Translate this page into:
Temporal, Probabilistic and Surprise Question Approaches of Clinician Predicted Survival in Advanced Hepatopancreaticobiliary Cancers: A Prospective Cohort Study
*Corresponding author: Jayita Deodhar, Department of Palliative Medicine, Tata Memorial Hospital, Homi Bhabha National Institute, Mumbai, Maharashtra, India. jukd2000@yahoo.co.uk
-
Received: ,
Accepted: ,
How to cite this article: Surendran S, Kuriakose j, Ponnamthodi P, Poojary SS, Deodhar J. Temporal, Probabilistic and Surprise Question Approaches of Clinician Predicted Survival in Advanced Hepatopancreaticobiliary Cancers: A Prospective Cohort Study. Indian J Palliat Care. doi: 10.25259/IJPC_323_2024
Abstract
Objectives:
Clinician-predicted survival for patients with hepatopancreaticobiliary cancers referred for specialist palliative care (SPC) has not been established. This study aimed to estimate and compare the three approaches of clinician predicted survival- temporal, probabilistic and surprise question (SQ) approaches at 7, 30, 60 and 90 days.
Materials and Methods:
A prospective observational study was conducted following ethical approval, involving 160 adult patients with metastatic cancers of the liver, pancreas, gallbladder and biliary system, who were not receiving any cancer-directed treatment and were referred to SPC from September 2022 to May 2023. Patients were prospectively followed up for 90 days.
Results:
A total of 160 patients were recruited, 134 (83.8%) of whom died by the end of the study period. The overall accuracy (OA) for the 7-day temporal, categorical and SQ approaches of Clinician Predicted Survival were 83.5%, 65.4% and 89.5%, respectively, whereas the 90-day OA was 86.5%, 86.3% and 93.4%, respectively. The c-statistic value for 60- and 90-day was 0.62 (95% confidence interval [CI]: 0.52–0.72) and 0.73 (95% CI: 0.60–0.86).
Conclusion:
The SQ approach was more accurate than the temporal and probabilistic approaches at all timepoints, in our study of patients with advanced hepatopancreaticobiliary cancers. The temporal approach displayed significantly moderate diagnostic accuracy on days 60 and 90. The least accurate prognostic estimation was recorded on day 30 for all three approaches. We further emphasise employing SQ as the preferred approach for determining the Clinician Predicted Survival.
Keywords
Gallbladder neoplasms
Liver neoplasms
Palliative care
Pancreatic neoplasms
Prognosis
INTRODUCTION
Hepatopancreaticobiliary (HPB) cancers, comprising primary and metastatic cancers of the liver, pancreas, gallbladder and biliary system, have historically been deemed to have a poor prognosis.[1] The innocuous and latent symptomatic phase, which delays medical attention, results in a tardy diagnosis and an advanced stage at detection with limited curative-intent treatment options. This profoundly affects the quality of life of patients, increases the symptom burden, and leads to financial and time-related toxicities. In previous studies, the median overall survival (OS) rates after diagnosis of advanced hepatocellular, gallbladder and pancreatic cancers were 2–3, 3–6 and 6–11 months, respectively.[2-4] For these patients, accurate prognostication is a crucial step in incorporating timely specialist palliative care (SPC) services, supporting patient-centred care and facilitating decision-making processes regarding treatments and the preferred place of care/death, thereby ensuring an adequate quality of End-of-Life (EOL) care and provision of dignified death. As death is a probabilistic event, it may not be possible to ascertain the exact time of death.[5] Moreover, with disease progression, the illness trajectory of HPB cancers could be interspersed with complications such as obstructive jaundice, encephalopathy, infections, hepatic failure, malignant ascites, peritoneal carcinomatosis and gastrointestinal haemorrhage, the outcomes of which would further limit prognosis.[6]
Research on prognostication has predominantly focused on cancers in general, relying on clinician prediction of survival (CPS) and various scales and indices.[7-10] However, there is a paucity of studies specifically addressing the prognostication of HPB cancers within the context of SPC. CPS is commonly used by physicians as it is simple, prompt and convenient.[11] It considers various patient- and disease-related factors, including illness trajectory, performance status, symptom burden and clinicians’ assessment of laboratory markers. Although it incorporates known prognostic factors, variability in the weighting given to each parameter, in conjunction with prior clinical knowledge, clinical experience and personality, often results in heterogeneous and inaccurate estimations.[12,13] Hui et al. have demonstrated that the accuracy of CPS is comparable to or exceeds that of other prognostic indices across various timeframes.[14] Perez-Cruz et al. compared probabilistic and temporal approaches in patients with advanced cancer during the final days of life. The findings showed the probabilistic approach was more accurate than the temporal approach.[15] With the establishment of early integration of palliative care as a standard practice for metastatic cancers and advancements in cancer therapeutics, SPC physicians must deploy the best available prognostication tool to efficiently guide patients and caregivers with respect to informed decision making, better healthcare utilisation, and advance care planning, and to align the goals of treatments with what matters the most to patients.
Objective
This study aimed to determine the accuracy of CPS in predicting survival in patients with metastatic HPB cancers who were planned for best supportive care (BSC) and were referred to SPC in a tertiary cancer centre in India.
MATERIALS AND METHODS
Study design and setting
This prospective observational cohort study was conducted in the Department of Palliative Medicine at Tata Memorial Hospital from September 2022 to May 2023, in accordance with the principles of the Declaration of Helsinki.
Study participants
We included adult patients aged ≥18 years who were diagnosed with metastatic cancers of the liver, pancreas, gallbladder and biliary system, with an Eastern Cooperative Oncology Group (ECOG) score ≥1, not planned for any disease-modifying treatment (DMT) and referred to SPC services (outpatient clinic/inpatient-palliative care unit/emergency department) for BSC. Patients with pre-existing psychiatric comorbidities were excluded from the study.
Outcomes and measures
The primary outcome of this study was the accuracy of survival predicted by SPC clinicians using the CPS. This was assessed by comparing CPS with actual survival (AS) at the end of 90 days by calculating the difference in days (temporal prediction), assessing the percentage chance of survival (probabilistic prediction) at 7, 30, 60 and 90 days, and using the surprise question (SQ) approach (Will you be surprised if the patient died within a given timeframe?). The secondary outcome was to compare the accuracy of the three CPS approaches. AS was defined as the time from the first assessment by an SPC physician to death or censoring at the end of the follow-up period (90 days).
The evaluation of prognostic accuracy for each approach has been described in previous studies.[12-16] The time points were chosen after consensus among the authors and were based on decisions on initiating or continuing invasive procedures, initiating goals of care discussion and advanced care planning (ACP) in the context of sociocultural differences observed in our setting, an appropriate time for deciding the place of care and death, and relocation. Patients with HPB cancers were chosen because they are among the most referred cancers to SPC in our setting.
Temporal prediction estimates the time to event (death) and is expressed either as a continuous variable (number of days the patient is expected to live) or as a categorical variable (≤7, 8–30, 31–60, 61–90 days). Prognostic accuracy for temporal continuous CPS was defined as an error of less than ±33.3% in the duration. Values were considered ‘accurate’ if the quotient of the AS divided by the estimated CPS value was between 0.67 and 1.33. Values <0.67 were taken as ‘optimistic/overestimated’ and those >1.33 were taken as ‘pessimistic/underestimated.’[12,13,16]
Probabilistic predictions estimate the percentage chance of an event occurring at a particular time (the percentage chance of a patient being alive at 7, 30, 60 and 90 days from the first assessment). The determination of the probability of survival was deemed accurate if either of the following conditions were met: (a) If the patient died, and the clinician predicted a survival probability ≤30% or (b) if the patient survived, and the clinician estimated survival probability ≥70%. The survival probability of 40–60% signified uncertainty and thus was considered inaccurate, regardless of the survival outcome. A similar approach was followed in a study of prognostication by Hui et al.[12]
The SQ approach asks whether the respondent would be surprised if the patient were to die within a specified time frame (Would you be surprised if the patient were to die in 7, 30, 60 and 90 days?). Responses of ‘Yes’ or ‘No’ were recorded at each time point at baseline.[17,18]
Study procedure
After screening, eligible participants were provided with a detailed information sheet regarding the study, and those who consented were enrolled. Consent was sought from the primary caregivers of the patients who presented with delirium. The patients received standard palliative care as part of their routine clinical assessments and management. Variables recorded at baseline included sociodemographic (age, sex and marital status) and clinical (primary cancer diagnosis, ECOG score and place of presentation). Seven SPC physicians with at least 3 years of clinical experience, who were blinded and not part of the study, answered the SQ and estimated probabilistic and temporal parts of CPS separately at baseline for each patient to minimise confirmation bias. To mitigate selection bias, the study’s principal investigators, who were not involved in prognostication, employed a randomised approach for selecting three physicians for every patient. Patients’ ECOG performance status and information regarding AS were assessed periodically, either in-person or telephonically, at 15, 30, 60, and 90 days.
Sample size estimation
Approximately 200–250 (40–45%) patients visiting the hospital with HPB cancers per month in 2022 were referred to SPC services. Of these, approximately 20–30 (10–15%) patients were referred for BSC only, as deemed unfit for any DMT. To obtain reliable sensitivity-specificity estimates (which require a minimum number of events to be at least 100) and considering a 10% attrition rate, we estimated the sample size to be 160.
Statistical analysis
Categorical variables were summarised using frequencies and percentages. Continuous variables were described using mean and standard deviation for normally distributed data and median and interquartile range (IQR) for data with a skewed distribution. Spearman’s correlation coefficient was used to compare temporal CPS and AS. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy (OA) were used to analyse the data for the SQ and temporal approaches of CPS. Paired comparisons of the accuracy of the temporal, probabilistic, and SQ approaches for CPS at 7, 30, 60 and 90 days were performed using Cochran’s-Q test, and post hoc analysis using McNemar’s test. Area under the receiver operator curve (AUROC) analysis was performed to analyse the discrimination ability of CPS. Survival curves were obtained using the Kaplan–Meier method. Statistical significance was set at P < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences software version 29.0 (IBM Corporation, USA). The study was performed in accordance with the STARD reporting guidelines.
RESULTS
Among the 186 patients evaluated for the study, 160 were found to meet the eligibility criteria and were subsequently enrolled. One hundred and thirty-four (83.8%) of the patients died at the end of 90 days. Of the remaining 26 patients who were censored, 19 (11.9%) were alive (right-censored) and 7 (4.4%) were lost to follow-up (left-censored).
The mean age of the patients in our study was 56 (±11.98) years, 81 (50.6%) were female, and 142 (88.8%) were married. The predominant sites of primary malignancy were the gallbladder (n = 80, 50%), liver (n = 60, 37.5%) and pancreas (n = 20, 12.5%). The median ECOG-PS score on presentation to the OPD was 3 (IQR = 1). The median number of years of experience of the seven Palliative Medicine physicians participating in our study was 5 years (Range: 3–25 years). The detailed characteristics are listed in Table 1.
| Characteristic | Number of patients (%) |
|---|---|
| Age (mean, SD) | 55.98 (11.98) |
| Gender | |
| Male | 79 (49.4) |
| Female | 81 (50.6) |
| Marital status | |
| Married | 142 (88.8) |
| Unmarried | 3(1.9) |
| Widow/widower | 14 (8.7) |
| Separated | 1 (0.6) |
| Primary cancer diagnosis | |
| Gallbladder carcinoma | 80 (50) |
| Hepatocellular cancer | 60 (37.5) |
| Pancreatic cancer | 20 (12.5) |
| ECOG-PS at baseline | |
| 1-2 | 59 (36.9) |
| 3 | 79 (49.4) |
| 4 | 22 (13.7) |
| Setting | |
| Out-patient clinic | 146 (91.2) |
| In-patient palliative care unit | 8 (5) |
| Emergency department | 6(3.8) |
ECOG-PS: Eastern cooperative oncology group-performance status, SD: Standard deviation
The median OS was 24 days (95% confidence interval [CI]: 21.16–26.83). The median OS for CPS range estimates for ≤7, 8–30, 31–60, 61–90 days were 36 days (95% CI: 0.00– 78.94), 16 days (95% CI: 12.20–19.79), 30 days (95% CI: 21.77–38.23) and 70 days (95% CI: 57.60–82.39), respectively (P < 0.001) [Figure 1].
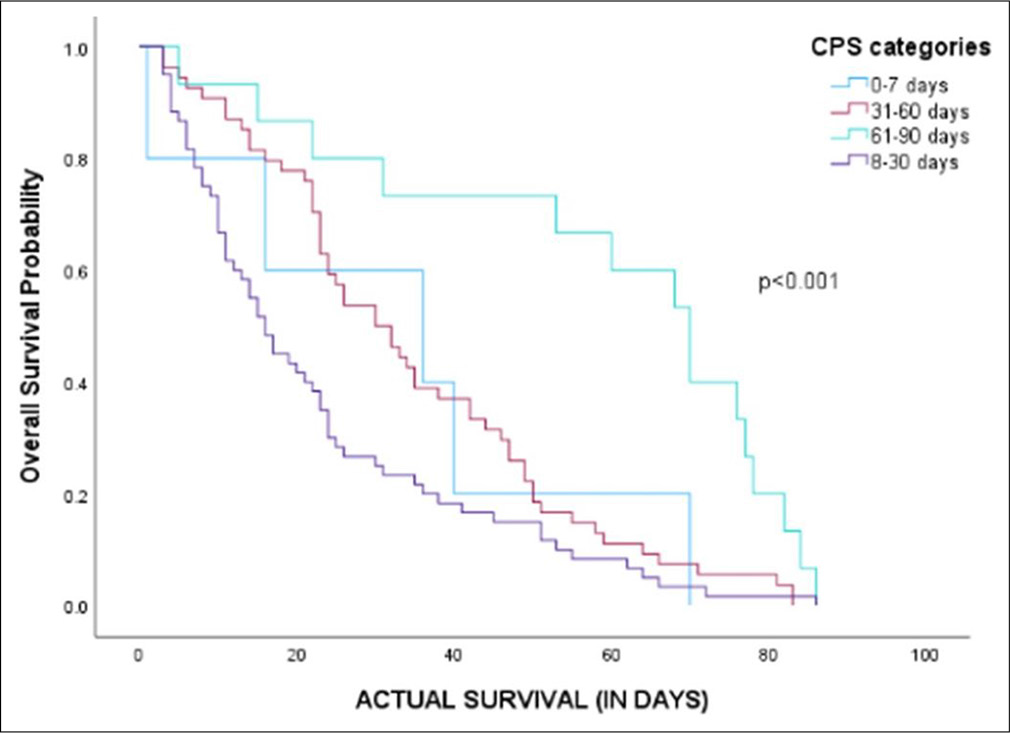
- Kaplan–Meier curve describing survival curve stratified for clinician prediction of survival categories. CPS: Clinician predicted survival.
Temporal clinician-predicted survival (CPS)
The median continuous temporal CPS was 40 days (95% CI: 33.75–46.25 days). The median difference between AS and temporal CPS was 16 days (95% CI: 12.59–19.42). The correlation coefficient between temporal CPS and AS is 0.48 (95% CI: 0.33–0.60; P < 0.001). Among 134 patients included in the analysis, 54 (40.3%) were accurate, 57 (42.5%) were optimistic/overestimated, and 23 (17.2%) were pessimistic/underestimated. The mean difference in the overestimated group was 23.4 days. The mean difference in the underestimated group was 29.3 days. The c-statistic value for CPS at day 60 was 0.62 (95% CI: 0.52–0.72, P = 0.03) and at day 90 was 0.73 (95% CI: 0.60–0.86, P = 0.001) [Table 2].
| Survival assessment timepoints | c-statistic (95%CI) | P-value |
|---|---|---|
| 7-day | 0.25 (0.11-0.38) | <0.001 |
| 30-day | 0.40 (0.30-0.49) | 0.05 |
| 60-day | 0.62 (0.52-0.72) | 0.03 |
| 90-day | 0.73 (0.60-0.86) | 0.001 |
AUROC: Area under receiver operating curve, CI: Confidence interval. Bold and italic values specify significant P-values (P<0.05)
Receiver operating characteristics for 7, 30, 60 and 90 days have been described in Figures 2-5.
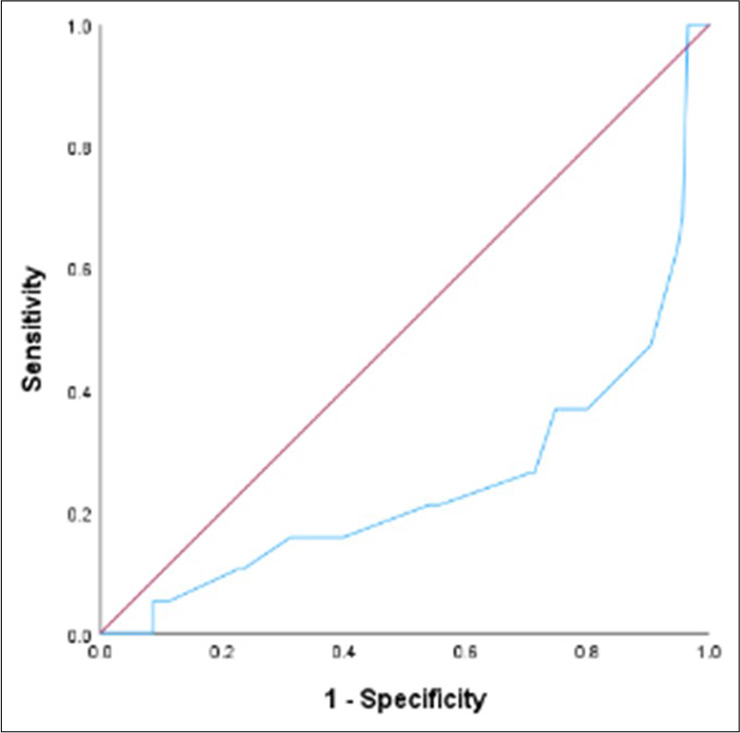
- Receiver operating Clinician predicted survival at day 7. Blue line signifies the model’s curve for Clinician predicted survival for the specified timepoint. Red line signifies a diagonal line that represents a classifier based on random guessing.
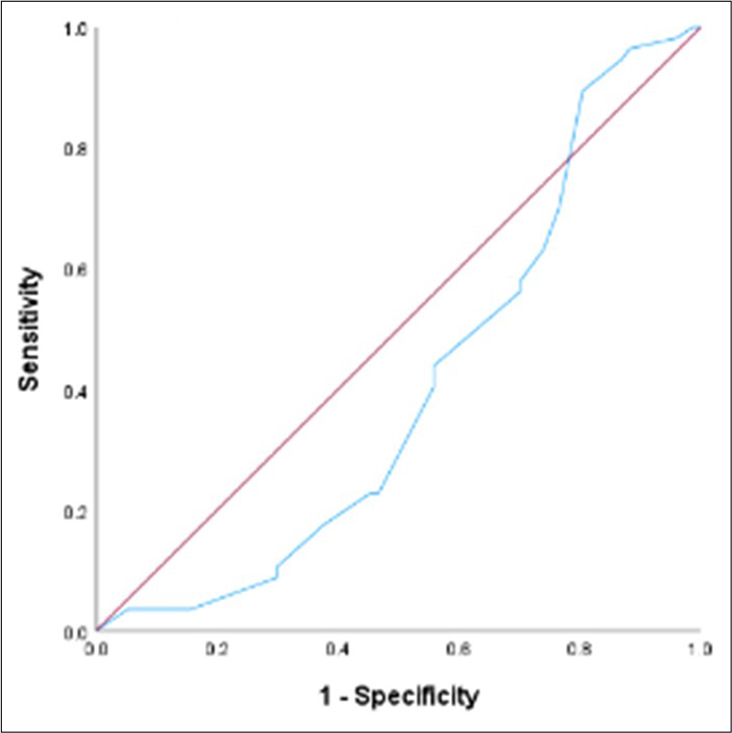
- Receiver operating characteristic curve for Clinician predicted survival at day 30.
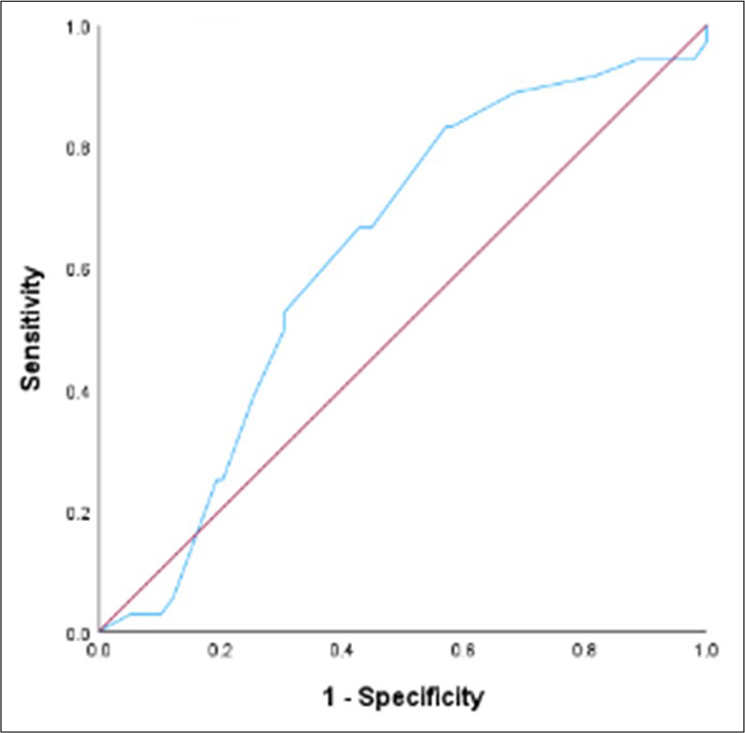
- Receiver operating characteristic curve for Clinician predicted survival at day 60.
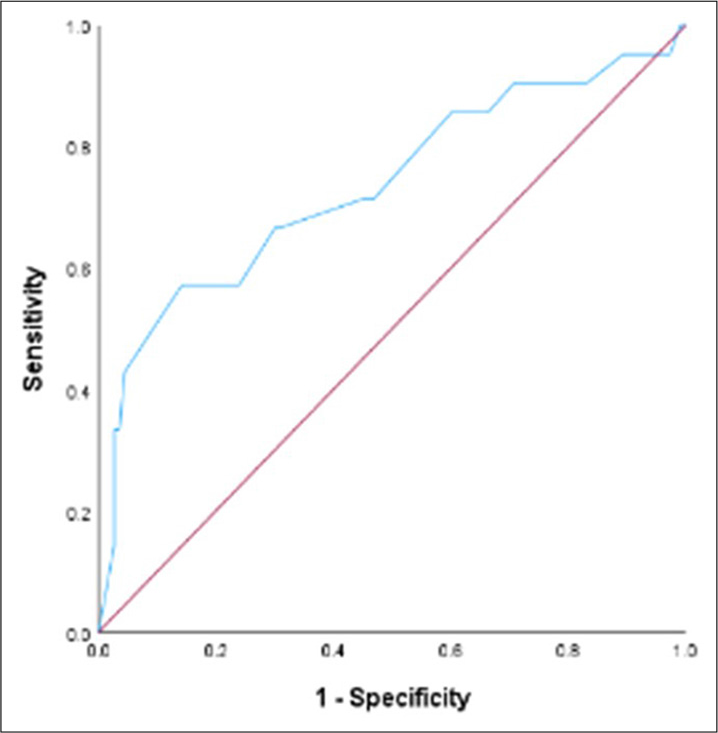
- Receiver operating characteristic curve for Clinician predicted survival at day 90.
The OA of the categorical temporal CPS prediction was 65(48.5%). The optimistic/overestimated estimates were 45 (33.6%), and the pessimistic/underestimated estimates were 24 (17.9%). McNemar’s test, used to compare the OA of continuous and categorical predictions, showed that the categorical approach was significantly more accurate than continuous temporal CPS prediction (48.5% vs. 40.3%, P = 0.04).
Probabilistic approach of CPS
The median probabilistic CPS values were 70%, 50%, 30% and 20% for 7, 30, 60 and 90 days, respectively. The accuracies of probabilistic prediction at 7, 30, 60 and 90 days were 65.4%, 24.2%, 56.9% and 86.3%, respectively. Uncertainty (responses in the range of 40–60%) was expressed as 28.1%, 65.4%, 36.6%, 9.8% at 7, 30, 60 and 90 days.
SQ approach and outcomes
The sensitivity and specificity on day-7 were 47.4% and 95.5%, respectively, whereas those on day-90 were 97.8% and 68.4%, respectively. The details are summarised in Table 3.
Paired comparisons of OAs of the temporal, probabilistic and SQ approaches for CPS at 7, 30, 60 and 90 days using Cochran’s-Q test revealed statistically significant differences in accuracy among the three groups at all time points (P < 0.001). In the post hoc analysis using McNemar’s test, the temporal approach was significantly more accurate than the probabilistic approach at days 30 and 90 (P < 0.001), while the SQ approach was significantly more accurate than the probabilistic approach at days-7, 30 and 60 (P < 0.005 ), and the SQ approach was significantly more accurate than the temporal CPS approach at all-time points (P < 0.001).
| Timepoints | CPS approach | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | P-value |
|---|---|---|---|---|---|---|---|
| 7-day | Temporal | 5.3 | 96.5 | 20 | 86 | 83.5 | 0.70 |
| SQ | 47.4 | 95.5 | 60 | 92.8 | 89.5 | <0.001 | |
| Probabilistic | - | - | - | - | 65.4 | 0.02 | |
| 30-day | Temporal | 54.4 | 62.3 | 51.7 | 64.9 | 58.9 | 0.05 |
| SQ | 73.7 | 67.5 | 69.1 | 72.2 | 70.6 | <0.001 | |
| Probabilistic | - | - | - | - | 24.2 | 0.53 | |
| 60-day | Temporal | 58.3 | 66.3 | 38.9 | 81.3 | 64.1 | 0.01 |
| SQ | 83.2 | 55 | 83.9 | 53.7 | 75.8 | <0.001 | |
| Probabilistic | - | - | - | - | 56.9 | 0.78 | |
| 90-day | Temporal | 42.9 | 94.7 | 60 | 89.9 | 86.5 | <0.001 |
| SQ | 97.8 | 68.4 | 95.6 | 81.3 | 93.4 | <0.001 | |
| Probabilistic | - | - | - | - | 86.3 | 0.004 |
SQ: Surprise question, PPV: Positive predictive value, NPV: Negative predictive value. Bold and italic values specify significant P-value (P<0.05)
DISCUSSION
Prognostication is pivotal in palliative care, particularly for advanced HPB cancers, which have a poor prognosis and limited survival. Accurate CPS predictions are linked with increased adherence to patient care preferences, less aggressive EOL care, timely hospice enrolment, initiation of ACP, reduced intensive care unit (ICU) admissions and length of stay in the ICU and increased compliance with Do-Not-Resuscitate outcomes, resulting in enhanced family satisfaction and improved patient outcomes.[19-22] We found that CPS was most accurate at day 90, followed by day 7- 86.5% and 83.5% for temporal, 86.3% and 65.4% for probabilistic, 93.4% and 89.8% for SQ approaches, respectively. The SQ approach was more accurate than temporal and probabilistic approaches. The least accurate prognostic estimation was recorded on day 30 for all three approaches. Temporal CPS displayed a significantly moderate diagnostic accuracy on days 60 and 90, as determined by AUROC analysis. The median survival for patients in our cohort was 24 days.
The median difference between AS and CPS was 16 days, with values ranging between −29.3 and +23.4 days. The continuous temporal estimates demonstrated various metrics according to the method of estimation-40% accuracy, as per the AS ± 33% method, a moderate correlation between AS and continuous temporal CPS and c-statistic values ranging from 0.25 to 0.73. The SN for 7-day prediction was 5.3%, but the accuracy was 83.5%, largely contributed by the ability to detect true negatives. In the AUROC analysis, temporal CPS displayed moderate diagnostic accuracy on days 60 and 90. However, the accuracy varied depending on the estimation method, as reported recently.[23] Hiratsuka et al., in their study, recorded a higher AUROC estimation (0.86 at 7 days, 0.82 at 30 days) as compared to estimation by the AS ± 33% method-30%. The accuracy of the categorical estimates at different time points ranged from 58.9% to 86.5%. The temporal CPS yielded OAs of 40% and 49% for the continuous and categorical estimates, respectively. The categorical estimates were more accurate than the continuous estimates (P < 0.001). The overall temporal CPS accuracy was similar to that reported in previous studies.[12,15,23,24] The possible reasons for low accuracy may be tied to the subtle nature of the temporal question, lack of upper threshold of clinician response, inclination to err on the side of caution to maintain hope and protect patients and caregivers from psychological distress contributed by poor prognosis, stringent accuracy criteria for patients with short, expected survival,[12] increased likelihood of acute complications in our subgroup, the non-compliance to horizon effect[25] and high clinician dependence, making it less reliable.[16,26] Physicians with experience who are not directly involved in patient management tend to provide more accurate prognostication than those actively engaged in the patients’ care. Inaccuracy attributed to underestimation is frequently associated with inherent unpredictability stemming from overall general conditions, comorbidities and sudden unexpected deaths.[27] In the Indian setting, the coping mechanisms of patients and caregivers are influenced by philosophical and religious beliefs, including stoicism, acceptance of fate, karma and spiritual convictions.[28] These factors contribute to a reduction in overall distress, possibly enhancing survival outcomes and changing the prognostic course for patients. Other factors contributing to CPS inaccuracy include resource constraints, high patient volume and inadequate training in the application of prognostic instruments. Although AUROCs are recommended for estimation, they may not capture clinically significant differences in prognostic discrimination, and the percentages shown in the AUROC are not the same as the predicted probability that the patient would die in the given period.[23] Notably, the low sensitivity and high specificity of day-7 prediction are concordant with the appearance of physical signs of death in the last week of life.[29]
The accuracy of probabilistic prediction exhibited a nonlinear pattern, with the lowest accuracy and maximum uncertainty observed on day 30. Conversely, the highest accuracy and least uncertainty were documented on day 90. Survival time was predicted to be accurate in the short term (days of survival) or long term (months of survival). In contrast, prediction in the intermediate time frame necessitates comprehending the risk of developing acute life-threatening events (infections, refractory dyselectrolytemia and acute thromboembolism), which frequently occur in the weeks preceding death. The outcomes of these events are challenging to predict, even in patients not on any DMT, due to inter-patient variability and pre-existing disease burden. This aligns with other studies that demonstrate the challenging nature of predicting intermediate survival.[12,30] Notably, previous studies did not exclusively consider patients receiving BSC only. The use of prognostic tools in this timeframe could potentially enhance the accuracy of clinicians. In addition, probabilistic estimation had the least accuracy among the three. This finding is contradictory to that of previous studies by Hui et al. and Perez-Cruz et al., who compared temporal and probabilistic predictions and found that probabilistic predictions were more accurate than temporal predictions.[12,15]
Our results showed that SQ demonstrated an increasing trend in sensitivity and PPV, with the highest values recorded on day 90. Meanwhile, specificity was highest on day 7, and accuracy was highest on day 90, followed by day 7. The lowest accuracy was recorded for the prediction on day 30. We found that sensitivity and PPV decreased as the timeframe for the SQ decreased, similar to the findings reported in previous studies.[31,32] The possible reason could be the hesitancy of physicians associated with answering the SQ with shorter time frames, to avoid ‘taking risks’, as being wrong could have a significantly undesirable impact on clinical decision making. Our results for day-7 SQ prediction are similar to those of a study by Kim et al., who assessed SQ at 7, 21 and 42 days in a multicentre prospective cohort study in a palliative care inpatient unit.[33] The specificity at different time points followed a nonlinear pattern, contrary to the finding observed by Hamano et al.[34] that as the duration of the prediction period decreases, the SQ demonstrates a lower accuracy in identifying patients who will die. Our study was performed primarily in an outpatient clinic setting with shorter SQ timeframes in a cohort of patients with advanced HPB cancer, which differs from previous studies, which focused on patients with diverse advanced stage cancers in hospital-based settings.[32-34] Further studies with a shorter timeframe SQ are recommended in patients with advanced cancer presenting to different settings, preferably multi-centric trials.
Prognostication ultimately translates to prognostic communication, which can be significantly influenced by inaccuracies. This is particularly evident in the facilitation of goals-of-care discussions and decision-making processes in the EOL phase, consequently affecting overall family satisfaction and confidence in the healthcare team.[35] Harding et al. highlight sociocultural factors affecting prognostic communication in India. Healthcare professionals demonstrate a greater inclination towards disclosing prognostic information, while families prefer a collaborative approach to decision-making centred around the family for patient care to alleviate anticipatory psychological stress. A paternalistic approach, in conjunction with patients’ passive reception of information and reliance on physicians for decision-making, is prevalent. Financial constraints and caregivers’ opportunity costs significantly impact information-giving and decision-making processes. Consequently, patients often lack disease insight, autonomy in decision-making and informed consent to treatment, which results in them accessing futile treatments and experiencing treatment poverty.[36]
The SQ approach was more accurate than the temporal and probabilistic approaches across all time points, highlighting the critical role of question framing in enhancing clinicians’ predictive abilities. To the best of our knowledge, this is the first study that focuses on prognostication in patients with advanced HPB cancers and on BSC in a low-middle income country (LMIC) setting. Notably, a novel feature of our study is that we have determined and compared all three approaches of CPS in variable timeframes, and an effort to determine which modality would be the most accurate approach for CPS, within the same disease-specific patient cohort, in diverse palliative care settings at a large tertiary cancer centre. In addition, we recorded continuous and categorical estimates as two separate questions, which is one of the limitations of the previous studies.
Limitations
Our study was conducted with a relatively modest number of patients at a single tertiary care cancer centre, in a peculiar patient population with limited OS, thus limiting the generalisability of our findings. Trainee estimations and comparative analysis based on years of experience have not been examined within the scope of this study. The primary objective was to assess the accuracy of CPS as a tool and to limit clinician errors during their subjective assessment. Future studies focusing on intra-clinician variability and differences in trainee estimations, as well as their impact on prognostic accuracy in our setting, warrant further investigation.
Perspectives of multidisciplinary teams, including nursing staff, social workers and psychologists, were not incorporated, which potentially limited the comprehensiveness of prognostic evaluations. The inclusion of multidisciplinary team (MDT) in prognostication can enhance prognostication as compared to only clinician-based prognostication.[37,38] Adequate training and real-world experience in prognostication, followed by studies on MDT prognostication, should be highly considered.
Given that our follow-up assessments were conducted telephonically with patients being in their preferred place of care, we were unable to accurately ascertain the incidence of sudden unexpected events contributing to mortality.[27] Further investigations in supervised settings (acute palliative care units or hospice) may facilitate the acquisition of data regarding the incidence and contribution of unexpected events in our patient population.
Implications and recommendations
The integration of CPS into routine clinical practice, accompanied by periodic reassessments of CPS throughout the patient’s disease trajectory, may yield valuable insights. A ‘prognostication log’ has been described by Hiratsuka et al., which can be a valuable experience-based self-learning tool.[39] Evidence-based education through various mediums- academic conferences, seminars, workshops and inclusion in curriculum, ensuring standard training can eventually improve prognostic confidence. In addition, consideration of patients’ preferences and decision-support systems can essentially enhance our understanding of CPS. Hui et al. have delineated focused future research on the incorporation of multiple variables in prognostic assessment, applying time trends, examination of signs of impending death and their application in prognostication and employment of advanced statistical models.[35]
Notably, recent clinical practice guidelines recommend using CPS in all patients with a survival of few months or less, regardless of their DMTs.[40]
The SQ approach, which demonstrated high accuracy in our study, is inherently subjective and influenced by clinician biases, underscoring the need to explore its integration with objective prognostic tools to further improve accuracy. Moreover, the observed challenges in predicting intermediate survival (30-day) point to a need for enhanced tools or strategies tailored to this specific timeframe. Addressing these gaps through structured training programs and technological innovations could help bridge the accuracy divide, particularly in resource-constrained settings.
CONCLUSION
Prognostication is a dynamic process, as opposed to a single event. Recent clinical practice guidelines recommend using CPS in all patients with a survival of a few months or less, regardless of their DMTs. We further emphasise through the means of this study to employ SQ as the preferred approach for determining CPS. Supplementing the 30-day and 60-day estimations with serial CPS or employing prognostic tools can potentially improve accuracy. In addition, more research focusing on prognostication should be conducted, especially in LMIC settings where survival estimates differ from the developed world and among diverse cancer groups.
Acknowledgements
We acknowledge the support of our patients, hospital administration and staff.
Authors’ contributions
SS, JK, JD: Conceptualisation, formulation of the research protocol, data acquisition, statistical analysis and drafting of the original manuscript. All authors participated in the interpretation of the data and revision of the manuscript and have participated sufficiently in appropriate portions of the content.
Ethics approval
The Institutional Ethics Committee (IEC) at Tata Memorial Hospital, Mumbai, reviewed and approved this study, approval number (4003/2022/00001) and registered it with the Clinical Trials Registry-India, number (CTRI/2022/08/044932), dated on 28th July 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Measuring Health-Related Quality of Life in Patients with Hepatobiliary Cancers: The Functional Assessment of Cancer Therapy-Hepatobiliary Questionnaire. J Clin Oncol. 2002;20:2229-39.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced Hepatocellular Carcinoma: A Regional Cancer Center Experience of 48 Cases. Indian J Cancer. 2017;54:526-9.
- [CrossRef] [PubMed] [Google Scholar]
- An Observational Study of Best Supportive Care with or without Oral Capecitabine in Patients with Metastatic Gallbladder Cancer. J Clin Oncol. 2023;41(4 Suppl):521.
- [CrossRef] [Google Scholar]
- Treatment Practices for Metastatic Pancreatic Cancer: Can we Deliver an Appropriately Efficacious and Safe Regimen in Indian Patients? Indian J Cancer. 2018;55:138-43.
- [CrossRef] [PubMed] [Google Scholar]
- Practical Model for Prognostication in Advanced Cancer Patients: Is Less More? J Clin Oncol. 2008;26:5843-4.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency and Factors Associated with Unexpected Death in an Acute Palliative Care Unit: Expect the Unexpected. J Pain Symptom Manage. 2015;49:822-7.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the Palliative Prognostic Index and Palliative Prognostic Score in a Palliative Care Consultation Team Setting for Patients with Advanced Cancers in an Acute Care Hospital in Japan. Am J Hosp Palliat Care. 2014;31:730-4.
- [CrossRef] [PubMed] [Google Scholar]
- Successful Validation of the Palliative Prognostic Score in Terminally ill Cancer Patients Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:240-7.
- [CrossRef] [PubMed] [Google Scholar]
- An Inflammation-Based Prognostic Score and its Role in the Nutrition-Based Management of Patients with Cancer. Proc Nutr Soc. 2008;67:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Development of Prognosis in Palliative Care Study (PiPS) Predictor Models to Improve Prognostication in Advanced Cancer: Prospective Cohort Study. BMJ. 2011;343:d4920.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostication of Survival in Patients with Advanced Cancer: Predicting the Unpredictable? Cancer Control. 2015;22:489-97.
- [CrossRef] [PubMed] [Google Scholar]
- The Accuracy of Probabilistic Versus Temporal Clinician Prediction of Survival for Patients with Advanced Cancer: A Preliminary Report. Oncologist. 2011;16:1642-8.
- [CrossRef] [PubMed] [Google Scholar]
- Terminal Cancer Duration and Prediction of Survival Time. Eur J Cancer. 2000;36:2036-43.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting Survival in Patients with Advanced Cancer in the Last Weeks of Life: How Accurate are Prognostic Models Compared to Clinicians' Estimates? Palliat Med. 2020;34:126-33.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal Temporal and Probabilistic Prediction of Survival in a Cohort of Patients with Advanced Cancer. J Pain Symptom Manage. 2014;48:875-82.
- [CrossRef] [PubMed] [Google Scholar]
- Extent and Determinants of Error in Doctors' Prognoses in Terminally ill Patients: Prospective Cohort Study. BMJ. 2000;320:469-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Significance of the “Surprise” Question in Cancer Patients. J Palliat Med. 2010;13:837-40.
- [CrossRef] [PubMed] [Google Scholar]
- The 'Surprise' Question in Advanced Cancer Patients: A Prospective Study among General Practitioners. Palliat Med. 2014;28:959-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicians' Prediction of Survival Is Most Useful for Palliative Care Referral. Palliat Med Rep. 2024;5:365-72.
- [CrossRef] [PubMed] [Google Scholar]
- The Accuracy of Clinician Predictions of Survival in the Prognosis in Palliative Care Study II (PiPS2): A Prospective Observational Study. PLoS One. 2022;17:e0267050.
- [CrossRef] [PubMed] [Google Scholar]
- The Importance of Prognostication: Impact of Prognostic Predictions, Disclosures, Awareness, and Acceptance on Patient Outcomes. Curr Treat Options Oncol. 2021;22:12.
- [CrossRef] [PubMed] [Google Scholar]
- Does Calculated Prognostic Estimation Lead to Different Outcomes Compared With Experience-Based Prognostication in the ICU? A Systematic Review. In: Crit Care Explor. Vol 1. 2019. p. :e0004.
- [CrossRef] [PubMed] [Google Scholar]
- How Different are Accuracies of Clinicians' Prediction of Survival by Assessment Methods? Ann Palliat Med. 2024;13:49-56.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of predictions of survival in palliative care: How accurate are clinicians and who are the experts? PLoS One. 2016;11:e0161407.
- [CrossRef] [PubMed] [Google Scholar]
- A Brief Review of Survival Prediction of Advanced Cancer Patients. Int J Palliat Nurs. 2014;20:530-4.
- [CrossRef] [PubMed] [Google Scholar]
- How Accurate are Physicians' Clinical Predictions of Survival and the Available Prognostic Tools in Estimating Survival Times in Terminally ill Cancer Patients? A Systematic Review. Clin Oncol (R Coll Radiol). 2001;13:209-18.
- [CrossRef] [PubMed] [Google Scholar]
- Unexpected Death in Palliative Care: What to Expect when you are not Expecting. Curr Opin Support Palliat Care. 2015;9:369-74.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Discussions in Advanced Cancer: A Qualitative Thematic Analysis of Patients' and Caregivers' Experiences in a Tertiary Cancer Center in India. Indian J Med Paediatr Oncol. 2024;46:183-90.
- [CrossRef] [Google Scholar]
- Clinical Signs of Impending Death in Cancer Patients. Oncologist. 2014;19:681-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Prediction Survival of Advanced Cancer Patients by Palliative Care: A Multi-Site Study. Int J Palliat Nurs. 2016;22:380-7.
- [CrossRef] [PubMed] [Google Scholar]
- Using the Surprise Question to Identify those with Unmet Palliative Care Needs in Emergency and Inpatient Settings: What do Clinicians Think? J Palliat Med. 2017;20:729-35.
- [CrossRef] [PubMed] [Google Scholar]
- The Prognostic Value of the 12-, 6-, 3-and 1-Month 'Surprise Question'in Cancer Patients: A Prospective Cohort Study in Three Hospitals. Eur J Cancer Care (Engl). 2022;31:e13551.
- [CrossRef] [PubMed] [Google Scholar]
- "The Surprise Questions" Using Variable Time Frames in Hospitalized Patients with Advanced Cancer. Palliat Support Care. 2022;20:221-5.
- [CrossRef] [PubMed] [Google Scholar]
- Surprise Questions for Survival Prediction in Patients with Advanced Cancer: A Multicenter Prospective Cohort Study. Oncologist. 2015;20:839-44.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostication in Advanced Cancer: Update and Directions for Future Research. Support Care Cancer. 2019;27:1973-84.
- [CrossRef] [PubMed] [Google Scholar]
- Health Literacy in Communication, Decision-Making and Outcomes among Cancer Patients, their Families and Clinicians in India: A Multicentre Cross-Sectional Qualitative Study. Psychooncology. 2022;31:532-40.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosticating in Patients with Advanced Cancer--Observational Study Comparing the Accuracy of Clinicians' and Patients' Estimates of Survival. Ann Oncol. 2013;24:482-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative Multidisciplinary Prediction of Survival in Patients with Advanced Cancer. Support Care Cancer. 2014;22:611-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of Survival in Patients with Advanced Cancer: A Narrative Review and Future Research Priorities. J Hosp Palliat Care. 2023;26:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Evaluation in Patients with Advanced Cancer in the Last Months of Life: ESMO Clinical Practice Guideline. ESMO Open. 2023;8:101195.
- [CrossRef] [PubMed] [Google Scholar]







