Translate this page into:
Factors Influencing Compliance to Radical Treatment of Middle Thoracic Esophageal Cancer: An Audit from a Regional Cancer Centre
Address for correspondence: Dr. Anshuma Bansal; E-mail: dranshubansal3@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The aim of this study is to identify the factors responsible for interruption of planned treatment in patients of carcinoma mid-thoracic esophagus and also discuss the strategies for improving treatment completion rates.
Materials and Methods:
Patients with nonmetastatic mid-thoracic esophageal cancer who received treatment by multimodality approach using chemotherapy, radiation, and/or surgery were retrospectively analyzed. Factors influencing compliance with planned treatment completion were evaluated, and their significance was determined using multivariate Cox regression analysis.
Results:
Ninety-one patients were reviewed. Median follow-up period was 11 months. Of 15 patients planned with neoadjuvant chemoradiation followed by surgery (Group 1), only 6 (40%) could complete the treatment. Similarly, only 19 out of 36 patients (52.8%) completed the planned definitive chemoradiation (Group 2). Furthermore, of forty patients planned with definitive radiotherapy (Group 3), 29 patients only (72.5%) completed this schedule. The rate of completion of therapy was worst in Group 1. The most common reason for noncompletion of planned treatment was nutritional inadequacy and excessive weight loss in all groups. In addition, chemotherapy-induced myelosuppression (P = 0.05) was the factor leading to treatment interruption in Group 2 and radiation-induced acute mucositis (P = 0.02) and lost to follow-up (P = 0.02) were the factors in Group 3.
Conclusions:
Rate of treatment completion significantly impacts survival rates. Nutritional inadequacy was the most common factor for noncompletion of planned treatment. A well-trained management team consisting of oncologist, dietitian, and psychotherapist can help overcome these factors and thereby improve the treatment completion rates.
Keywords
Compliance
Esophageal cancer
Multimodality treatment
Treatment completion
INTRODUCTION
Esophageal cancer presents as a locally advanced disease in majority of the patients.[1] About 50% of cases of esophageal cancer occur in the middle thoracic esophagus which extends from 25 cm to 30 cm as defined in the most recent American Joint Committee on Cancer report.[2] The standard of care for these patients includes a multimodality approach involving surgery, chemotherapy, and radiotherapy in various combinations. For patients who are potential surgical candidates, commonly employed treatment strategy includes neoadjuvant chemoradiation followed by surgery, and adjuvant chemotherapy if indicated. For those patients with inoperable disease or at high-surgical risk, radical chemoradiation is the standard treatment.[34]
Multiple studies have demonstrated significantly better outcomes with multimodality approaches compared to surgery or radiotherapy alone,[56] emphasizing the fact that completion of the planned treatment protocol plays an important role in determining the outcomes of the patients. Unfortunately, many patients suffer from disease-related malnutrition and cachexia even before the initiation of the treatment.[7] Furthermore, the performance status of these patients often deteriorates during chemoradiation due to the associated acute toxicities, compromising the compliance of the patients to the treatment protocol, resulting in unexpected treatment breaks and shift of the treatment intent from cure to palliation.[8] The purpose of the present audit is to determine the factors which influence the treatment compliance and might potentially predict unintended discontinuation of the planned treatment protocol in the resource constrained scenario of a developing country. The difference in treatment outcomes between the patients who completed their planned treatment and those who could not was analyzed as the secondary endpoint.
MATERIALS AND METHODS
All the patients registered in the Department of Radiotherapy and Oncology of our institute between January 2008 and December 2012 with a diagnosis of locally advanced nonmetastatic middle thoracic esophageal cancer were included in this audit. Of the 1248 patients of esophageal cancer registered in the department, 91 patients (7.3%) of nonmetastatic middle thoracic esophageal cancer who planned for radical treatment were retrospectively analyzed.
Apart from histopathological examination of the biopsied specimen, initial evaluation of these patients included endoscopy and barium study to assess the mucosal extent of the disease and contrast enhanced computed tomography (CECT) scan of the chest to assess the extra-esophageal extent and nodal spread of the disease. Baseline evaluation of bone marrow, liver, and renal functions was carried out to assess the tolerance for chemotherapy.
Treatment groups
Potentially surgical candidates were planned for neoadjuvant chemoradiotherapy (Group 1) and received 30 mg/m2 of cisplatin and 325 mg/m2 of 5-flurouracil (5-FU) for 4 days, followed by external radiation of 30 Gy in ten fractions over 2 weeks as per the departmental protocol. Surgery was planned depending on the radiological response after 4-6 weeks. Adjuvant chemotherapy was offered based on the pathological response on histological examination.
Patients who were deemed inoperable per se were planned for definitive chemoradiotherapy (Group 2) with external radiation of 60 Gy in 30 fractions over 6 weeks, concurrently with 30 mg/m2 of cisplatin and 325 mg/m2 of 5-FU every week as per the departmental protocol.
Inoperable patients who were not suitable candidates for concurrent chemotherapy because of deranged renal functions but otherwise with a good performance status were given radical radiotherapy (Group 3) with external radiation of 60 Gy in 30 fractions over 6 weeks as per the departmental protocol.
Treatment planning
All the patients in Groups 1, 2, and 3 were planned on conventional simulator using open-posterior fields. The patients in Groups 2 and 3 were planned using anteroposterior fields till a dose of 40 Gy in twenty fractions, covering 5 cm of normal esophagus proximal and distal to the disease as determined by barium contrast, with a lateral margin of 3 cm on either side. The residual disease along with a proximal and distal margin of 2 cm and a circumferential margin of 2 cm is boosted using one anterior and two posterior oblique fields to a dose of 20 Gy.
Monitoring
All the patients were monitored once a week for acute treatment-related toxicity during the entire course of treatment. The monitoring included subjective and objective assessment of treatment-related toxicities using version 3 of Common Terminology Criteria for Adverse Events. U.S. Department of Health and Human Services. National Institutes of Health and National Cancer Institute.[9] Weekly monitoring of complete blood counts and renal function tests was done for those undergoing concurrent chemotherapy. Nutritional intake of the patients was also monitored and appropriate dietary advice was given. Any interruption in the planned treatment was documented, and the reason for the interruption was specified. The patients were followed up clinically at 2 monthly intervals, and response assessment was performed using barium study and endoscopy at the first follow-up (2 months posttreatment) and CECT chest at the third follow-up (6 months posttreatment). Any clinical suspicion of recurrence was evaluated with appropriate radiological investigations and documented.
The patients who could not complete all or a part of their initial plan of treatment were considered to be defaulters, and the factors determining the treatment compliance were evaluated for all the three groups separately.
Statistical analysis
Descriptive statistics was obtained for characterization of the treatment groups. Various factors potentially influencing the compliance of the patients to the planned treatment protocol were evaluated in multivariate analyses using Cox proportional hazard model. As the secondary endpoint, the loco-regional control and survival were calculated using Kaplan-Meier method and significance determined using the log-rank test. SPSS 19, (IBM, Armonk, NY, United States of America) was used for the analysis.
RESULTS
Patients profile
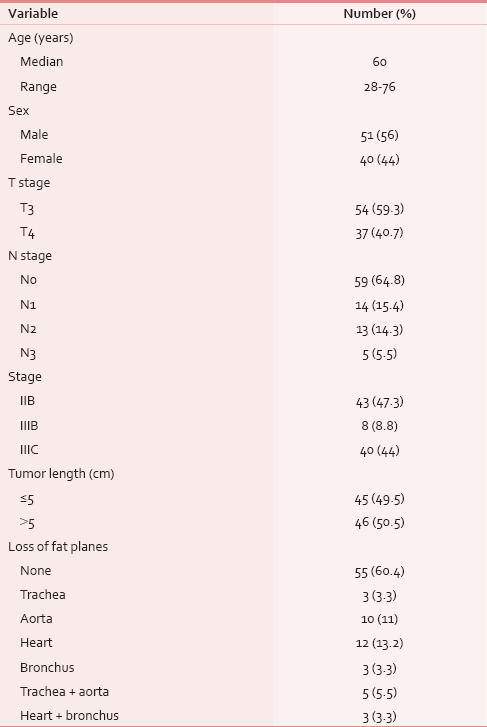
The patient and disease characteristics are shown in Table 1.
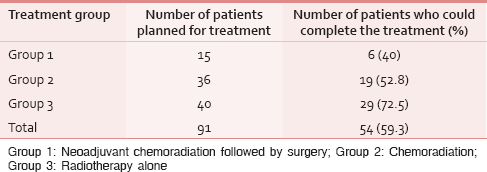
Treatment characteristics
Table 2 shows the treatment details of the patients. Among the studied population, only 59.3% could complete the entire course of intended treatment. About 40.7% of the patients could not complete the treatment completely, and the factors determining the treatment compliance were analyzed separately for each group.
Factors hindering treatment completion
Table 3 shows the factors hindering treatment completion.
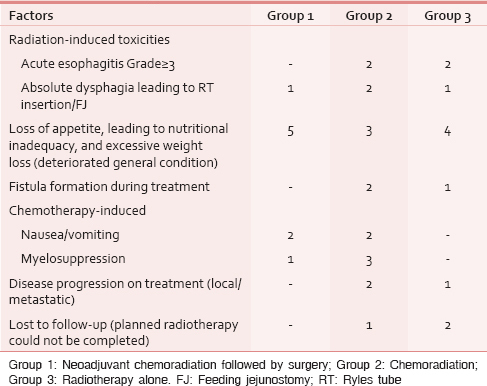
Among the 15 patients in Group 1, nine patients could not tolerate the adjuvant chemotherapy though indicated. Postoperative weight loss, extreme loss of appetite, severe nausea, and poor hematological tolerance were the common causes of treatment interruption in this group. On multivariate analysis, loss of appetite postoperatively leading to nutritional inadequacy and excessive weight loss was the only factor which was significantly correlated to treatment interruption in the form of inability to take adjuvant treatment (P = 0.02).
Among the 36 patients in Group 2, 17 patients could not complete planned treatment. The only factors which significantly correlated with noncompliance to treatment completion in this group were loss of appetite and worsened nutritional intake (P = 0.05) and chemotherapy-induced myelosuppression (P = 0.05).
Among the forty patients in Group 3, 11 patients could not complete planned treatment. The factors which significantly correlated with noncompliance to treatment completion were radiation-induced acute mucositis (P = 0.02), loss of appetite and worsened nutritional intake (P = 0.000), and lost to follow-up (leading to noncompletion of planned radiotherapy) (P = 0.02).
Outcome
As the secondary endpoint, the patterns of failure, local control, and survival of each group were analyzed separately for the subsets who completed their intended treatment and the defaulters.
The majority of the patients failed locally and the maximum failures were seen in Group 3 [Table 4]. The only patient in Group 1 who failed treatment failed at anastomotic site.

Local control and survival rates are significantly higher in patients who completed their planned treatment compared to the defaulters [Table 5a].
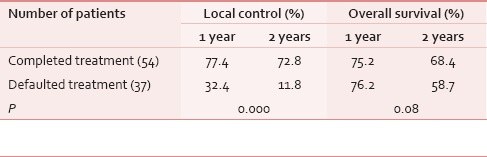
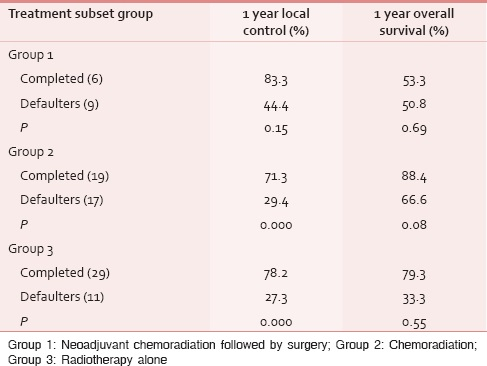
Table 5b shows that there are significantly higher local control and survival rates among the patients who completed their planned treatment in Groups 2 and 3, compared to the defaulters. However, the difference could not reach statistical significance in Group 1 patients. This can be attributed to relatively small number of patients analyzed in Group 1.
Attempt to salvage therapy
The patient in Group 1 who failed at anastomotic site and all patients in Group 2 who failed locally were further planned for surgery. When surgery was not feasible, palliative systemic taxol-based chemotherapy was planned. However, all the patients treated by radiation alone (Group 3) were given supportive care only.
DISCUSSION
The benefit of multimodality therapy is clearly established for esophageal carcinomas, but its impact on toxicity is not well defined. The purpose of this study is to address the impact of multimodality therapy on treatment toxicity for patients of esophageal carcinoma treated at our institute and thereby identify the factors hindering the planned treatment completion.
Taken individually, surgery, radiotherapy, and chemotherapy, each has its own toxicities and complications. When used in combination, not only can there be an additive effect of the adverse effects, but often, one modality can intensify the toxicities of the other.
The incidence of toxic effects of radiotherapy can vary largely depending on dose, fractionation, treatment volumes, techniques, and whether administered concomitantly with chemotherapy. Still, the nature of toxic effects is consistent with the most common acute toxic effect being esophagitis. In patients of carcinoma esophagus, who often presents with dysphagia and poor nutritional status, the addition of esophagitis often results in dehydration and malnutrition requiring feeding tubes in half of the patients.[10] Other common acute toxicities include gastrointestinal effects and fatigue. In the radiotherapy-alone arm of Radiation Therapy Oncology Group (RTOG) 85-01, 28% of patients experienced severe acute toxicities. The most common acute toxicities were upper aerodigestive (18%).[11] In our study also, acute radiation toxicity in the form of esophagitis and absolute dysphagia led to treatment interruption in three patients (7.5%). In four other patients (10%), loss of appetite during radiation led to excessive weight loss and overall deteriorated general condition, and thus further radiotherapy could not be delivered.
Although acute esophagitis is most commonly ascribed as an adverse effect of radiotherapy, the addition of radiosensitizing chemotherapy does increase the incidence and severity of esophagitis. In RTOG 85-01 trial, the addition of concurrent cisplatin/5-FU chemotherapy increased the incidence of severe upper aerodigestive toxicity from 18% with 64 Gy of definitive radiotherapy to 33% with 50 Gy of definitive chemoradiotherapy.[11] The rates of severe (Grade ≥3) esophagitis have ranged from 16% to 63% in studies using chemoradiotherapy.[11121314] Other common gastrointestinal side effects are nausea, vomiting, and diarrhea. In clinical practice, patients receiving chemoradiotherapy, whether definitive or neoadjuvant, will almost universally experience some degree of esophagitis. In our study also, four patients (11.1%) on chemoradiation had acute esophagitis Grade ≥3 and two patients (5.5%) had chemotherapy-induced severe nausea and vomiting because of which they could not complete their planned treatment.
Hematologic toxicity is a well-expected toxicity observed with strategies using chemotherapy, the most common toxicity being neutropenia/granulocytopenia. Based on the trial comparing radiotherapy with chemoradiotherapy (RTOG 85-01), the addition of chemotherapy to radiotherapy significantly increases acute hematologic toxicity from 3% to 48%.[11] Myelosuppression leading to treatment interruption was seen in three patients (8.33%) in our study.
The recent meta-analysis of neoadjuvant chemotherapy or chemoradiotherapy prior to surgery for esophageal carcinoma showed no significant association between the surgical complications and neoadjuvant interventions.[15] Yet, the addition of chemoradiotherapy to surgery alone will induce both acute and late adverse effects that would not be seen with surgery alone. In the trials of multimodality therapy, the rate of Grade 3 toxicity ranges from 41% to 46%.[16171819]
In our study, patients who could not complete the planned trimodality therapy can be categorized into two groups. The first group of defaulters (4 in number [26.7%]) experienced severe acute hematological and gastrointestinal toxicities after neoadjuvant chemoradiotherapy after which they could not proceed for surgery. Another group (five patients = 33.33%) completed neoadjuvant chemoradiotherapy and surgery but had excessive loss of weight postoperatively due to loss of appetite and inadequate diet and hence could not be delivered adjuvant chemotherapy. This aspect of treatment interruption can be seen commonly in resource constrained set up in developing countries where adequate postoperative nutritional counseling is lacking.
In addition, fistula formation, disease progression during treatment, and lost to follow-up (leading to noncompletion of radiotherapy schedule) were the three factors which were unrelated to the toxicity of chemoradiotherapy but led to treatment interruption in nine of our patients.
To achieve cure which is the main aim of treatment in all cancer patients, the treatment completion rates must be improved. Various interventions can be made to improve these rates.
Nutritional counseling should ideally be offered to all the patients before any cancer-directed therapy is initiated. Ours being a setup with heavy patient load, the nutritional counseling is done by oncologist only. We also have a palliative care section where patients with poor nutritional intake, are admitted, and are built up before starting radical treatment. However, due to limited resources in setup like ours, every patient cannot be provided such facilities. Therefore, for adequate diet counseling, a dietitian or nutritionist should be the part of the team management. European Society for Clinical Nutrition and Metabolism published their guidelines on enteral nutrition (EN) for nonsurgical cancer patients in 2006.[7] EN referred to oral nutritional supplements (ONSs) or tube feeding. According to the guidelines, EN should be started if undernutrition already exists or if food intake is markedly reduced for more than 7-10 days. EN is indicated preoperatively for 5-7 days in cancer patients undergoing major abdominal surgery. During radiotherapy of gastrointestinal regions, dietary counseling and ONS prevent weight loss and interruption of radiotherapy.
Treatment-related acute mucositis might lead to exaggeration of dysphagia in these patients, and it should be aggressively managed with enteral feeding through Ryles tube (RT) or feeding jejunostomy (FJ) or percutaneous gastrostomy (PEG) depending on the institutional protocols.
Esophageal stenting is another option to relieve malignant dysphagia before neoadjuvant or radical chemoradiation so as to maintain adequate nutrition during treatment.[2021] However, stenting is an expensive procedure compared to FJ/PEG and is associated with complications such as stent migration, tumor ingrowth, and overgrowth, esophagorespiratory fistula, for which stent replacements are required.[22] Therefore, it should be judiciously used in palliative setting only.
Patients on chemotherapy, especially when combined with radiation, may require prophylactic treatment so as to prevent chemotherapy-induced nausea-vomiting and myelosuppression.
For patients who receive concurrent chemoradiation, antiemetic therapy is dictated by the emetogenicity of chemotherapy unless the emetic risk of radiation therapy is higher.[23]
In our setup, patients who are given concurrent cisplatin are given a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist for 5 days postchemotherapy. The recent update on the use of antiemetics by “American Society of Clinical Oncology (ASCO)” however recommends that all patients who receive highly emetogenic chemotherapy regimens should be offered a three-drug combination of a neurokinin 1 receptor antagonist, 5-HT3 receptor antagonist, and dexamethasone.[23] Preferential use of palonosetron is recommended for moderate emetic risk regimens, combined with dexamethasone. For low-risk agents, patients can be offered dexamethasone before the first dose of chemotherapy.
Chemotherapy-induced myelosuppression is considered to be a significant negative prognostic factor for overall survival as it results in earlier discontinuation of the preoperative chemoradiation schedules, often requiring dose reduction of chemotherapeutic agents.[24] Still according to the ASCO 2015 update of recommendations for the use of Colony-stimulating factors (CSFs) in cancer patients receiving chemotherapy, the use of CSFs to prevent and treat chemotherapy-induced myelosuppression should be avoided in patients receiving concomitant chemoradiation, particularly involving the mediastinum.[25] In the absence of chemotherapy, therapeutic use of CSFs may be considered in patients receiving radiation therapy alone if prolonged delays secondary to neutropenia are expected.[25]
Psychosocial support is another aspect of treatment counseling which is often neglected in developing countries and at the centers with patient overload. It has been proven that patients with gastrointestinal cancer, who undergo surgery, benefit from a formal program of psychotherapeutic support during the inpatient hospital stay in terms of long-term survival.[26] A well-trained psychotherapist should therefore be a part of the management protocols designed to treat patients. In resource-constrained centers, again, an oncologist can take over the role as is being done at our institute. Patients should be counseled both before and during the treatment by providing educational information regarding the need for adequate diet, disease prognosis, benefits of treatment, and a supportive relationship should be developed. They should also be provided on-going emotional and cognitive support.[27] Patients on radiation sometimes get relief from dysphagia in the middle of the course of radiotherapy schedule and stop treatment in between considering it to be the cure of disease. Some patients who develop radiotherapy- and chemotherapy-induced toxicities also leave the treatment in between. Adequate counseling should be done in such patients from the beginning of treatment for the need and impact of completing the entire treatment. Especially before discharge, the therapist should explore the patient's emotional and cognitive interpretation of the treatment and assist him in planning for future.
Based on the results of this audit, to improve compliance rates in our patients, we have introduced following major changes. Before starting treatment, we nowadays refer all the patients with esophageal cancer to the dietitian for adequate diet counseling. An attempt is made to encourage all patients with more than or equal to Grade 2 dysphagia for RT insertion or at least FJ. All such patients are assessed twice weekly for the adequacy of their dietary intake. All patients on concurrent chemotherapy with cisplatin are given a three-drug combination of neurokinin 1 receptor antagonist, 5-HT3 receptor antagonist, and dexamethasone if emesis is not controlled by 5-HT3 receptor antagonist alone.
CONCLUSIONS
Patients of carcinoma esophagus by the nature of their disease are bound to have nutritional inadequacy either due to primary dysphagia or cancer-related loss of appetite. Radiation- and chemotherapy-induced toxicities further decrease the compliance of patients to treatment completion. Timely intervention by the management team consisting of an oncologist, dietitian, and psychotherapist can help overcome these factors and thereby improve the treatment completion rates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology. 2015;149:302-17.e1.
- [Google Scholar]
- Postchemoradiotherapy pathologic stage classified by the American Joint Committee on the Cancer staging system predicts prognosis of patients with locally advanced esophageal squamous cell carcinoma. J Thorac Oncol. 2015;10:1481-9.
- [Google Scholar]
- Role of chemoradiotherapy in oesophageal cancer – Adjuvant and neoadjuvant therapy. Clin Oncol (R Coll Radiol). 2014;26:522-32.
- [Google Scholar]
- Multimodal treatment of esophageal carcinoma. Dtsch Med Wochenschr. 2014;139:2141-7.
- [Google Scholar]
- The impact of multimodality therapy of distal esophageal and gastroesophageal junction adenocarcinomas on treatment-related toxicity and complications. Semin Radiat Oncol. 2013;23:60-73.
- [Google Scholar]
- ESPEN guidelines on enteral nutrition: Non-surgical oncology. Clin Nutr. 2006;25:245-59.
- [Google Scholar]
- Importance of nutritional screening in treatment of cancer-related weight loss. Lancet Oncol. 2005;6:334-43.
- [Google Scholar]
- CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176-81.
- [Google Scholar]
- Phase II evaluation of preoperative chemoradiation and postoperative adjuvant chemotherapy for squamous cell and adenocarcinoma of the esophagus. J Clin Oncol. 2000;18:868-76.
- [Google Scholar]
- Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-7.
- [Google Scholar]
- INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-74.
- [Google Scholar]
- Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551-6.
- [Google Scholar]
- Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-8.
- [Google Scholar]
- Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011;12:681-92.
- [Google Scholar]
- Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-92.
- [Google Scholar]
- Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol. 2005;6:659-68.
- [Google Scholar]
- Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-13.
- [Google Scholar]
- A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-7.
- [Google Scholar]
- Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc. 2012;76:44-51.
- [Google Scholar]
- Nutritional support with endoluminal stenting during neoadjuvant therapy for esophageal malignancy. Ann Surg Oncol. 2009;16:3161-8.
- [Google Scholar]
- Malignant dysphagia: Palliation with esophageal stents – Long-term results in 100 patients. Radiology. 1998;207:513-8.
- [Google Scholar]
- Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol. 2016;34:381-6.
- [Google Scholar]
- Impact of chemoradiation-induced myelosuppression on prognosis of patients with locally advanced esophageal cancer after chemoradiotherapy followed by esophagectomy. Anticancer Res. 2015;35:4889-95.
- [Google Scholar]
- Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199-212.
- [Google Scholar]
- Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol. 2007;25:2702-8.
- [Google Scholar]
- Patients’ adjustment to cancer: The mental adjustment to cancer (MAC) scale vs clinical ratings. J Psychosom Res. 1989;33:373-7.
- [Google Scholar]






