43 (9.04) | 69.85 (5.91) | 0.224 |
| Median (Q-range) | 71 (68–74) | 70 (65–73) | |
| WHO QOL Last Visit (Day 10) | Control | Intervention | P-value |
| Domain 1 | |||
| Mean (SD) | 32.768 (10.29) | 60.256 (7.517) | <0.001 |
| Median (Q-range) | 33.928 (25–33.928) | 60.714 (53.57–60.71) | |
| Domain 2 | |||
| Mean (SD) | 35.31 (13.44) | 58.974 (8.47) | <0.001 |
| Median (Q-range) | 33.33 (29.166–33.33) | 58.33 (54.166–58.33) | |
| Domain 3 | |||
| Mean (SD) | 53.33 (13.84) | 59.188 (9.325) | 0.0309 |
| Median (Q-range) | 50 (50–50) | 58.333 (50–58.33) | |
| Domain 4 | |||
| Mean (SD) | 44.218 (9.376) | 52.484 (6.69) | <0.001 |
| Median (Q-range) | 43.75 (37.5–43.75) | 50 (46.875–50) | |
| Overall | |||
| Mean (SD) | 67.83 (7.77) | 81.56 (5.67) | <0.001 |
| Median (Q-range) | 67 (64–70) | 80 (78–86) | |
| WHO QOL FU | Control | Intervention | P-value |
| Domain 1 | |||
| Mean (SD) | 34.46 (11.48) | 67.03 (7.749) | <0.001 |
| Median (Q-range) | 35.71 (26.785–35.714) | 67.857 (64.285–67.857) | |
| Domain 2 | |||
| Mean (SD) | 33.02 (12.567) | 65.064 (8.089) | <0.001 |
| Median (Q-range) | 33.33 (25–33.33) | 66.666 (58.33–66.666) | |
| Domain 3 | |||
| Mean (SD) | 53.54 (14.60) | 63.03 (9.127) | <0.001 |
| Median (Q-range) | 50 (50–50) | 66.666 (58.33–66.666) | |
| Domain 4 | |||
| Mean (SD) | 42.109 (9.702) | 55.769 (7.838) | <0.001 |
| Median (Q-range) | 40.625 (37.5–40.625) | 53.125 (50–53.125) | |
| Overall | |||
| Mean (SD) | 65.95 (7.72) | 84.64 (6.31) | <0.001 |
| Median (Q-range) | 65.5 (61.25–69) | 84 (81–89) |
[Graph 1] compares the quality of life in the four domains as well as the overall QOL of the control arm versus the intervention arm at baseline, day 10 and FU. The graph shows that for all domains as well as overall QOL, at baseline, the mean quality of life in both arms was largely similar, with the control arm scoring slightly higher in all domains. However, on day 10 and again at FU, the intervention arm’s mean quality of life for all domains and overall QOL was higher than the control arm’s means.

- 4 Domains of QOL on Day 1, Day 10 and FU. D1: Domain 1 (physical health); D2: Domain 2 (psychological health): D3: Domain 3 (social relationships); D4: Domain 4 (environmental health); OQ: Overall QOL.
In both arms, quality of life evolved throughout the treatment. [Graph 2] represents the evolutions in quality of life in each domain for both arms. Interestingly, for each domain, quality of life improved for the intervention arm, while it decreased for the control arm.
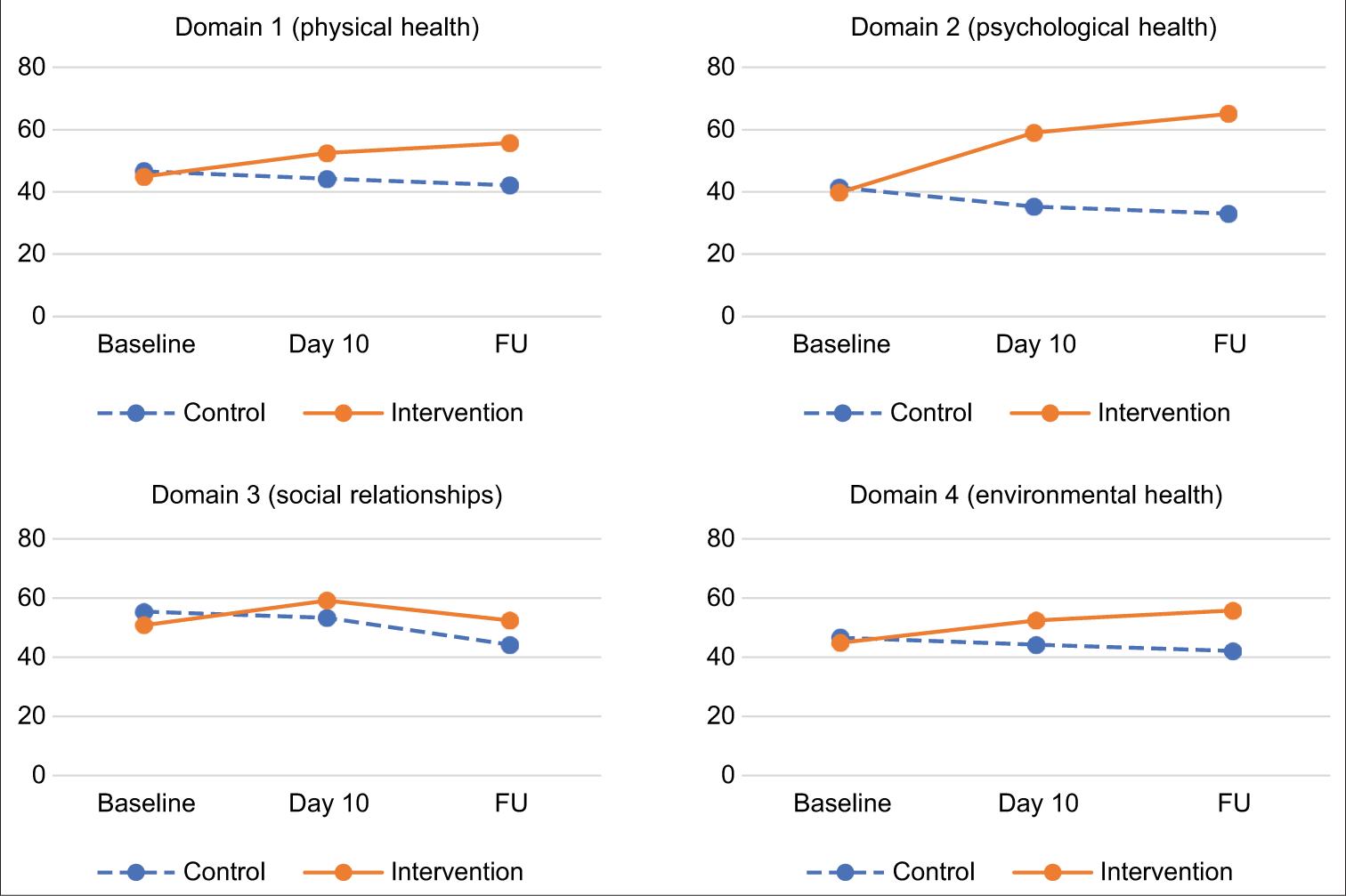
- Evolution of each QOL Domain from Day 1 through FU.
Repeated measure ANOVA showed that the positive changes over time were significant in the intervention arm from baseline to day 10 (P < 0.01), baseline to FU (P < 0.01) and day 10 to FU (P < 0.01) in all four domains of quality of life as well as overall quality of life. The opposite significant change was observed in the control arm. To understand the effect of ST on QOL over time, overall QOL was plotted along with morphine intake and pain for both the intervention arm and the control arm [Graph 3].
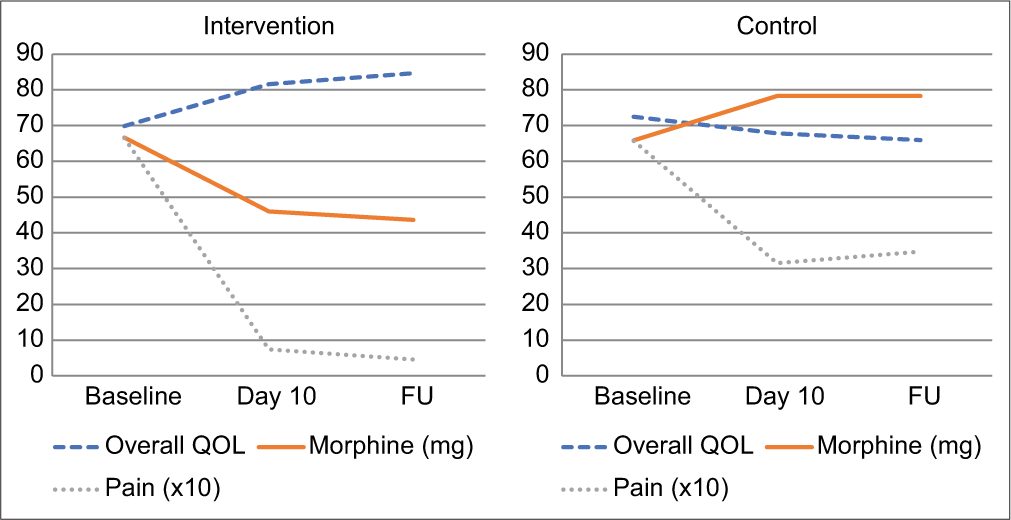
- Evolution of overall QOL versus pain and morphine intake. For visual clarity, pain scores have been multiplied by factor 10 in the graphs.
The graph shows how pain decreased in both the control and the intervention arm. If physical pain was the decisive factor of QOL for the studied patients, one would expect an increase of QOL in both arms based on reduced pain. Nevertheless, overall QOL significantly increased in the intervention arm, while it slightly decreased in the control arm. Morphine consumption saw the opposite evolution: It decreased in the intervention arm, while it increased in the control arm. Interestingly, in both arms, QOL seems to mirror morphine intake.
As shown in [Table 2], for both arms together, the mean change in morphine intake was –3.91 mg from baseline to day 10 and –5 mg from baseline to FU. The mean change in QOL was 3.43 from baseline to days 10 and 4 from baseline to FU. There was a significant negative correlation between changes in quality of life and morphine intake from baseline to day 10 (r = –0.57, P < 0.001) and from baseline to FU (r = –0.55, P < 0.001), indicating that a decrease in morphine intake was accompanied by an increase in quality of life and vice versa. However, in the intervention and control arm separately correlations were not significant.
| Day 1–10 | Day 1 to FU | |
|---|---|---|
| Total sample | ||
| Change in morphine (mg) | –3.91 (39.18) | –5 (46.1) |
| Change in QOL | 3.43 (9.49) | 4 (11.91) |
| Correlation | r=–0.57, P<0.001 | r=–0.55, P<0.001 |
| Intervention | ||
| Change in morphine (SD) (mg) | –22.12 (47.59) | –24.42 (58.73) |
| Change in QOL (SD) | 11.67 (5.3) | 3.08 (2.87) |
| Correlation | R=–0.055, P=0.79 | R=0.0228, P=0.263 |
| Control | ||
| Change in morphine (SD) (mg) | 12.41 (18.83) | 12.41 (18.83) |
| Change in QOL (SD) | –4.6 (4.33) | –6.48 (5.35) |
| Correlation | R=0.64, P=0.996 | R=–0.244, P=0.203 |
Although psychometric assessment of WHOQOL-BREF was not an explicit goal of this study, Cronbach’s Alpha was calculated for each quality of life domain at the first, tenth and at FU visit. The values of Cronbach’s Alpha are found in [Table 3].
| Day 1 | Day 10 | Follow-up | |
|---|---|---|---|
| Domain 1 (physical health) | 0.7309 | 0.9010 | 0.9282 |
| Domain 2 (psychological health) | 0.6989 | 0.8779 | 0.9093 |
| Domain 3 (social relationships) | 0.6584 | 0.6371 | 0.6870 |
| Domain 4 (environmental health) | 0.6945 | 0.7185 | 0.8119 |
The table shows that the Alpha values varied tremendously, from a low of 0.64 on day 10 for domain 3, to a high of 0.93 on FU for domain 1. Overall, Alpha values at FU seemed to be substantially better in comparison to those on day 1.
DISCUSSION
This study has shown that ST improves the quality of life in patients suffering from mild to severe pain caused by head and neck and thoracic cancer. Moreover, this is the first randomised controlled trial on ST in cancer pain to include an assessment of the quality of life. However, the observations align with earlier lower-level evidence that has been described in several case reports and a single-arm trial. Han and Lee published a case report describing the effect of ST on chronic low back pain and depression. Depression is not equal to the quality of life, but it is associated with it. ST was given to a 52-year-old patient who was diagnosed with chronic Lower back pain (LBP). The ST sessions were given every day over 10 days with each session having a duration of 40 min. A visual analogue score (VAS) was used to measure pain. Depression was measured using the Beck depression inventory (BDI). It was observed that ST decreased the symptoms of pain and depression in this patient with chronic LBP. Pain measured on VAS score decreased from 8 to 1 and BDI score decreased from 22 to 7.[26]
Jeong et al. described a case of a patient suffering from degenerative gonarthritis. The investigators intended to identify the effects of ST on pain and quality of life. The therapy was given daily for 40 min over 15 days. The pain was measured with a visual analogue scale. The SF-36 questionnaire was used for the assessment of the patient’s quality of life. The visual analogue score decreased from 9/10 to 1/10 after the completion of ST sessions. Higher scores on quality of life after the ST session indicated improvement when compared with the score before the session. Over that period, the SF-36 score increased from 79 to 95.[27]
Kim et al. described a case that showed an improvement in quality of life through the administration of ST in a patient suffering from shingles. This study aimed to analyse the effect of ST on antineuralgic pain and quality of life after shingles. A 54-year female was included in this study. She had antineuralgic pain after shingles. Ten sessions of ST were given to her. The pain was measured using the visual analogue scale. Quality of life was evaluated using SF-36. Significant pain relief was observed with VAS rating scores dropping from 7 points to 1 after completion of 10 ST sessions. Similar positive results were observed in the quality of life with a 26 points increase in SF-36 score from 102 points baseline score. The results of this study indicate that ST decreased pain and improved quality of life.[28]
More comprehensive insight on the efficacy of ST on quality of life can be derived from the single-arm trial conducted by Compagnone and Tagliaferri they presented a multicentre retrospective analysis on the effectiveness of ST for the management of chronic pain. The pain was measured immediately after treatment and at 3 months. 201 chronic pain patients from eight different centres were treated with ST from January to December 2012. Adult patients of age 18 years or more and having chronic pain due to postherpetic neuralgia, chronic low back pain, polyneuropathy and peripheral neuropathy for more than 6 months were included in this study. The mean number of sessions per patient was 10 but 39 patients reported complete pain relief earlier and hence, their treatment sessions were stopped. On the contrary, seven patients (3%) reported no or lack of improvement and hence left the study. The mean NRS score of all the patients reduced from a mean of 7.41 before the treatment to 1.60 after the completion of the sessions. The investigators did not assess the quality of life directly, but for the long-term outcome changes in brief pain inventory score were recorded at 3 months for various parameters such as general activity, mood, walking ability, normal work, relationship, life-enjoyment, pain and sleep. These parameters overlap with dimensions of quality of life. There was an improvement in all of these various parameters.[25]
As interesting as these observations on the effect of ST on quality of life are, they do not explain how ST improves the quality of life. It may seem obvious to assume that ST improves the quality of life through the reduction of physical pain. Pain treatment has indeed been observed to improve QOL.[13] The association between pain and QOL is not so surprising if we look at how the quality of life has been understood in the literature. The WHO has defined quality of life as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and concerning their goals, expectations, standards and concerns.”[35] This definition makes clear that quality of life is a personal assessment of life considering the degree to which specific aspirations, such as wishes, desires and ideals, have been fulfilled. When persons feel that they may not be able to realise aspirations that are considered essential to living a meaningful life, they may experience suffering and reduced QOL.[36] The experience of pain, in particular, may interfere with the ability to realise life-fulfilling aspirations and, thus, contribute to reducing QOL. Conversely, pain reduction may lead to an improvement in QOL.
In this way, pain reduction may explain the significant improvement in QOL in the intervention arm. However, the negative association between pain and QOL is not always a given. In the current trial, pain improved in both the control arm as well as the intervention arm, yet QOL significantly improved in the intervention arm, while it significantly deteriorated in the control arm. This means that among the studied cancer patients, improvement in physical pain did not automatically lead to improved QOL. This phenomenon can be explained by considering morphine intake and the increase of morphine-associated side effects that accompany larger doses of the drug. In the intervention arm, ST led to a decrease in morphine intake, while morphine intake increased in the control arm to control persisting pain.[24]
The significant differences between the intervention arm and control arm in all dimensions of quality of life effect of ST on QOL on day 10 and at FU may, therefore, at least partially be explained by the decrease in morphine intake. The fact that this study found a significant correlation between changes in morphine intake and QOL across both arms but not for the control arm and intervention arm separately, may indicate that morphine reduction needs to be substantial to have a significant impact on QOL in individual patients. ST indeed led to a significant decrease in morphine intake in the intervention arm when compared to the control arm.[24] Yet, the absence of a significant correlation between changes in morphine intake and QOL in the intervention arm, may, also, indicate that other factors work in tandem with reduced morphine intake to improve QOL in patients undergoing ST. Improvement in pain may be another factor, along with the psychological impact of increased attention and care for patients undergoing ST.
Although these findings are important and points toward the broader impact that ST may have on cancer patients’ quality of life, the observations need to be dealt with cautiously. Cronbach’s Alpha’s varied tremendously, from a low 0.64 on day 10 for domain 3, to a high of 0.93 on FU for domain 1. While Alpha values more than. 80 are considered very good, 0.65–0.70 is interpreted as minimally acceptable and any value below.65 is undesirable.[37] The Alpha values may indicate that there are some reliability issues with WHOQOL-BREF among the studied population. Particularly in domain 3 (social relationships), Alpha values remained consistently low throughout the study. On the other hand, the scores across the domains of quality of life show a logical pattern, which seems to imply that the data on QOL is reliable after all.
CONCLUSION
ST significantly improved QOL among patients suffering from moderate to severe pain caused by head and neck and thoracic cancer. The beneficial effect of ST on QOL may be explained by the combined positive effect of ST on pain as well as morphine intake. To lend further support to this finding, future studies would need to document to what extent morphine-related side effects are reduced when ST is administered to cancer patients. The fact that ST may reduce dependency on morphine, thereby lessening morphine’s side effects, illustrates the urgency to further integrate nonpharmacological therapies such as ST into cancer pain management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- WHOQOL-BREF: Introduction, Administration, Scoring, and Generic Version of the Assessment Geneva: World Health Organization; 1996.
- [Google Scholar]
- Understanding of the concept of “total pain”: A prerequisite for pain control. J Hosp Palliat Nurs. 2008;10:26-32.
- [CrossRef] [Google Scholar]
- A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015;51:888-900.
- [CrossRef] [PubMed] [Google Scholar]
- Depressive symptoms, social anxiety, and perceived neck function in patients with head and neck cancer. Head Neck. 2018;40:1443-52.
- [CrossRef] [PubMed] [Google Scholar]
- Psychosocial issues in patients with head and neck cancer: An updated review with a focus on clinical interventions. Curr Psychiatry Rep. 2017;19:56.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life in patients with advanced non-small-cell lung cancer receiving palliative chemotherapy. Adv Exp Med Biol. 2019;1160:11-8.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life of breast cancer patients in the Eastern Mediterranean region: A systematic review and meta-analysis. Breast Cancer Res Treat. 2019;174:585-96.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life in Asian patients with breast cancer: A systematic review. BMJ Open. 2018;8:e020512.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic understanding and quality of life in patients with advanced lung cancer: A multicenter study. Clin Lung Cancer. 2019;20:e369-e75.
- [CrossRef] [PubMed] [Google Scholar]
- Stigma and quality of life in patients with advanced lung cancer. Oncol Nurs Forum. 2019;46:318-28.
- [Google Scholar]
- The impact of pain management on quality of life. J Pain Symptom Manage. 2002;24:S38-47.
- [CrossRef] [Google Scholar]
- Relationship between pain and quality of life In: Preedy VR, Watson RR, eds. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer; 2010. p. :3933-53.
- [CrossRef] [Google Scholar]
- Recent advances in understanding and managing cancer pain. F1000Res. 2017;6:945.
- [CrossRef] [PubMed] [Google Scholar]
- A trial of Scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother. 2013;27:359-64.
- [CrossRef] [PubMed] [Google Scholar]
- EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952-70.
- [CrossRef] [PubMed] [Google Scholar]
- Inside the scrambler therapy, a noninvasive treatment of chronic neuropathic and cancer pain: From the gate control theory to the active principle of information. Integr Cancer Ther. 2019;18:1534735419845143.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for the efficacy of scrambler therapy for cancer pain: A systematic review. Pain Physician. 2020;23:349-64.
- [CrossRef] [Google Scholar]
- Impact of scrambler therapy on pain management and quality of life in cancer patients: A study of twenty cases. Indian J Palliat Care. 2017;23:18-23.
- [CrossRef] [PubMed] [Google Scholar]
- Scrambler therapy for the management of chronic pain. Support Care Cancer. 2016;24:2807-14.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of scrambler therapy for the management of head, neck and thoracic cancer pain: A randomized controlled trial. Pain Physician. 2020;23:495-506.
- [CrossRef] [Google Scholar]
- Chronic pain treatment and scrambler therapy: A multicenter retrospective analysis. Acta Biomed. 2015;86:149-56.
- [Google Scholar]
- Effects of scrambler therapy on pain and depression of patients with chronic low back pain: Case study. J Phys Ther Sci. 2018;30:913-4.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of pain scrambler therapy on the pain and quality of life of degenerative gonarthritis patients. J Phys Ther Sci. 2018;30:479-80.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of pain scrambler therapy on antineuralgic pain and quality of life after shingles. J Phys Ther Sci. 2017;29:1113-5.
- [CrossRef] [PubMed] [Google Scholar]
- Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: A systematic literature review. J Pain Symptom Manage. 2011;41:1073-93.
- [CrossRef] [PubMed] [Google Scholar]
- Does awareness of diagnosis influence health related quality of life in north Indian patients with lung cancer? Indian J Med Res. 2016;143:S38-44.
- [CrossRef] [PubMed] [Google Scholar]
- A cross-sectional comparison of quality of life between physically active and underactive older men with prostate cancer. J Aging Phys Act. 2016;24:642-8.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life after first-line anti-cancer treatments for advanced non-small cell lung cancer in clinical practice. Qual Life Res. 2016;25:1441-9.
- [CrossRef] [PubMed] [Google Scholar]
- The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299-310.
- [CrossRef] [PubMed] [Google Scholar]
- WHOQOL-Hindi: A questionnaire for assessing quality of life in health care settings in India. Natl Med J India. 1998;11:160-5.
- [Google Scholar]
- WHOQOL: Measuring Quality of Life. 2018. Geneva: World Health Organization; Available from: https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en [Last accessed on 2018 Dec 21]
- [Google Scholar]
- The Nature of Suffering and the Goals of Medicine New York, United States: Oxford University Press USA OSO; 2004.
- [CrossRef] [Google Scholar]







