Translate this page into:
Translational Research in Oncology: Implications for Palliative Care
Address for correspondence: Dr. Arunangshu Ghoshal, 503B, Iris Brook House, Talbot Yard, London SE1 1XT, UK. E-mail: arun.bata@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The label “translational research” (TR) has become ever more popular in the biomedical domain in recent years. It is usually presented as an attempt to bridge a supposed gap between knowledge produced at the laboratory bench and its use at the clinical bedside. This is claimed to help society harvest the benefits of its investments in scientific research. The past decade has witnessed a remarkable acceleration in the pace of translational cancer medicine – genome sequencing of various human cancers has been broadly deployed in drug discovery programs, diagnostic tests have been developed to predict molecularly targeted anticancer agents, advent of cancer immunotherapies, an enhanced appreciation of the complex interactions that exist between tumor cells and their microenvironment have revolutionized the management of cancers. Treatment for cancer and palliative care (PC) go hand in hand and the role of TR in PC can no longer be ignored. This paper discusses about the scientific discourse of TR in cancer care and its implications for the practice of PC. It starts with a brief reconstruction of the history of the concept and subsequently unravels how the label is used in clinical/research practice. In conclusion, TR seems to be driven by a changed relationship between science and society. “Translation” has become important because society is thought to deserve a tangible return in terms of health and quality of life on its investment in basic biomedical science.
Keywords
Cancer
palliative care medicine
translational research
INTRODUCTION
Recent decades have yielded unprecedented advances in understanding the biological mechanisms underlying cancer development, progression, and symptomatology. Translation of this knowledge to the clinic promises new treatments tailored to exploit the molecular intricacies of a patient's tumor. In the cancer field, this is what is generally understood by translational cancer research. Translational research (TR) has been described in various ways such as – “taking research from bench-to-bedside;”[1] “bridging basic research and medical innovation;”[2] “translating research into medical practice (…);”[3] “translating science into better healthcare.”[4] This article discusses about the translational gap, the models of the translational process, current issues, challenges, and successes with clinical implementation of translational science. It focusses on TR in oncology and its implications for palliative care (PC) medicine.
TRANSLATIONAL RESEARCH IN THE BIOMEDICAL DOMAIN
A nonsystematic search with the term “TR” in PubMed shows that the term came in biomedical journals around 1950 and grew exponentially from about the year 2000 onward [Figure 1].
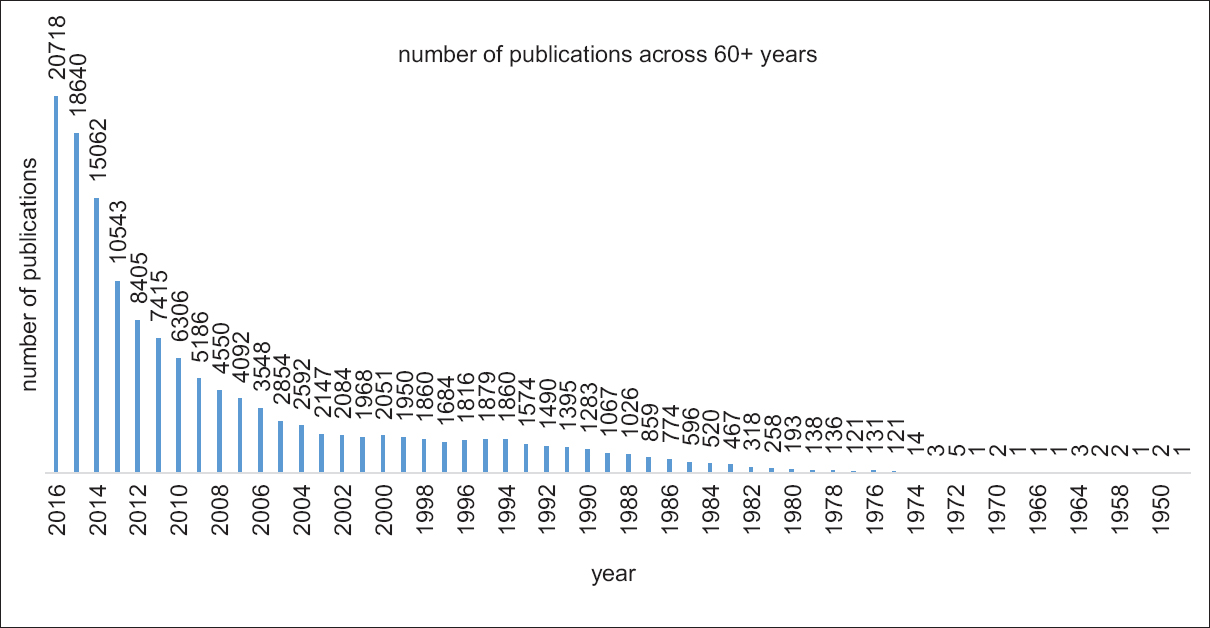
- Number of published articles with the terms “translational research”, “translational science,” or “translational medicine” each year as appeared in a PubMed search (carried out in December 2016)
The early publications were mostly focused on research in oncology.[5678910] Its inception goes back to the establishment of Specialized Programs of Research Excellence by the U.S. National Cancer Institute in 1992, which phrased TR as “from bench to bedside”[10] though the translational process can be linear or even bidirectional. Lately, many institutions have started academic programs in Clinical and Translational Science such as the British Medical Research Council,[11] the Advisory Council on Health Research (RGO) in the Netherlands,[12] and the European Advanced Translational Research Infrastructure in Medicine in 2009.[2]
THE TRANSLATIONAL GAP
TR attempts to link basic sciences with new approaches for preclinical, clinical science, and medical applications. It “bridges” the “gap” between basic life sciences and clinical medicine.[13] The “gap” can has been represented as “narrow” T1 and “broader” T2, T3, and T4 [Figure 2].[14]
![“Blue Highways” on the NIH Roadmap[14]](/content/137/2017/23/4/img/IJPC-23-462-g002.png)
- “Blue Highways” on the NIH Roadmap[14]
There are two identifiable causes of translational gap which can be found in biomedical scientific practice – “external” and “internal.” The “external” views are centered around individuals who can further the translational process and deals with lack of funds, paucity of communication between laboratory researchers and clinicians, or strict regulatory guidelines for human research. Most frequently, the translational gap is within the research and development phase in linear model. However, lack of awareness among clinicians about the latest biomedical advances can hinder translation as well.[15] Thus, developing clinical guidelines can be an essential translational endeavor.[16] Again, a gap in between implementation and improved health can be related to the expenses with new drugs or diagnostics, where insurance companies might need to look at current policies of reimbursement.[17] Lack of backward translation is, on the other hand linked to “external” causes, like restricted access to results of scientific research[18] and nonpublication of important negative results.[19] The “internal” views involve scientific researchers and technology developers.[202122] Several authors have argued that in vitro and animal models are not par with the complex mechanisms in humans[2324] and more realistic models are needed.[2526] Other authors criticize randomized clinical trials,[162728] citing them to be population selective with limited external validity.[272930] In these papers, a constant urge has been the need for integration between life sciences,[18] different experimental approaches,[31] clinical sciences,[32] and even population studies.[33] Such integration will need better computation power to handle big databases which can only be provided with better technology systems. Thus, there is a need to focus on new research methods along with traditional ones.[333435]
MODELS OF THE TRANSLATIONAL PROCESS
The underlying model of the translational process is useful to distinguish different interpretations of TR. It can be a linear model, a bidirectional model, or a complex one. Linear model in innovation involves the achievement of the last step in individual or public health through preceding ones in a stepwise pattern.[303637] It assumes that innovation starts with basic science and is translated through medical knowledge in pathophysiology before resulting in clinically relevant technologies. Thus, different steps in innovation would run on a time-dependent manner with a clear division in the role played by each person involved and thus might be a systematic way of achieving results. However, some authors have criticized this approach as inadequate. Stokes[38] have pointed out “use-inspired basic research” in the history of biomedical innovation. He brings about the contribution of Louis Pasteur in the field of microbiology. Bijker[39] mentions about a “pull and push”[40] mechanism among various social groups to bring about multidimensional development in technology. Many authors have criticized the unidirectional view on translation as they believe basic knowledge in science can be modified and researched with findings from clinical and population studies. They have coined this term as “backwards translation.” This phenomenon can be conceptualized either from a “narrow” view or from a “broad (er)” viewpoint. The “narrow” view conceptualizes that the knowledge gained in a specific stage of research is “fed back” to the earlier phases.[4142] The “broader” views want the early phases to be designed hand-in-hand with clinical practice, individual, and/or population behavior.[3037434445] There have also been pleas to involve endusers,[464748] communities[4950] in service utilization-based research. Some authors have even gone a step ahead to coin the translational process as “multidirectional” and “dynamic,” increasing the jargons.[434651] There the translational process is regarded as a continuous data exchange within and between various research and nonresearch practices.
CURRENT PRACTICE
TR encompasses a heterogeneous set of activities such as clinical biomarker development, introducing novel techniques in clinical studies, the research and development of a novel technique, formulation of guidelines for clinical practice, and subsequent development of scientific knowledge.[5253] The value of TR is incontestable. It has resulted in a “translational ethos” in biomedical science[54] and an obligation for biomedical researchers.[51] TR encompasses a heterogeneous set of activities such as clinical biomarker development, research, and development of novel techniques, introducing novel designs in clinical studies, formulation of guidelines for clinical practice, and subsequent development of scientific knowledge[53] but has seen few publications in the fields of PC, social sciences, arts, and humanities.[54] There is a need to open this domain of research to PC as well. There have been notable successes of TR in cancer. This brings up new challenges as well as opportunities for PC:
-
Microarray technology, next-generation sequencing, and whole-exome/genome/RNA sequencing platforms generate huge amounts of genomic data to unravel the complex biology of cancers and variations in clinical response.[55] This has led to the development of newer therapies such as cancer immunotherapy and targeted gene therapy.[56] Many of these therapies are offered in clinical trials to patients with advanced cancer and that has increased the responsibility of PC.[57] It is important to mention here that PC is different from end-of-life care. Patients with cancer can present to hospitals with acute deteriorations in their health, and in this rapidly shifting situation, it can be challenging to distinguish correctly between a treatable cause (leading to recovery), a transition to PC, or a transition to the last days of life. A timely recognition of both the transition to PC and the transition to the very end of life are required for optimal care as has been pointed out in a discussion paper[58]
-
Humanized mice models have been developed to overcome the ethical and technical constraints of studying human biology in vivo to study cancers, implication of rational chemotherapy, and in pain research. However, the complexity of the phenomenon of pain has made it difficult to assess the true value of these advances as pain studies are importantly affected by a wide range of modulatory factors, including sex, genotype, and social communication, all of which must be taken into account when using these models[59]
-
PC drugs are often used outside their prescribing license because strict adherence to recommendations may be impractical.[60] Novel research techniques might help avoid untoward implications of such necessary practice. Use of predictive models such as isolated, paced Langendorff-perfused heart model in female rabbit to understand drug-induced electrophysiological effects,[61] “high content screening technology” in humans,[62] programs such as Simcyp®63 and pharmacokinetic-pharmacodynamic modeling[64] can be contemplated. The extrapolation of these model outputs can help in better planning, efficacy, and reduced side effects in drug trials
-
Biomarkers are used for diagnosis, selection of therapy, prognosis, and for providing insights in disease progression in oncology.[65] The Neuberger review made several recommendations to improve end of life care, including research into the biology of dying. An important aspect of the biology of dying is the identification of biomarkers as indices of disease processes. Biomarkers have the potential to inform the current, limited understanding of the dying process and assist clinicians in recognizing dying, in particular how to distinguish dying from reversible acute deterioration.[66] An exploratory analysis of prospective or retrospective clinical data could be a way forward to determine the prognostic-predictive clinical utility of a biomarker. A well-designed randomized controlled clinical trial (RCT) could follow this for further validation
-
Functional neuroimaging and related neuroimaging techniques are becoming important tools for rehabilitation research in PC. They can be used to determine the effects of brain injury or disease on cognition and behavior. These techniques include functional magnetic resonance imaging, positron emission tomography, electroencephalography, magnetoencephalography, near-infrared spectroscopy, and transcranial magnetic stimulation. Related diffusion-weighted magnetic resonance imaging techniques, including diffusion tensor imaging and high angular resolution diffusion imaging can quantify white matter integrity[67]
-
Research in PC is a heterogeneous field that encompasses both qualitative and quantitative methods and descriptive as well as interventional study designs. Despite providing valuable evidence, its progress has been impeded by a persistent uncertainty about the ethics of these studies.[68] For instance, there have been concerns raised about whether patients near the end of life should ever be asked to participate in research although others have objected to this extreme position.[69] Again, while administering informed consent of the participants in PC, balance between the ethical issues of right of the individual to receive compassionate care and the scientific quest for efficacy and safety of specific therapies needs to be maintained. There are many difficulties in conducting RCTs in PC, and use of innovative approaches such as response-adaptive randomization procedures has been considered. Such designs focus on minimizing the expected treatment failures while maintaining the power and randomization benefits.[70] “Add-on” trials, where a new treatment is added to the current treatments, the patient is receiving, and rescue strategies may reduce the consequences to the individual patients from being randomized to a placebo treatment.[71] Crossover trial designs may also be useful in studies of diseases but are only applicable when symptoms are relatively stable. Using patients as their own control markedly increases the power of the study, but concerns about carryover effects between treatment periods are a serious risk to the validity of the study.[72] Again, reluctance of participants may result in under-enrolment or selective enrolment which may fail to provide adequate precision in quantifying the treatment effect and increase the probability of type II errors. By including people with relatively early or mild symptoms, the response rate may be higher than expected; whereas novel interventions for recalcitrant subjects may have a lower response rate than standard therapy, thereby underestimating the treatment's potential usefulness. Poor participant adherence and dropout are another problem which can substantially bias the results of a trial.[73] Although intention-to-treat analyses may mitigate this bias if nonadherence or dropout rates are higher in one group than in the other, such analyses may also prevent a true effect of treatment from being detected.[74] New kind of trials has been designed to overcome these challenges, for instance the “N-of-1” trial, standard parallel-arm RCTs, fast-track RCTs where controls receive delayed access to the intervention and adaptive RCTs.[6775] They can be either individual patient or cluster randomization types. Pragmatic trials, implementation research, community-based participatory research, and demonstration projects are new approaches to research that aim to close the gap and bring research close to the real-life healthcare.[7677]
CONCLUSION
New medical knowledge and technologies are important building blocks to enhance health and quality of life in the society. TR in cancer has led to improvements in molecular diagnosis, tumor heterogeneity, and newer systematic therapies. This has brought challenges to the clinic in terms of patient education, toxicity management, workflow, and prognostication for PC. The process of “translation” is a nexus of many ideas involved in the design of scientific work, which needs anticipation and coordination with the requirements for applicability in the future. Thus, collaboration between researchers and stakeholders is vital to ensure the success of TR and benefits to science and society.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I would like to thank the Department of Palliative Medicine, Tata Memorial Hospital, Mumbai, India and King's College London, Division of Cancer Studies, London, UK. The views expressed are those of the author.
REFERENCES
- From bench to bedside: The growing use of translational research in cancer medicine. Am J Transl Res. 2010;2:1-18.
- [Google Scholar]
- Translational Medicine | EATRIS. Available from: http://www.eatris.eu/
- Shattuck lecture – Clinical research to clinical practice – lost in translation? N Engl J Med. 2003;349:868-74.
- [Google Scholar]
- CTMM Website. Available from: http://www.ctmm.nl/pro1/general/start.asp?i=1&j=3&k=0&p=0&itemid=53
- Translational research in the San Antonio breast cancer SPORE. 1994. Breast Cancer Res Treat. 32:1-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7819578
- [Google Scholar]
- Scientific basis for cancer prevention. Intermediate cancer markers. Cancer. 1993;72(3 Suppl):978-83.
- [Google Scholar]
- Behavioral science in translational research and cancer control. Cancer. 1994;74(4 Suppl):1409-17.
- [Google Scholar]
- Trends in program project grant funding at the National Cancer Institute. Cancer Res. 1993;53:477-84.
- [Google Scholar]
- Home – Medical Research Council. Available from: http://www.mrc.ac.uk/
- Advisory Council on Health Research. Translational research in the Netherlands. Between laboratory and clinical practice. The Hague: Advisory Council on Health Research (RGO) 2007 publication no. 55. ISBN 978-90-5549-649-5
- [Google Scholar]
- A decade of exploring the cancer epigenome – Biological and translational implications. Nat Rev Cancer. 2011;11:726-34.
- [Google Scholar]
- Practice-Based Research – “Blue Highways” on the NIH Roadmap. JAMA. 2007;297:403-6.
- [Google Scholar]
- Translational research. 2011. Anesthesiology. 115:909-11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21934485
- [Google Scholar]
- Building research programs in diagnostic radiology. Part III. Clinical and translational research. Radiology. 2007;243:5-9.
- [Google Scholar]
- Translational medicine 2.0: From clinical diagnosis-based to molecular-targeted therapies in the era of globalization. Clin Pharmacol Ther. 2010;87:642-5.
- [Google Scholar]
- Translational research: Is there a future? J Thorac Cardiovasc Surg. 2007;133:1409-11.
- [Google Scholar]
- Emerging subspecialties in neurology: Translational research in movement disorders. Neurology. 2009;73:e40-1.
- [Google Scholar]
- Configuration challenges: Implementing translational research policies in electronic medical records. Acad Med. 2007;82:661-9.
- [Google Scholar]
- Translational research on vaccines: Influenza as an example. Clin Pharmacol Ther. 2007;82:745-9.
- [Google Scholar]
- Promoting translational research in academic health centers: Navigating the “roadmap”. Acad Med. 2005;80:1012-8.
- [Google Scholar]
- Translational research in the development of novel sepsis therapeutics: Logical deductive reasoning or mission impossible? Crit Care Med. 2009;37(1 Suppl):S10-5.
- [Google Scholar]
- Manual exfoliation plus immunomagnetic bead separation as an initial step toward translational research. Arch Pathol Lab Med. 2006;130:74-9.
- [Google Scholar]
- Vaccine epidemiology: Efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201:1607-10.
- [Google Scholar]
- Translational research. 2008. Br Med J. 337:a863. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18755767
- [Google Scholar]
- Translational research: Bridging the gap between long-term weight loss maintenance research and practice. J Am Diet Assoc. 2010;110:1511-22.:1522.e1-22.
- [Google Scholar]
- Cancer stem cells: The centrality of translational research to cancer control. CMAJ. 2007;176:29-30.
- [Google Scholar]
- Clinical pharmacology as a foundation for translational science. Clin Pharmacol Ther. 2011;90:10-3.
- [Google Scholar]
- APM. Translating basic discoveries into better health care: The APM's recommendations for improving translational research. Am J Med. 2004;116:431-4.
- [Google Scholar]
- Principal Investigators of National Institutes of Health Clinical and Translational Science Awards. Linking scientific discovery and better health for the nation: The first three years of the NIH's Clinical and Translational Science Awards. Acad Med. 2010;85:457-62.
- [Google Scholar]
- Clinical and translational science awards: A framework for a national research agenda. Transl Res. 2006;148:4-5.
- [Google Scholar]
- Translational research: Understanding the continuum from bench to bedside. Transl Res. 2011;157:1-5.
- [Google Scholar]
- Translational medicine in neurology: The time is right. Arch Neurol. 2010;67:1263-6.
- [Google Scholar]
- 2011. Pasteur's Quadrant: Basic Science and Technological Innovation. Available from: https://www.books.google.com/books?hl=en&lr=&id=TLKDbvJX86YC&oi=fnd&pg=PA1&ots=TUKYAnPUXW&sig=e-wFjzKSfDE3v-8SoLRySx6C-Rc
- 1997. Of Bicycles, Bakelites, and Bulbs: Toward a Theory of Sociotechnical Change. Available from: https://www.books.google.com/books?hl=en&lr=&id=IsbmwN8-m1cC&oi=fnd&pg=PR9&ots=4QeMQ7WuUL&sig=QL0H15zz_Nekl5Pfw0LT2AcX_HA
- Ethics and technology in the making: An essay on the challenge of nanoethics. 2007. Nanoethics. 1:21-30. Available from: http://www.link.springer.com/10.1007/s11569-007-0006-7
- [Google Scholar]
- The National Heart, Lung, and Blood Institute bench to bassinet program: A new paradigm for translational research. J Am Coll Cardiol. 2010;55:1262-5.
- [Google Scholar]
- Defining translational research: Implications for training. Acad Med. 2010;85:470-5.
- [Google Scholar]
- Translational research for cardiovascular diseases at the National Heart, Lung, and Blood Institute: Moving from bench to bedside and from bedside to community. Circulation. 2010;121:929-33.
- [Google Scholar]
- Linking practice-based research networks and Clinical and Translational Science Awards: New opportunities for community engagement by academic health centers. Acad Med. 2010;85:476-83.
- [Google Scholar]
- Creating a culture of discovery through clinical trials and translational research. Am J Med Sci. 2009;337:155.
- [Google Scholar]
- Translational research: Moving discovery to practice. Clin Pharmacol Ther. 2007;81:126-8.
- [Google Scholar]
- The role of the clinical and translational science awards program in improving the quality and efficiency of clinical research. Chest. 2011;140:764-7.
- [Google Scholar]
- Translational research in environmental health sciences. 2008. Transl Res. 151:57-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18201672
- [Google Scholar]
- From bench to bedside? Biomedical scientists' expectations of stem cell science as a future therapy for diabetes. Soc Sci Med. 2006;63:2052-64.
- [Google Scholar]
- Challenges and pathways for clinical and translational research: Why is this research different from all other research? Acad Med. 2009;84:411-2.
- [Google Scholar]
- A new generation of cancer genome diagnostics for routine clinical use: Overcoming the roadblocks to personalized cancer medicine. Ann Oncol. 2015;26:1830-7.
- [Google Scholar]
- Targeted cancer therapy: The next generation of cancer treatment. Curr Drug Discov Technol. 2015;12:3-20.
- [Google Scholar]
- 2014. Targeted Cancer Therapies Fact Sheet – National Cancer Institute. :1-6. Available from: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet
- Principles of drug use in palliative care. 2009. Oxford Handbook of Palliative Care. Ch. 4. Oxford: Oxford University Press; Available from: http://www.oxfordmedicine.com/view/10.1093/med/9780199234356.001.0001/med-9780199234356-chapter-5
- [Google Scholar]
- Review of the predictive value of the Langendorff heart model (Screenit system) in assessing the proarrhythmic potential of drugs. J Pharmacol Toxicol Methods. 2004;49:171-81.
- [Google Scholar]
- High-content screening technology combined with a human granuloma model as a new approach to evaluate the activities of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59:693-7.
- [Google Scholar]
- The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5:211-23.
- [Google Scholar]
- Biomarkers in translational research: Focus on discovery, development and translation of protein biomarkers to clinical immunoassays. Expert Rev Mol Diagn. 2007;7:545-53.
- [Google Scholar]
- A systematically structured review of biomarkers of dying in cancer patients in the last months of life; An exploration of the biology of dying. PLoS One. 2017;12:e0175123.
- [Google Scholar]
- The n-of-1 clinical trial: The ultimate strategy for individualizing medicine? Per Med. 2011;8:161-73.
- [Google Scholar]
- 2015. Understanding clinical trials in palliative care research. Oxford: Oxford University Press; Vol. 1 Available from: http://www.oxfordmedicine.com/view/10.1093/med/9780199656097.001.0001/med-9780199656097-chapter-193
- Are special ethical guidelines needed for palliative care research? J Pain Symptom Manage. 2000;20:130-9.
- [Google Scholar]
- Maximizing power and minimizing treatment failures in clinical trials. Clin Trials. 2004;1:141-7.
- [Google Scholar]
- Add-on or step-up trials for new drug development in rheumatoid arthritis: A new standard? Arthritis Rheum. 2003;48:1481-3.
- [Google Scholar]
- Which treatment is better? Ascertaining patient preferences with crossover randomized controlled trials. J Pain Symptom Manage. 2015;49:625-31.
- [Google Scholar]
- Clinical trials in palliative care: A systematic review of their methodological characteristics and of the quality of their reporting. BMC Palliat Care. 2017;16:10.
- [Google Scholar]
- Analyzing phase III studies in hospice/palliative care. a solution that sits between intention-to-treat and per protocol analyses: The palliative-modified ITT analysis. J Pain Symptom Manage. 2012;44:595-603.
- [Google Scholar]
- Study protocol: Phase III single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. Trials. 2011;12:130.
- [Google Scholar]
- Scientific challenges in the application of randomized trials. JAMA. 1984;252:2739-45.
- [Google Scholar]
- Methodological challenges in supportive and palliative care cancer research. Semin Oncol. 2011;38:460-6.
- [Google Scholar]






