Translate this page into:
Palliative Radiotherapy in Locally Advanced Head and Neck Cancer after Failure of Induction Chemotherapy: Comparison of Two Fractionation Schemes
Address for correspondence: Dr. Swaroop Revannasiddaiah; E-mail: swarooptheone@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Among patients with locally advanced head and neck squamous cell cancers (LAHNSCC), the prognosis after nonresponse or progression despite induction chemotherapy (IC) is dismal, and further treatment is often palliative in intent. Given that nonresponse to chemotherapy could indicate subsequent radioresistance, we intended to assess the outcomes with two different fractionation schemes.
Aims:
To compare the outcomes of two fractionation schemes- ’standard’ (consisting 3GyX5 daily fractions for 2 consecutive weeks) versus ‘hybrid’ (6GyX3 fractions on alternate days during the 1st week, followed by 2GyX5 daily fractions in the 2nd week).
Settings and Design:
Prospective randomized controlled two-arm unblinded trial.
Materials and Methods:
Patients with locally advanced oropharyngeal, laryngeal, and hypopharyngeal cancers treated with a minimum of two cycles of taxane, platinum, and fluorouracil-based IC were eligible if residual disease volume amounted <30 cm3. Kaplan-Meier survival curves were compared by the log-rank test. Response rates were compared using the unpaired t-test. Quality of life (QOL) was measured via patient reported questionnaires.
Results:
Of the initially enrolled 51 patients, 45 patients (24 from standard arm, and 21 from the hybrid arm) were eligible for analysis. Despite being underpowered to attain statistical significance, there still seemed to be a trend towards improvement in progression-free (Hazard ratio (HR) for progression: 0.5966; 95% CI 0.3216-1.1066) and overall survival (HR for death: 0.6062; 95% CI 0.2676-1.3734) with the hybrid arm when compared to the standard arm. Benefits were also observed with regards to response rates and QOL. Rate of complications were similar in both arms.
Conclusions:
In comparison to the routinely used palliative fractionation scheme of 30 Gray (Gy) in 10 fractions (Fr), the use of hybrid fractionation which integrates hypofractionation in the 1st week, followed by conventional fractionation in the 2nd week, could possibly offer better response rates, QOL increments, and potential survival benefits among LAHNSCC patients even after failing to respond to IC.
Keywords
Hybrid regimen
Induction chemotherapy
Radioresistance
INTRODUCTION
Locally advanced head and neck squamous cell cancer (LAHNSCC) which is inoperable at diagnosis is often treated with a course of induction chemotherapy (IC) with an intention to downsize tumor volume prior to further treatment. The integration of taxanes along with platinum and fluorouracil have enhanced response rates with IC.[1],[2],[3] Even though a majority of patients attain good response rates with IC, the occasional patients who do not respond to IC are often treated with palliative radiotherapy. Current understanding states that nonresponse to IC often is a harbinger of radioresistance.[4],[5]
The most commonly utilized scheme for palliative radiotherapy in LAHNSCC is to deliver 30 Gray (Gy) over a period of 2 weeks, with 10 fractions (Fr) of 3 Gy each; wherein the intention of using 3 Gy per fraction instead of the conventional 2 Gy per fraction is to achieve optimal palliation within minimal time duration. Other common palliative schemes such as 20 Gy in 5 Fr and the ‘quad-shot’ also utilize hypofractionation mainly with the intention to reduce treatment time.[6],[7],[8],[9],[10],[11]
However, no study has experimented upon the specific population of LAHNSCC who have not responded to taxane based IC. Given that ‘LAHNSCC not responding to IC’ is different from ‘chemotherapy naive LAHNSCC’, it can be said that the use of a standard palliative dose fractionation schemes such as 30 Gy in 10 Fr may not be expected to give optimal results. Reasons within the complex domain of tumor biology may indicate that ‘LAHNSCC which could repair chemotherapy induced damage can as well repair radiation induced damage’, given that chemotherapy and radiotherapy both target the tumor deoxyribose nucleic acid (DNA).[4],[5]
Cells with enhanced ability to repair cytotoxic damage can be expected to behave like late responding tumors in the radiobiological sense, that is with a lower α/β ratio, implying a higher ability to repair sublethal damage (SLD), and thus being resistant to standard doses per fraction. Thus, assuming a larger ability to damage SLD would mean a lower α/β ratio, meaning that the use of larger doses per fraction (hypofractionation) can be expected to be more effective.[12],[13],[14],[15]
In our experimental arm (hybrid arm), we have incorporated hypofractionation in the 1st week, as well as conventional fractionation in the 2nd week. This is with the assumption that a tumor consists of cell having varying radiosensitivities.[16],[17],[18],[19],[20] The use of hypofractionation in the 1st week (with 18 Gy in 3 Fr on alternate days) can be theoretically expected to be very effective against late responding tumor cells, the same which did not respond to preceding IC. The subsequent use of 10 Gy in 5 Fr on consecutive days in the 2nd week, is with an intention to target any tumor cells activated into mitosis via proliferation signals produced during the 1st week of RT. The use of standard fractionation should suffice to tackle these cells which if actively mitotic, would be behaving akin to early responding cells.[21],[22],[23],[24],[25] The control arm (standard arm) was so chosen since 30 Gy in 10 Fr is the most commonly used dose fractionation scheme for palliation in head and neck cancers.[6]
MATERIALS AND METHODS
This prospective randomized controlled, un-blinded trial is unique in that this is the first ever study assessing the outcomes with two radiation therapy (RT) fractionation schemes among a specific patient population of LAHNSCC who had a residual gross disease volume of at least 30 cm3 measured on contrast-enhanced computed tomography (CECT) at least after 4 weeks of completion of a minimum of two cycles of IC containing all three of docetaxel, 5-fluorouracil, and cisplatin (TPF). In retrospect, the pre-IC stages of all patients were stage IVA/IVB, with all patients having N2b/N3 pretreatment nodal status.
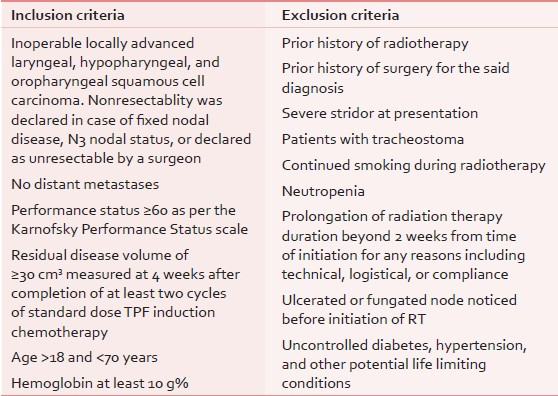
Subject to fulfilment of the eligibility criteria [Table 1], the patients were randomized on a 1:1 basis into the two arms: The ‘hybrid arm’ and the ‘standard arm’.
Patients in the ’standard arm’ were treated to a dose of 30 Gy delivered over 2-weeks, with 10 Fr of 3 Gy each. Patients in the hybrid arm were treated with 3 Fr of 6 Gy each during the 1st week, followed by 5 Fr of 2 Gy each during the 2nd week. Radiotherapy was delivered by conventional radiotherapy with multileaf collimator based field shaping as determined by virtual simulation.
CT was obtained for each patient in either arm, prior to the initiation of radiotherapy as well as on the completion of 4-weeks after radiotherapy. The CT images were used to calculate disease volume, which was obtained by the sum the contoured gross primary and nodal disease volumes. The pretreatment and posttreatment disease volumes of each patient were compared to assess percentage change in the disease volume after radiotherapy.
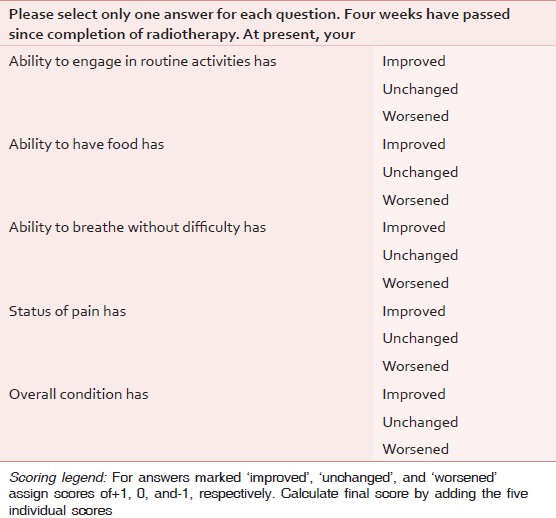
Quality of life (QOL) questionnaire was specifically devised for the purpose of this study [Table 2] and scores were obtained at 4-weeks post radiotherapy. The questionnaire contains five simple questions, each with three discrete options: ‘Improved’, ‘unchanged’, and ‘worsened’; which would be assigned scores of ‘+1’, ‘0’, and ‘−1’, respectively. The sum of scores from the five questions was recorded as the final QOL score of the individual patient. Thus, the maximum and minimum possible QOL scores would be +5 and −5, respectively. The difference in results of the two arms was tested for significance by the independent samples t-test.
The 1st day of radiotherapy was selected as the starting point for all time-based measures in the study. Patients were followed-up for 2-weekly intervals for the first 3 months, followed by 4-weekly intervals beyond 3 months.
Differences in the two arms of response in the form of measured tumor volume changes were compared using the independent samples t-test. Additionally, responses were classified into discrete categories also-; as ‘complete’ (100% regression), ‘near-complete’ (75-99% regression), ‘partial’ (31-74% regression), ‘minor’ (1-30% regression), and ‘nonresponse’ (for zero-response and progressive). Differences in the distribution in between the two arms were tested for significance by using the Fisher's exact test.
Kaplan-Meier survival curves (for progression free and overall survival) of the two arms were compared using the log-rank test. Additionally, number of patients surviving beyond 3 and 6 months in each of the arms were compared using the Fisher's exact test.
RESULTS
Of the enrolled 51 patients, 45 were eligible for final analysis. Two patients from the standard arm and four patients from the hybrid arm were excluded from final analysis. The necessity for exclusion arose from an inability to complete planned course of radiotherapy within the strict schedule of 2 weeks. Of the six patients excluded, four patients could not complete treatment in planned time schedule due to an unexpected treatment machine downtime. Two patients cited logistical reasons for not being able to comply with the schedule.
The pre-RT characteristics of patients were similar between the two arms. The distributions with regards to post chemotherapy residual disease volume were similar in both arms (initial mean volumes of 44 vs 46.9 cm3 and ranges of 31-70 vs 31-69 cm3 for the hybrid arm and standard arm, respectively). The distributions with regards to age were also comparable (mean age 54.3 vs 53.3 years and ranges of 39-65 vs 36-64 years for the hybrid arm and standard arm, respectively). The 95% confidence interval (CI) for the median with regards to Karnofsky Performance Status (KPS) of patients in both arms was similar, lying in the 80-90 value. Majority of patients in the study had been referred to us after having received three cycles of induction docetaxel, 5-fluorouracil, and cisplatin (TPF), while only three patients in either arm had received two cycles of TPF. The initiation of RT in all patients was strictly ensured at the 5th week after completion of IC.
The tolerances to both arms were equally good. This could possibly be due to the very strict selection criteria. There were no radiotherapy related deaths in the study. There were no incidences of acute toxicities of grade-3 or higher (toxicity as measured by the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3).[26]
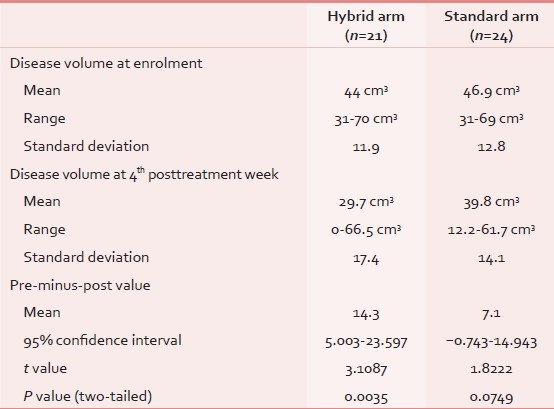
The mean disease volumes at presentation were 44 cm3(range: 31-70 cm3; standard deviation (SD): 11.9) and 46.9 cm3(range: 31-69 cm3; SD: 12.8) in the hybrid arm and the standard arm, respectively. Measured at 4th posttreatment week, the disease volumes were 29.7 cm3(range: 0-66.6 cm3; standard deviation: 17.4) and 39.8 cm3(range: 12.2-61.7 cm3; standard deviation: 14.1) in the hybrid arm and the standard arm, respectively. The amount of volumetric regression was seen to be greater in the hybrid arm in comparison to the standard arm, with the mean pre-minus-post value being 14.3 cm3(P = 0.0035; 95% CI: 5.003-23.597) vs 7.1 cm3(P = 0.0749; 95% CI: −0.743-14.943), respectively [Table 3].
There was only one case of complete response (100% volumetric regression) in the entire study population, and the patient belonged to the hybrid arm. If <30% volume regression was considered as ‘response’, the rates of response were 47.6 vs 25% (P = 0.13) in the hybrid and the standard arms, respectively. Further, 14.3 vs 0% (P = 0.09) of patients in the hybrid and the standard arms achieved <70% regression.
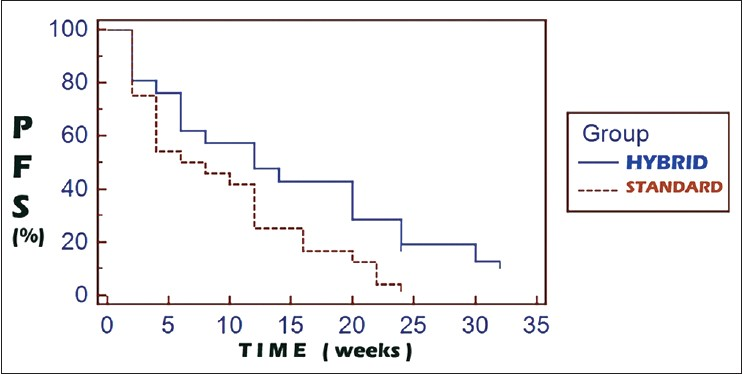
On comparison of the survival curves of the hybrid arm and the standard arm, the hazard ratio for progression was 0.5966 (95% CI: 0.3216-1.1066). The progression free survival (PFS) comparisons [Figure 1] between the hybrid and the standard arms were 47.6 vs. 25% and 19 vs 0% at 3 and 6 months follow-up, respectively (P = 0.0613). Comparisons with regards to overall survival (OS) [Figure 2] between the hybrid and the standard arm revealed the hazard ratio for death as 0.6062 (95% CI: 0.2676-1.3734). The OS comparisons were 85.7 vs 79.2% and 61.9% vs 42.1% at 3 and 6 months, respectively (P = 0.2019).
- Kaplan-Meier curves for progression free survival
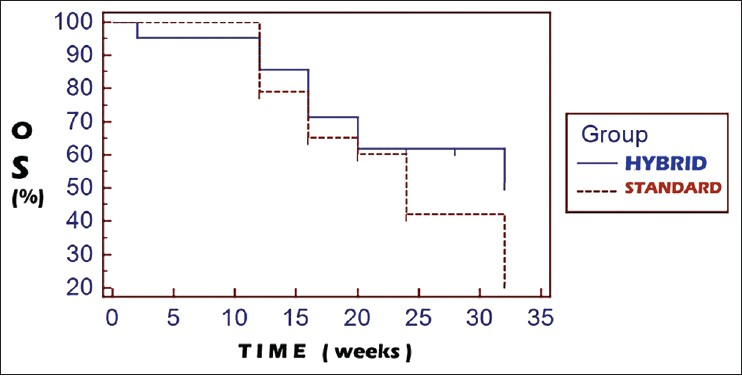
- Kaplan-Meier curves for overall survival
QOL was measured by a simple questionnaire designed to be easy and unambiguous [Table 2]. The QOL questionnaire contained five questions with three discrete answers. Each answer would be scored + 1, 0, or −1 as per whether the patient perceives ‘improved’, ‘unchanged’, or ‘worsened’ condition at 4-weeks after completion of radiotherapy. The sum of the five answers would lead to the final score, which could range from a maximum of +5 to a minimum of −5. The range of scores obtained were 0-4 in both arms of the study. The range of QOL scores in both the arms was from 0 to 4. No patient from either arm experienced a QOL worsening. The mean QOL scores were 2.67 vs 1.96 (P = 0.0396) for the hybrid and the standard arms, respectively.
DISCUSSION
IC is utilized often with the goal of reduction of tumor volume prior to definitive treatments. Other biological advantages include a potential efficacy against systemic micrometastases. However, prolongation of the overall treatment time has been a point against the widespread acceptance of IC as a routine standard of care. Even though survival benefit has not been noted, several studies have demonstrated a benefit in terms of tumor volume reduction and as well as in the reduction of distant metastases.[27],[28] While initial studies were mostly with the use of doublet-IC, recent studies have shown more favorable outcomes with the use of triplet regimens, by the addition of taxanes to the usual cisplatin and 5-fluorouracil.[29],[30] Response rates with IC are reportedly as high as <80%, with about half of the responding patients demonstrating complete responses.[27],[28]
Despite the good response rates with IC, the prognosis of the nonresponding population can be regarded as poor. It is due to the rationale that nonreponse to IC often is an indicator of subsequent radioresistance. Reasons include potentially enhanced repair mechanisms against cytotoxic insults such as radiation and chemotherapy. Another reason could be possibly because of a high proportion of dormant cells in the tumor, which could compromise radiosensitivity and chemosensitivity, since cytotoxicity is maximum upon actively dividing cells.[4],[5],[27],[28]
No trial has specifically studied the radioresponsiveness to palliative RT among LAHNSCC patients not responding to IC with TPF. Given that nonresponding tumors are in general considered to have low α/β ratios, it is possible that these tumors could respond better to larger doses per fraction (hypofractionation) than with standard fractionation.[12],[13],[14],[15],[31],[32]
Since the ability to repair SLD could be the reason for nonresponsiveness, the tumors are more likely to respond with large fraction sizes, at least theoretically. This hypothesis was to be tested in this trial.
In general, palliative RT is hypofractionated, but with the main intention of reducing overall treatment time. Further, many altered fractionation schemes utilize larger doses per fraction since late toxicities are often not anticipated given that patients receiving palliative RT are expected to have survival periods short enough to not allow manifestation of late toxicities. Most commonly used regimens include 30 Gy in 10 Fr, 35 Gy in 15 Fr, and 20 Gy in 5 Fr. A few altered fractionation schemes too exist, such as the ‘hypo-tail’, which utilizes hypofractionation during the final fractions of treatment; and the ‘quad-shot’, which delivers a large dose in a short period of time.[6],[7],[8],[9],[10],[11],[33]
We however attempted to devise a palliative RT scheme which would be effective, while at the same time not depositing excessive doses upon the critical organs, so as to prevent late toxicities in the (occasional) long-term survivor. Further, we intended to utilize different dose-fractions during the 1st and 2nd week of treatment given that tumors are presumed to contain different populations of cell which vary in their radioresponsiveness. Thus, in the experimental arm (hybrid arm), we utilized 3 Fr of 6 Gy each delivered on Mondays, Wednesdays, and Thursdays during the 1st week; while we utilized 5 Fr of 2 Gy each from Monday to Friday during the 2nd week. The rationale for utilizing hypofractionation during the 1st week is to elicit maximum response from slow responding and dormant tumor cells (these cells hold a higher ability to repair SLD, and hence are better tackled by hypofractionation). The rationale of utilizing standard fractionation during the 2nd week was to be able to deliver 5 Fr of 2 Gy each, which would be able to target any remaining tumor cells which could be induced into proliferation during the initial week of radiotherapy. Since proliferating cells can be expected to have larger α/β ratios, the use of smaller fractions provides optimal therapeutic ratio.[32],[34]
In the control arm (standard arm), we utilized the scheme of 30 Gy in 10 Fr, which happens to be the most commonly utilized scheme for head and neck palliative RT.
With the hybrid arm, assuming the spinal cord α/β ratio to be 1.5 Gy, using the Wither's linear-quadratic (LQ) isoeffect equation[34](based on the LQ model), a total dose of 48 Gy EqD2(equivalent dose if delivered in 2 Gy per fraction) would be delivered to the spinal cord, which happens to be an organs at risk (OAR) with the lowest threshold for late toxicity in the head and neck region. This is in comparison to 38 Gy EqD2 that would be delivered in the ’standard arm’. The EqD2 for a population of early responding cells (assuming α/β ratio of 10 Gy for early responding cells) would be 34 and 32 Gy for the hybrid and the standard arms, respectively. Thus, no differences in early toxicity could be expected.
Since we had not expected a large number of good responses, especially among a population of patients who were nonresponsive to IC, we had not generated a flowchart outlining the further management of patients with good responses. Thus, further management was continued as per the individual patient assessment, preferences, and prevailing circumstances. Further radiotherapy, when required was continued with three-dimensional conformal radiation therapy (3DCRT) or intensity-modulated radiation therapy (IMRT) so as to spare OARs prone for late toxicity.
Shortcomings
We have decided to terminate the study before the initially planned accrual of at least 150 patients. The actual accrual of only 52 patients (of which only 45 were eligible for analysis) has been much lesser than the initially expected accrual of <150 due to the unexpected rarity of patients fulfilling the eligibility criteria. Given that majority of LAHNSCC patients treated with IC with TPF respond very well, the absolute number of patients with a residual disease volume of more than 30 cm3 turned out to be very low. The cut-off value of 30 cm3 was selected arbitrarily, with the intention of reducing selection ambiguities and to induce a semblance of uniformity.
The flowchart for further treatment of patients with good responses to palliative radiotherapy post failure of IC was not devised by us before the initiation of trial. Given that poor outcomes are generally expected with palliative radiotherapy after failure of IC (since chemoresistance is often postulated to imply intrinsic radioresistance), we had not expected the possibility of significant number of patients enjoying responses well enough for them to be eligible for further treatment with radiotherapy. Thus, patients with good performance status and good response to radiotherapy were offered further treatment with continuation of radiotherapy on an individual patient basis (continuation RT was strictly given with intensity modulated radiotherapy only).
The questionnaire utilized in this study for QOL was devised by us to be simple and to be answerable with minimum time and effort for the patient. Though endowed with simplicity and ease of use, the said questionnaire lacks the detailed structure as would more standardized questionnaires, such as the EORTC-H and N35 and FACT-G, for examples.[35],[36],[37]
Failures in this study were noted upon clinical or radiological detection of local progression, or the detection of distant metastases. Since the use of18 F-fluorodeoxyglucose-positron emission tomography would offer higher sensitivity for detection of relapse, we regard the non-use of fluorodeoxyglucose-positron emission tomography (FDG-PET) during follow-up to have potentially caused a possible underestimation of disease progression.
With the recent understandings of the importance of p16/HPV status upon the radiosensitivity of HNSCC, future study designs will also have to accommodate it as a factor for stratification.[38],[39]
CONCLUSION
LAHNSCC failing to respond to IC can be expected to have intrinsic resistance towards subsequent radiotherapy, which according to radiobiological rationale could be due to a more robust ability to repair radiation induced sublethal damage upon the tumor cell DNA. Given that higher doses per fraction are more effective than lower doses per fraction, the use of three 6 Gy fractions in the 1st week could hold good efficacy against late responding tumor cells, whereas the use of five 2 Gy fractions in the 2nd week would be effective against any remaining tumor cells which could be initiated into proliferation during the prior week of radiotherapy.
Thus this study possibly validates that tumors could contain a dynamic mixture of dormant and progressive cells, thus utilizing a single fraction size for the entire treatment course could be ineffective against tumor cells of certain different radiosensitivities.
Despite being underpowered to detect any statistically significant benefit in response rates and survival, we have observed a strong trend towards enhanced outcomes with the use of the hybrid-fractionation approach. We are now in the planning stages of initiating a bigger multi-institutional trial, involving similar patient population as with this study, while also refining out the above mentioned deficiencies of this particular study.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Combination of taxanes, cisplatin and fluorouracil as induction chemotherapy for locally advanced head and neck cancer: A meta-analysis. PLoS One. 2012;7:e51526.
- [Google Scholar]
- EORTC 24971/TAX 323 Study Group. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695-704.
- [Google Scholar]
- Induction chemotherapy in patients with resectable head and neck squamous cell carcinoma: A meta-analysis. World J Surg Oncol. 2013;11:67.
- [Google Scholar]
- Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54:811-4.
- [Google Scholar]
- Diffusion weighted imaging in predicting progression free survival in patients with squamous cell carcinomas of the head and neck treated with induction chemotherapy. Acad Radiol. 2011;18:1225-32.
- [Google Scholar]
- Palliative radiation therapy for head and neck cancer: Toward an optimal fractionation scheme. Head Neck. 2008;30:1586-91.
- [Google Scholar]
- Hypofractionated radiotherapy denoted as the “Christie scheme”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. 2009;48:562-70.
- [Google Scholar]
- The ‘QUAD SHOT’: A phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137-42.
- [Google Scholar]
- Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment “Hypo Trial”. Radiother Oncol. 2007;85:456-62.
- [Google Scholar]
- Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol. 2004;71:275-80.
- [Google Scholar]
- Quad shot: A short but effective schedule for palliative radiation for head and neck carcinoma. Indian J Palliat Care. 2009;15:137-40.
- [Google Scholar]
- A review of alpha/beta ratios for experimental tumors: Implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys. 1985;11:87-96.
- [Google Scholar]
- Quantitative clinical radiobiology of early and late lung reactions. Int J Radiat Biol. 2000;76:453-62.
- [Google Scholar]
- Cellular responses to multifractionated irradiations. Gan No Rinsho. 1987;33:1571-5.
- [Google Scholar]
- Radiation related prognostic factors in radiation oncology. Eur J Gynaecol Oncol. 2000;21:7-12.
- [Google Scholar]
- Effect of heterogeneity in radiosensitivity on LQ based isoeffect formalism for low alpha/beta cancers. Acta Oncol. 2004;43:499-502.
- [Google Scholar]
- Heterogeneity of in vitro radiosensitivity in human bladder cancer cells. Radiat Oncol Investig. 1999;7:66-76.
- [Google Scholar]
- Radiosensitivity testing of primary cervical carcinoma: Evaluation of intra- and inter-tumour heterogeneity. Radiother Oncol. 1990;18:349-56.
- [Google Scholar]
- In vitro radiosensitivity of tumour cells and fibroblasts derived from head and neck carcinomas: Mutual relationship and correlation with clinical data. Br J Cancer. 1999;79:1074-84.
- [Google Scholar]
- Intratumoral heterogeneity as a confounding factor in clonogenic assays for tumour radioresponsiveness. Radiother Oncol. 1996;39:145-53.
- [Google Scholar]
- Activation of epidermal growth factor receptor and its downstream signaling pathway by nitric oxide in response to ionizing radiation. Mol Cancer Res. 2008;6:996-1002.
- [Google Scholar]
- Radiation-induced enhanced proliferation of human squamous cancer cells in vitro: A release from inhibition by epidermal growth factor. Clin Cancer Res. 1995;1:1557-62.
- [Google Scholar]
- Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170-6.
- [Google Scholar]
- Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283-300.
- [Google Scholar]
- Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells. Mol Biol Cell. 2002;13:2233-44.
- [Google Scholar]
- Cancer Therapy Evaluation Program. 2006. Common Terminology Criteria for Adverse Events (CTCAE). Version 3.0, DTCD, NCI, NIH, DHHS. Available from: http://ctep.cancer.gov/forms/CTCAEv3.pdf
- [Google Scholar]
- Prediction of distant metastasis in head neck cancer patients: Implications for induction chemotherapy and pre-treatment staging? Strahlenther Onkol. 2008;184:580-5.
- [Google Scholar]
- Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4-14.
- [Google Scholar]
- Induction chemotherapy for head and neck cancer: Recent data. Oncologist. 2010;15(Suppl 3):3-7.
- [Google Scholar]
- Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705-15.
- [Google Scholar]
- Cell surival Curves. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the Radiologist (6th ed). Philadelphia: Lippincott Williams and Wilkins; 2006. p. :30-46.
- [Google Scholar]
- The linear-quadratic approach in clinical practice. In: Joiner M, Van der Kogel A, eds. Basic Clinical Radiobiology (4th ed). London: Edward Arnold; 2002. p. :120-34.
- [Google Scholar]
- Retrospective study of palliative radiotherapy in newly diagnosed head and neck carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:958-63.
- [Google Scholar]
- A new isoeffect curve for change in dose per fraction. Radiother Oncol. 1983;1:187-91.
- [Google Scholar]
- Assessing quality of life in patients with head and neck cancer: Cross-validation of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Head and Neck module (QLQ-H and N35) Arch Otolaryngol Head Neck Surg. 2000;126:459-67.
- [Google Scholar]
- Validity and reliability of the FACT-G scale for use in the older person with cancer. Am J Clin Oncol. 2001;24:591-6.
- [Google Scholar]
- Reporting of “Quality of life”: A systematic review and quantitative analysis of research publications in palliative care journals. Indian J Palliat Care. 2012;18:59-67.
- [Google Scholar]
- Human papillomavirus-related head and neck tumors: Clinical and research implication. Curr Opin Oncol. 2009;21:201-5.
- [Google Scholar]






