Translate this page into:
The Benefit of Zinc Sulfate in Oropharyngeal Mucositis during Hyperfractionated Accelerated Concomitant Boost Radiotherapy with Concurrent Cisplatin for Advanced-Stage Oropharyngeal and Hypopharyngeal Cancers
Address for correspondence: Dr. Rahamathulla Mudassar Sharief, Department of Dental Surgery, Government Arignar Anna Memorial Cancer Institute, Karapettai, Kanchipuram - 631 552, Tamil Nadu, India. Affiliated to the Tamil Nadu Dr. MGR Medical University, No. 69, Anna Salai, Guindy, Chennai - 600 032 Tamil Nadu, India. E-mail: mudassarsharief@rediffmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Radiation-induced oropharyngeal mucositis is a major problem causing widespread clinical symptoms and may interfere with treatment plans, ultimately jeopardizing patient outcome. Zinc supplementation may be considered beneficial in preventing acute toxicity during chemoradiation.
Aims and Objective:
The aim of the study is to determine the effect of zinc supplementation on radiation-induced oropharyngeal mucositis in Stage III and IV-A oropharynx and hypopharynx cancers treated by hyperfractionated accelerated concomitant boost radiotherapy with weakly cisplatin. The objective behind the study is to know any changes in the onset, duration, and severity of oropharyngeal mucositis by implementation of oral zinc sulfate.
Materials and Methods:
The study is double-blinded randomized controlled assessment involving 120 patients (60 – control and 60 – experimental) treated with chemoradiation for oropharyngeal and hypopharyngeal cancers. The experimental group received oral zinc sulfate 150 mg once daily during and after treatment, whereas the control group patients were given placebo. The categorical data were analyzed using the Chi-square test and Pearson correlation. The Friedman test was used for comparison of oral mucositis grading between the groups.
Results:
A statistically significant difference was found in the zinc-supported experimental group showing delay in onset, decrease in severity, and duration of oropharyngeal mucositis.
Conclusion:
Zinc supplementation could be beneficial in managing oropharyngeal mucositis during chemoradiation of head-and-neck cancers with no untoward side effects.
Keywords
Chemoradiotherapy
head-and-neck cancers
hyperfractionated radiotherapy with concomitant boost
oral mucositis
zinc supplementation
INTRODUCTION
Oropharyngeal mucositis (OM) due to the treatment of head-and-neck cancers by radiotherapy (RT) and systemic therapies represents a major problem causing widespread clinical symptoms such as debilitating oral pain, bleeding, dysphagia, infections, and reduced quality of life and may interfere with delivery of the programmed treatment plans, ultimately jeopardizing patient outcome.[1] Oropharyngeal mucositis is a complex biological process involving direct damage to the dividing cells of oral epithelium, with depletion of the basal epithelium, modulation of the immune system, inflammatory process, and superadded infection by oral bacterial flora.[2] The multidisciplinary treatment strategies in treating head and neck cancer patients such as surgery, RT and systemic therapies- including chemotherapy (CT) and targeted therapy (TT) has resulted in substantial increase in acute toxicity. Various risk factors are considered for the development of OM, which include poor oral hygiene, periodontal disease, alcohol or tobacco use, xerostomia, low body mass index <18.5, comorbidities such as diabetes mellitus, immunodeficiency, vascular, renal, liver, and lung function impartment, and older patients.[1] During the past two decades, several trials have shown altered fractionation RT like accelerated fractionation schedules, hyperfractionated RT with or without CT has resulted in significant improvement in tumor response and overall survival.[34] Although accelerated/hyperfractionated RT improved the response rate and disease-free survival, it is usually associated with greater incidence of oral ulcerative mucositis resulting in dysphagia and odynophagia.[5] Moreover, adding concurrent CT usually by weekly cisplatin 40 mg/m3 to hyperfractionated RT has resulted in high exponential rate of toxicity.[5] The higher grades of toxicity can cause planned or unplanned treatment breaks that may reduce the beneficial effect of chemoradiotherapy by reducing biological effective dose due to cancer cell repopulation during the break period. It is estimated that approximately 60% of patients receiving conventional RT and more than 90% of those receiving experimental modalities (i.e., altered fractionation and combination of CT and RT) will develop OM.[2]
Zinc has been shown as an essential trace element in diverse physiological process such as growth and development, maintenance and priming of the immune system, and tissue repair. It acts as a catalytic component for more than 300 enzymes, structural constituent of many proteins, and regulatory ion for the stability of proteins and in preventing free radical formation.[2] The antioxidant role of the zinc can be elucidated by following two mechanisms – the protection of sulfhydryl groups against oxidation and the inhibition of the production of reactive oxygen species (ROS) by transition metals.[2] Zinc ions may induce the synthesis of metallothionein which plays a pivotal role in metal-related cell homeostasis, because of its high affinity for free radical, thereby forming protective roles against cytotoxic effect of reactive oxygen species, ionizing radiation, electrophilic anticancer drugs, mutagens, and metals. Iron and copper ions catalyze the production of hydroxyl radicals from H2O2, and zinc is known as an eminent competitor for iron and copper in binding to cell membranes, thus decreasing the production of hydroxyl radicals.[6] Zinc plays a part in the maintenance of epithelial and tissue integrity by promoting cell growth and suppressing apoptosis and by its underappreciated role as an antioxidant protecting against free radical damage during inflammatory responses.[7]
Studies reveal that a slight decrease in zinc status may first influence the immune system, owing to an increased number of infections. The impact of zinc on immunocompetence is greater in cell-mediated immunity than in humoral immunity. In particular, chemotaxis by neutrophils and monocytes, thymic endocrine activity, antigen presentation by MHC Class II molecules, natural killer activity, cytokine production, and TH1/TH2 balance are the immune functions affected by zinc. Zinc stabilizes the structures of DNA, RNA, and ribosome and activates numerous enzymes associated with nuclear material synthesis.[68]
The benefit of zinc supplementation has been clearly illustrated in several randomized control trials, and excess oral ingestion to the point of zinc toxicity is rare.[29101112131415] Hence, the aim of the study is to determine the effect of zinc supplementation on chemoradiation-induced oral/oropharyngeal mucositis in Stage III and IV-A oropharynx and hypopharynx cancers treated by hyperfractionated accelerated concomitant boost RT with weakly cisplatin. The objective behind the study is to know any changes in the onset, duration, and severity of oropharyngeal mucositis by implementation of oral zinc sulfate.
MATERIALS AND METHODS
Sample
A total of 120 patients with Stage III and IV-A oropharynx and hypopharynx cancers who were treated by chemoradiotherapy in Government Arignar Anna Memorial Cancer Hospital, Regional Cancer Center, Kanchipuram, India, during January 2018–December 2019 were included in the study. The participants were randomly allocated to experimental (n = 60) or control (n = 60) groups by equal allocation method. Inform consent from each patient and ethical approval for the study were obtained from the institutional ethical committee. The inclusion criteria were the patients under the age of 70 years with good performance status having adequate bone marrow function which is defined as an absolute peripheral granulocyte count (AGC ≥4000 cells/mm3), platelet count of ≥1 lakh cells/mm3, hepatic function with bilirubin ≤1.5 mg%, serum creatinine ≤1.5 mg%, creatinine clearance ≥50 ml/min based on 24-h urine collection, SGOT or SGPT ≤2 times the upper limit of normal, and no symptomatic coronary heart disease. The exclusion criteria were patients with histologically other than squamous cell carcinoma, evidence of distant metastasis other than the regional lymph nodes, prior CT or RT to the head-and-neck region, patients who were surgically operated before, patients with more than one primary tumor, patients with other reasons for oral mucositis, pregnant women, and comorbid conditions such as renal or hepatic disorders. Patients who discontinued/expired during treatment regimen were also excluded. The sample size was based on the prevalence of the disease recorded in the cancer register of the Regional Cancer Center. Each patient was evaluated and staged in the Department of Otorhinolaryngology with the help of direct and indirect laryngoscopy and imaging, and for the required patients, nasogastric feeding tube was inserted for feeding purpose. Predental examination and active intervention were undertaken in the Department of Dental Surgery before chemoradiotherapy. To increase response rate and survival based on the previous trials,[45] all the patients received experimental modality of chemoradiotherapy with field covering more than one-third of the buccal mucosa through three-dimensional conformal radiation therapy.
Treatment regimen
The patients were administered according to concomitant boost regimen, wherein a second fractionation of 1.5 Gy was delivered to smaller volume, making it tolerable to the last 13 treatment days. The advantage of this concomitant boost is to act against the accelerated repopulation after initial radiation.[5] The initial target volume encompassing primary tumor and neck nodes draining above clavicles received 1.8 Gy per fraction (FX) 5 FX per week to 50.4 Gy in 28 fractions over 5.5 weeks (day 1–38). An additional extra boost of 1.5 Gy irradiation was delivered routinely from 16th treatment day onwards (16th-28th treatment day) to the gross tumor volume and clinically/radiologically involved lymph nodes. The total cumulative dose by adding the regular fraction along with extra boost radiation will be 69.5 Gy. Dose adjustment for spinal cord area and clinically/radiologically involved lymph nodes was undertaken based on the RT Oncology Group (RTOG) guidelines. Concurrent CT with weekly cisplatin (40 mg/m3) was given to all the patients through standard precautionary procedures. Zinc supplementation with oral zinc sulfate tablets 150 mg once daily was given to the 60 experimental group patients, and placebo tablets with similar color and odorless were given to the 60 control group patients in the same manner for all the days (including the nontreatment days) during the entire course of chemoradiotherapy and continued till 2 weeks after treatment. Each patient received standard oral care instructions, and oral hygiene was monitored regularly by a physician and dental hygienist during the entire course of treatment. All the patients were evaluated weekly starting from the 1st to the 6th week of treatment and till 2 weeks post chemoradiotherapy for acute toxicity. Oropharyngeal mucositis was graded based on the RTOG for acute toxicity scale by two independent physicians (Department of Radiation Oncology and Department of Dental Surgery) [Table 1]. The examiners recording the grade of mucositis and the patients were made blind (allocation concealment) to the experimental versus control group to eliminate any systematic bias.
| Tissue toxicity | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Mucous membrane | No change over baseline | Irritation, might experience mild pain not requiring analgesic | Patchy mucositis that produces an inflammatory serosanguinous discharge, might experience moderate pain requiring analgesic | Confluent, fibrinous mucositis, might include severe pain requiring narcotics | Ulceration, hemorrhage, and necrosis |
Statistical analysis
Statistical analyses were performed with IBM SPSS version 17 (SPSS Inc., Chicago, IL, USA). Descriptive statistics was computed. The Friedman test was used for comparison of oropharyngeal mucositis grading before, during, and after treatment in oropharynx and hypopharynx cancers among the experimental and control groups. Categorical data were analyzed using the Chi-square test and Pearson correlation. P < 0.05 is considered as statistically significant.
RESULTS
The demographic data including age, gender, tumor stage, and site were not statistically significant between the control and experimental groups [Table 2]. All the patients including the control and experimental groups developed mucositis regardless of grade, but there were differences on the onset, duration, and severity [Figure 1]. Among the 120 patients, 64 were treated for carcinoma oropharynx and 56 for hypopharynx. The patients were diagnosed as advanced-stage cancers, of which 38 (31.6%) were in Stage III in and 82 (68.4%) were in Stage IV.
| Characteristics | Experimental group (n=60) | Control group (n=60) | P |
|---|---|---|---|
| Age (years) | 56.3±6.4 | 57.2±6.6 | 0.623 |
| Gender | |||
| Male | 6 (10.0) | 14 (23.3) | 0.299 |
| Female | 54 (90.0) | 46 (76.7) | |
| Classification of cancer | |||
| Posterior one-third of tongue | 22 (36.7) | 18 (30.0) | 0.063 |
| Postcricoid | 4 (6.7) | 8 (13.3) | |
| Posterior pharyngeal wall | 6 (10.0) | 4 (6.7) | |
| Pyriform fossa | 18 (30.0) | 16 (26.7) | |
| Soft palate | 2 (3.3) | 12 (20.0) | |
| Tonsil | 8 (13.3) | 2 (3.3) | |
| Stage III | 14 (23.3) | 24 (40.0) | 0.267 |
| Stage IV | 46 (76.7) | 36 (60.0) |
Mean±SD; Chi-square test; not significant. SD: Standard deviation

- The changes in mucositis grade between the control and experimental groups of both oropharyngeal and hypopharyngeal cancers
The onset of mucositis [Figure 2a] encountered was during the 2nd to the 3rd week and lasted till the end of the treatment. All the members of the control group developed mucositis in the beginning of the 2nd week (mean: 1260 cGY) in contrast to 30% of the patients from the experimental group. Similarly, the mucositis can be found even after 2 weeks on completion of chemoradiotherapy in 93.3% of the controls in conflict with 16.6% of the experimental group. Hence, the duration of mucositis is relatively less in the zinc-supplemented experimental group [Table 3 and Figure 1].
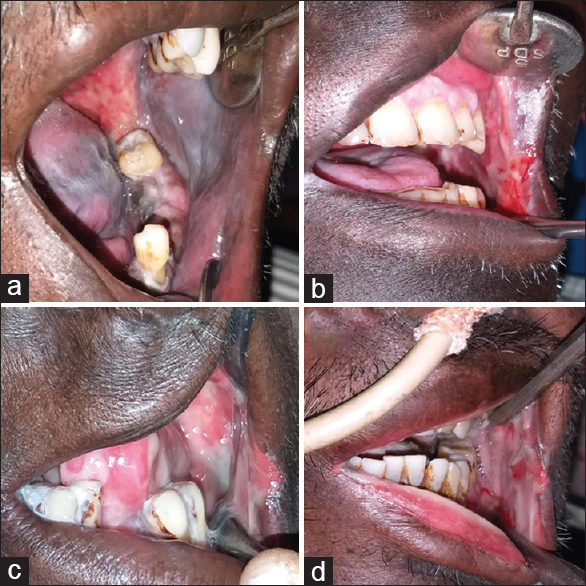
- Different grades of oral mucositis (Radiotherapy Oncology Group scoring criteria), (a) Grade 1 mucositis showing mild changes in the lining mucosa, (b) Grade 2 patchy mucositis that produces inflammatory serosanguinous discharge in the buccal mucosa, (c) Grade 3 confluent mucositis involving buccal mucosa, lip, and tongue, (d) Grade 4 mucositis showing ulceration and hemorrhage alimentation not possible
| Grade of oral mucositis | Experimental group (n=60) | Control group (n=60) | ||
|---|---|---|---|---|
| Oropharynx (n=32) | Hypopharynx (n=28) | Oropharynx (n=32) | Hypopharynx (n=28) | |
| Baseline | Grade 0 | Grade 0 | Grade 0 | Grade 0 |
| At 1st week | Grade 0 | Grade 0 | Grade 0 | Grade 0 |
| At 2nd week | Grade 0 | Grade 0 | Grade I | Grade I |
| At 3rd week | Grade I | Grade I | Grade II | Grade II |
| At 4th week | Grade I | Grade I | Grade III | Grade II |
| At 5th week | Grade II | Grade II | Grade III | Grade III |
| At 6th week | Grade II | Grade II | Grade III | Grade III |
| At post-RT 1st week | Grade I | Grade I | Grade II | Grade II |
| At post-RT 2nd week | Grade 0 | Grade 0 | Grade II | Grade I |
| P | 0.001* | 0.001* | 0.001* | 0.001* |
Friedman test; *P<0.05. RT: Radiotherapy
Grade II and Grade III mucositis [Figure 2b and c] appeared sooner (P = 0.001) in the placebo group than in the experimental group. The difference in the mucositis scores between the zinc (experimental) and placebo (control) groups continued till 2 weeks posttreatment [Friedman test, Table 3]. In the subgroup analysis by the region (oropharynx and hypopharynx) and stage (III and IV), no significant differences could be found in the development and progress of oral mucositis.
The severe form, i.e., Grade IV mucositis [Figure 2d], was noted in 12 patients (10%), all from the control group enforcing absolute treatment break for a week. In the subgroup assessment, the patients who developed Grade IV mucositis had carcinoma of oropharynx (posterior one-third of the tongue).
The Friedman test makes it conspicuous the significant difference in duration and severity, which is less in the zinc-supported experimental group [Table 3 and Figure 1]. The relative change in oral mucositis grading was represented in a graph [Figure 1]. The side effects due to orally administered zinc sulfate such as gastrointestinal discomfort and changes in the cell count were not noted.
DISCUSSION
Oropharyngeal mucositis (OM) is defined as inflammation of oropharyngeal mucosa resulting from cancer therapy typically manifesting as atrophy, swelling, erythema, and ulceration.[16] The condition may be exacerbated by local factors such as traumas from the teeth, improper oral hygiene methods, or microbial colonization. The four phases of mucositis are initial inflammatory/vascular, epithelial breakdown, ulcerative/bacteriological, and healing phase.[13] Oral mucositis is considered as one of the most debilitating side effects resulting in difficulty of eating, swallowing, requiring analgesics and supportive care, reducing the quality of life, and ultimately leading to discontinuation of treatment and increased hospitalization.[1718]
The clinical course of OM during conventional RT (2Gy/day in a single fraction, 5 fraction/week to a total cumulative dose of 60-70 Gy) is often predictable-i.e., erythema of the mucosa appears along with mild-to-moderate pain after the cumulative dose of 10 Gy, atrophic changes of the epithelium after the dose of 16-22 Gy and ulcerative lesions develop in the nonkeratinized mucosa during the later phases of radiation.[12] The involvement of orthokeratinized mucosa in the regions of hard palate, gingiva, and dorsum of the tongue is uncommon. The lesion may persist 2–4 weeks even after completion of the RT.[191718] This study utilized an experimental modality of hyperfractionated concomitant boost RT with concurrent weakly cisplatin 40 mg/m3 to increase the response rate in advanced-stage oropharyngeal and hypopharyngeal cancers. Previous studies[45] have shown an increase in incidence and severity of oral mucositis in experimental modalities, which was in accordance with this study as all the patients irrespective of groups developed mucositis during the course of treatment.[22021] The clinical course of the development of OM in the zinc-supported experimental group and the placebo added control group varied. The onset of the OM in the control group was much early during the 2nd week with a mean radiation dose of 12.6 Gy when compared to the zinc-supported (experimental) group at a mean radiation dose of 21.6 Gy. Similarly, the average duration of the mucositis was greater in the control group 6.8 versus 5.2 weeks in the experimental group. Moreover, all the members in the zinc group had completed a planned regimen without any treatment breaks or increased hospital stay.
As per the prevalence of oropharyngeal mucositis in general,[322] it was expected to find a lesser grade of OM in hypopharynx cancer (including the experimental and control groups), but the data revealed no significant difference in onset and progression of OM between oropharynx and hypopharynx cancers, which makes it suspicious to consider the field of the radiation along with backscattering effect is proportionally large and raises the question of conformity in hypopharynx cancer patients. The other factors involved in the pathogenesis of OM such as release of inflammatory mediators, nutritional status, and oral hygiene measures might require evaluation.
Various treatment modalities were being tried to prevent/decrease the severity of mucositis which include antibiotics, anti-inflammatory agents, cytokines (granulocyte-colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor), mouth-coating agents (sucralfate), vitamins (A and E), amino acids (glutamine), prostaglandins (PGE-1 and PGE-2), immune regulatory agents (immunoglobulin and pentoxifylline), cytoprotectors (amifostine), hormones (melatonin), and honey or traditional preparations, but no agent has been universally approved.[12] The Multinational Association of Supportive Care in Cancer/International Society of Oral oncology guideline recommends patients to use an oral care program such as dental flossing, frequent soft toothbrushing, and nonmedicated alcohol-free mouthwash (e.g., normal saline or bicarbonate). Benzydamine hydrochloride is a nonsteroidal drug that has exhibited a local anti-inflammatory, analgesic, anesthetic, and antimicrobial activity with clinical trials showing effectiveness and got approved to be used as mouthwash in larger trials by the panel of multidisciplinary experts in head-and-neck cancer (Milan, 2013).
Zinc is considered as a trace element which has properties of antioxidant, essential role in improving immunity, increases the gastrointestinal epithelial barrier function, and consequently, decreases the cell death and detachment.[7] It also acts as a cofactor for more than 300 enzymes such as DNA polymerase, RNA polymerase, protein synthesis, and cell proliferation. Zinc is also known to be a substantial element for collagen synthesis, fibroblast, and keratinocyte proliferation and enhances re-epithelialization.[15] The main role in preventing OM is that the zinc aids in scavenging ROS through antioxidant enzymes (glutathione peroxidase, glutathione reductase, superoxide dismutase, and catalase), inhibition of inflammatory cytokine production/secretion, particularly tumor necrosis factor-alpha, interleukin-1 (IL-1) β, IL-6, and nuclear factor-kappa B, and prevention of apoptosis by antagonizing caspases, specifically caspase-3. The recommended daily dosage is 15 mg. The experimental daily dosage of zinc utilized in our study was 150 mg of zinc sulfate tablet equivalent to 68 mg of elemental zinc which is higher than the recommended dose similar to the previous studies,[291012131415] but no studies have proved zinc overdose-related toxicity. We believe that this high dose could aid in ulcer healing propriety by having increased re-epithelialization of mucosa and anti-inflammatory effect. However, an appropriate therapeutic dose needs to be calculated by assessment through further trails.
The main objective behind the study is to know whether zinc supplementation can be used to delay the onset and reduce the duration and severity of oral mucositis. Previous studies favor beneficial effect of zinc supplementation in oral mucositis following RT and CT (Moslemi et al., 2014, and Rambod et al., 2018). On the contrary, other studies go against zinc prescription (Mansouri et al., 2012, and Sangthawan et al., 2013) [Table 4]. This study elucidates the beneficial importance of zinc in preventing, decreasing the severity and ulcer healing of oral mucositis.[31213] Moreover, Mehdipour et al.[14] utilized zinc-based mouthwash and made evident its usefulness.
| Study | Year of publication | Number of patients | Method of zinc supplementation and dose | Treatment regimen | Beneficial (yes/no) |
|---|---|---|---|---|---|
| Ertekin et al.[2] | 2004 | 30 | Zinc sulfate tablets 50 mg 3 times daily | Conventional RT | Yes |
| Lin et al.[10] | 2006 | 100 | Pro-Z powder 25 mg 2 to 4 times daily | Concurrent CT and RT | Yes |
| Lin et al.[9] | 2010 | 100 | Pro-Z powder 25 mg 2 to 4 times daily | Concurrent CT and RT | Yes |
| Watanabe et al.[30] | 2010 | 31 | Polaprezinc solution rinse and swallow | Concurrent CT and RT | Yes |
| Mosalaei et al.[23] | 2010 | 58 | Zinc sulfate tablets 220 mg 3 times daily | Conventional RT | Yes |
| Mehdipour et al.[11] | 2011 | 30 | 10 ml of 0.2% zinc sulfate mouthwash 3 times daily | CT for leukemia patients | Yes |
| Mansouri et al.[15] | 2012 | 60 | Zinc sulfate tablets 220 mg twice daily | CT for bone marrow disorders | No |
| Gorgu et al.[24] | 2013 | 40 | Zinc C tablets 25 mg 4 times daily | Conventional RT | No |
| Sangthawan et al.[14] | 2013 | 144 | Zinc sulfate 50 mg tablets 3 times daily | Conventional RT | No |
| Moslemi et al.[12] | 2014 | 40 | Zinc sulfate 30 mg tablets 3 times daily | Concurrent CT and RT | Yes |
| Rambod et al.[13] | 2018 | 86 | Zinc sulfate 50 mg tablets 3 times daily | CT for leukemia patients | Yes |
| Shuai et al.[25] Meta-analysis | 2019 | 162 | Zinc sulfate tablets | Concurrent CT and RT | No |
RT: Radiotherapy, CT: Chemotherapy
The commonly used measuring systems for oral mucositis are as follows: the National Cancer Institute-Common Toxicity Criteria from the USA, the Toxicity criteria of the RTOG, the European Organization for Research and Treatment of Cancer, and the criteria set out by the WHO in 1979. The scoring system should be valid, reliable, daily usable, less interobserver variability, clearly described parameters, fewest possible aids, and comfortable to the patients.[2627] Each scoring system is based on ordinal and numerical parameters. The ordinal scale classifies the symptoms into described categories and always subjected to patients' interpretation. Numerical system is those where the severity is based on the clinical measurement. An ideal scoring system should record objective (erythema, lesion, and edema), subjective changes (pain or sensitivity and dryness), and functional changes (voice, swallowing, and chewing). This study utilized a RTOG system for measuring mucositis to include patient- and clinician-oriented measurement of both subjective and objective changes. However, recording functional changes occur during the course of OM, will further help in better clinical approach.
Zinc reduces the incidence and severity of mucositis, pain, xerostomia, taste disturbance, and food intake problems.[2829] On the other hand, the tumor response rate in patients undergoing chemoradiotherapy was not significantly reduced by zinc (P = 0.281). This phenomenon of sparing the tumor cells, making is susceptible to chemoradiation has been demonstrated.[30] Alam et al., 2018, proposed zinc deficiency in 65% of head-and-neck cancer patients[316] and importance of zinc in preventing cancer. No patient developed zinc-related toxicity. Moreover, the zinc serum level assay estimation would have been an added advantage.
CONCLUSION
Zinc supplementation could be beneficial in managing oropharyngeal mucositis during chemoradiation of head-and-neck cancers with no untoward side effects. It is inexpensive and easily administered to patients. However, further evaluation using randomized control trials in larger group of population will be necessary and helpful in appropriate therapeutic dose calculation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Mucositis in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus statements. Crit Rev Oncol Hematol. 2016;100:147-66.
- [Google Scholar]
- Zinc sulphate in prevention of radiation-induced oropharyngeal mucositis: A prospective, placebo-controlled randomized study. Int J Radiat Oncol Biol Phys. 2004;58:167-74.
- [Google Scholar]
- Concomitant cisplatin chemotherapy and radiotherapy in advanced mucosal squamous cell carcinoma of the head and neck.Long-term results of the Radiation Therapy oncology group study 81-17. Cancer. 1990;66:1861-8.
- [Google Scholar]
- Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798-804.
- [Google Scholar]
- Final results of loco-regional control and late toxicity of RTOG 9003: A randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiation Oncol Biol Phy. 2014;89:13-20.
- [Google Scholar]
- Zinc and gastrointestinal disease. World J Gastrointestinal Pathophysiol. 2014;5:496-513.
- [Google Scholar]
- Discrepancy of the effects of zinc supplementation on the prevention of radiotherapy-induced mucositis between patients with nasopharyngeal carcinoma and those with oral cancers: Subgroup analysis of a double-blind, randomized study. Nutr Cancer. 2010;62:682-91.
- [Google Scholar]
- Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head and neck cancers: A double-blind, randomized study. Int J Radiat Oncol Biol Phy. 2006;65:745-50.
- [Google Scholar]
- A comparison between zinc sulfate and chlorhexidine gluconate mouthwashes in the prevention of chemotherapy-induced oral mucositis. Daru. 2011;19:71-3.
- [Google Scholar]
- Oral zinc sulphate and prevention of radiation-induced oropharyngeal mucositis in patients with head and neck cancers: A double blind randomized controlled clinical trial. Int J Radiat Res. 2014;12:235-41.
- [Google Scholar]
- The effect of zinc sulfate on prevention, incidence, and severity of mucositis in leukemia patients undergoing chemotherapy. Eur J Oncol Nurs. 2018;33:14-21.
- [Google Scholar]
- A randomized double-blind, placebo-controlled trial of zinc sulfate supplementation for alleviation of radiation-induced oral mucositis and pharyngitis in head and neck cancer patients. J Med Assoc Thai. 2013;96:69-76.
- [Google Scholar]
- The effect of zinc sulfate in the prevention of high-dose chemotherapy-induced mucositis: A double-blind, randomized, placebo-controlled study. Hematol Oncol. 2012;30:22-6.
- [Google Scholar]
- Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support Care Cancer. 2015;23:3249-55.
- [Google Scholar]
- Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145-53.
- [Google Scholar]
- Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6 and amp; amp; 7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother Oncol. 2012;103:69-75.
- [Google Scholar]
- Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886-98.
- [Google Scholar]
- Effect of oral zinc sulphate in prevention of radiation induced oropharyngeal mucositis during and after radiotherapy in patients with head and neck cancers. Middle East J Cancer. 2010;1:69-76.
- [Google Scholar]
- The effect of zinc sulphate in the prevention of radiation induced oral mucositis in patients with head and neck cancer. Int J Radiat Res. 2013;11:111-6.
- [Google Scholar]
- Prophylaxis with oral zinc sulfate against radiation induced oral mucositis in patients with head and neck cancers: A systematic review and meta-analysis of four randomized controlled trials. Frontiers Oncol. 2019;9:165. [Doi: 103389/fonc201900165]
- [Google Scholar]
- A scoring system for the assessment of oral mucositis in daily nursing practice. Eur J Cancer Care (Engl). 2006;15:228-34.
- [Google Scholar]
- Cancer treatment-induced oral mucositis: A critical review. Int J Oral Maxillofac Surg. 2012;41:225-38.
- [Google Scholar]
- A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer. 1998;82:1938-45.
- [Google Scholar]
- Mechanism-based management for mucositis: Option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016;9:2007-16.
- [Google Scholar]
- Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer. 2010;127:1984-90.
- [Google Scholar]
- Correlation between zinc intake and zinc serum levels with C-reactive protein levels in head and neck cancer patients. World Nutr J. 2018;2:9-14.
- [Google Scholar]






